Relationship of Acylcarnitines to Myocardial Ischemic Remodeling and Clinical Manifestations in Chronic Heart Failure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Considerations
2.2. Laboratory Evaluations
2.3. Statistical Analysis
3. Results
3.1. General Characteristics of the Groups
3.2. Evaluation of Echocardiographic Parameters
3.3. Differences in AC Levels between Patients with CHF of Ischemic Etiology with LVEF <50% and Patients with CHD
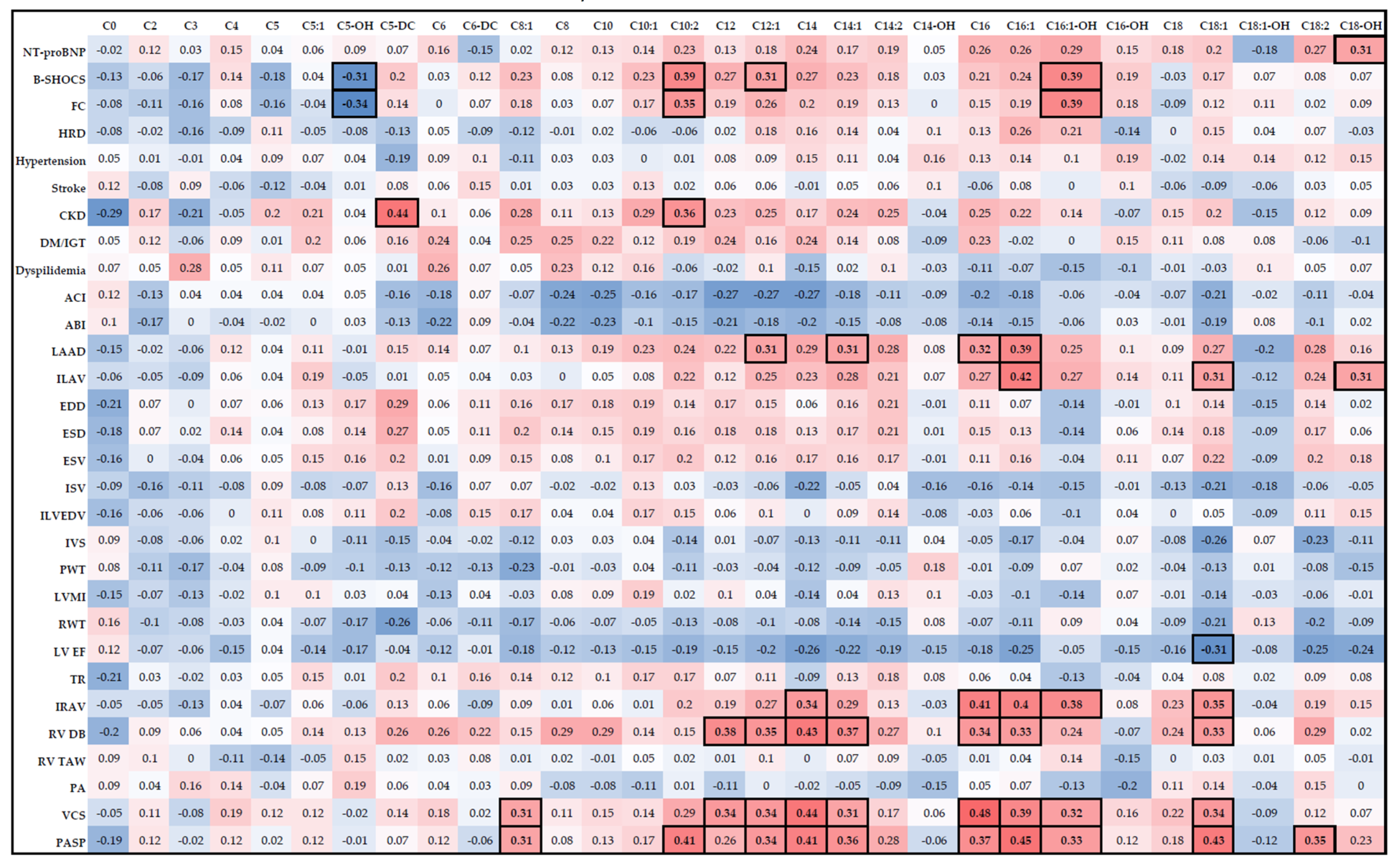
3.4. Relationship between AC Levels, Clinical Characteristics, and Remodeling Parameters in Patients with CHF of Ischemic Etiology with LVEF <50%
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Girerd, N.; Cleland, J.; Anker, S.D.; Byra, W.; Lam, C.S.P.; Lapolice, D.; Mehra, M.R.; van Veldhuisen, D.J.; Bresso, E.; Lamiral, Z.; et al. Inflammation and Remodeling Pathways and Risk of Cardiovascular Events in Patients with Ischemic Heart Failure and Reduced Ejection Fraction. Sci. Rep. 2022, 12, 8574. [Google Scholar] [CrossRef]
- Bertero, E.; Maack, C. Metabolic Remodelling in Heart Failure. Nat. Rev. Cardiol. 2018, 15, 457–470. [Google Scholar] [CrossRef]
- Makrecka-Kuka, M.; Sevostjanovs, E.; Vilks, K.; Volska, K.; Antone, U.; Kuka, J.; Makarova, E.; Pugovics, O.; Dambrova, M.; Liepinsh, E. Plasma Acylcarnitine Concentrations Reflect the Acylcarnitine Profile in Cardiac Tissues. Sci. Rep. 2017, 7, 17528. [Google Scholar] [CrossRef]
- Djoussé, L.; Benkeser, D.; Arnold, A.; Kizer, J.R.; Zieman, S.J.; Lemaitre, R.N.; Tracy, R.P.; Gottdiener, J.S.; Mozaffarian, D.; Siscovick, D.S.; et al. Plasma Free Fatty Acids and Risk of Heart Failure: The Cardiovascular Health Study. Circ. Heart Fail. 2013, 6, 964–969. [Google Scholar] [CrossRef] [PubMed]
- Berezhnov, A.V.; Fedotova, E.I.; Nenov, M.N.; Kasymov, V.A.; Pimenov, O.Y.; Dynnik, V.V. Dissecting Cellular Mechanisms of Long-Chain Acylcarnitines-Driven Cardiotoxicity: Disturbance of Calcium Homeostasis, Activation of Ca2+-Dependent Phospholipases, and Mitochondrial Energetics Collapse. Int. J. Mol. Sci. 2020, 21, 7461. [Google Scholar] [CrossRef]
- Azevedo, P.S.; Polegato, B.F.; Minicucci, M.F.; Paiva, S.A.R.; Zornoff, L.A.M. Cardiac Remodeling: Concepts, Clinical Impact, Pathophysiological Mechanisms and Pharmacologic Treatment. Arq. Bras. Cardiol. 2016, 106, 62–69. [Google Scholar] [CrossRef]
- Uskach, T.M.; Safiullina, A.A.; Makeev, M.I.; Saidova, M.A.; Shariya, M.A.; Ustyuzhanin, D.V.; Zhirov, I.V.; Tereshchenko, S.N. The effect of angiotensin receptors and neprilysin inhibitors on myocardial remodeling in patients with chronic heart failure and atrial fibrillation. Kardiologiia 2019, 59, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Reis Filho, J.R.D.A.R.; Cardoso, J.N.; Cardoso, C.M.D.R.; Pereira-Barretto, A.C. Reverse Cardiac Remodeling: A Marker of Better Prognosis in Heart Failure. Arq. Bras. Cardiol. 2015, 104, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Abboud, A.; Januzzi, J.L. Reverse Cardiac Remodeling and ARNI Therapy. Curr. Heart Fail. Rep. 2021, 18, 71–83. [Google Scholar] [CrossRef]
- Russian Society of Cardiology (Rsc). (Rsc) 2020 Clinical practice guidelines for Chronic heart failure. Russ. J. Cardiol. 2020, 25, 4083. [Google Scholar] [CrossRef]
- Moskaleva, N.E.; Shestakova, K.M.; Kukharenko, A.V.; Markin, P.A.; Kozhevnikova, M.V.; Korobkova, E.O.; Brito, A.; Baskhanova, S.N.; Mesonzhnik, N.V.; Belenkov, Y.N.; et al. Target Metabolome Profiling-Based Machine Learning as a Diagnostic Approach for Cardiovascular Diseases in Adults. Metabolites 2022, 12, 1185. [Google Scholar] [CrossRef] [PubMed]
- Kukharenko, A.; Brito, A.; Kozhevnikova, M.V.; Moskaleva, N.; Markin, P.A.; Bochkareva, N.; Korobkova, E.O.; Belenkov, Y.N.; Privalova, E.V.; Larcova, E.V.; et al. Relationship between the Plasma Acylcarnitine Profile and Cardiometabolic Risk Factors in Adults Diagnosed with Cardiovascular Diseases. Clin. Chim. Acta 2020, 507, 250–256. [Google Scholar] [CrossRef]
- Li, M.; Ning, Y.; Tse, G.; Saguner, A.M.; Wei, M.; Day, J.D.; Luo, G.; Li, G. Atrial Cardiomyopathy: From Cell to Bedside. ESC Heart Fail. 2022, 9, 3768–3784. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Fernandez, C.; Melander, O.; Ottosson, F. Altered Acylcarnitine Metabolism Is Associated With an Increased Risk of Atrial Fibrillation. J. Am. Heart Assoc. 2020, 9, e016737. [Google Scholar] [CrossRef]
- Deda, O.; Panteris, E.; Meikopoulos, T.; Begou, O.; Mouskeftara, T.; Karagiannidis, E.; Papazoglou, A.S.; Sianos, G.; Theodoridis, G.; Gika, H. Correlation of Serum Acylcarnitines with Clinical Presentation and Severity of Coronary Artery Disease. Biomolecules 2022, 12, 354. [Google Scholar] [CrossRef]
- Beuchel, C.; Dittrich, J.; Pott, J.; Henger, S.; Beutner, F.; Isermann, B.; Loeffler, M.; Thiery, J.; Ceglarek, U.; Scholz, M. Whole Blood Metabolite Profiles Reflect Changes in Energy Metabolism in Heart Failure. Metabolites 2022, 12, 216. [Google Scholar] [CrossRef]
- Kozhevnikova, M.V.; Belenkov, Y.N. Biomarkers in Heart Failure: Current and Future. Kardiologiia 2021, 61, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.G.; Kelly, J.P.; McGarrah, R.W.; Khouri, M.G.; Craig, D.; Haynes, C.; Ilkayeva, O.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.J.; et al. Metabolomic Profiling Identifies Novel Circulating Biomarkers of Mitochondrial Dysfunction Differentially Elevated in Heart Failure With Preserved Versus Reduced Ejection Fraction: Evidence for Shared Metabolic Impairments in Clinical Heart Failure. J. Am. Heart Assoc. 2016, 5, e003190. [Google Scholar] [CrossRef]
- Karagiannidis, E.; Moysidis, D.V.; Papazoglou, A.S.; Panteris, E.; Deda, O.; Stalikas, N.; Sofidis, G.; Kartas, A.; Bekiaridou, A.; Giannakoulas, G.; et al. Prognostic Significance of Metabolomic Biomarkers in Patients with Diabetes Mellitus and Coronary Artery Disease. Cardiovasc. Diabetol. 2022, 21, 70. [Google Scholar] [CrossRef]
- Gander, J.; Carrard, J.; Gallart-Ayala, H.; Borreggine, R.; Teav, T.; Infanger, D.; Colledge, F.; Streese, L.; Wagner, J.; Klenk, C.; et al. Metabolic Impairment in Coronary Artery Disease: Elevated Serum Acylcarnitines Under the Spotlights. Front Cardiovasc. Med 2021, 8, 792350. [Google Scholar] [CrossRef]
- Chen, W.-S.; Liu, M.-H.; Cheng, M.-L.; Wang, C.-H. Decreases in Circulating Concentrations of Short-Chain Acylcarnitines Are Associated with Systolic Function Improvement After Decompensated Heart Failure. Int. Heart J. 2020, 61, 1014–1021. [Google Scholar] [CrossRef]
- Selvaraj, S.; Fu, Z.; Jones, P.; Kwee, L.C.; Windsor, S.L.; Ilkayeva, O.; Newgard, C.B.; Margulies, K.B.; Husain, M.; Inzucchi, S.E.; et al. Metabolomic Profiling of the Effects of Dapagliflozin in Heart Failure With Reduced Ejection Fraction: DEFINE-HF. Circulation 2022, 146, 808–818. [Google Scholar] [CrossRef]
- Dimasi, C.G.; Darby, J.R.T.; Morrison, J.L. A Change of Heart: Understanding the Mechanisms Regulating Cardiac Proliferation and Metabolism before and after Birth. J. Physiol. 2023, 601, 1319–1341. [Google Scholar] [CrossRef] [PubMed]
- Gibb, A.A.; Hill, B.G. Metabolic Coordination of Physiological and Pathological Cardiac Remodeling. Circ. Res. 2018, 123, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Xie, X.; Cao, F.; Wang, Y. Mitochondrial Metabolism in Myocardial Remodeling and Mechanical Unloading: Implications for Ischemic Heart Disease. Front. Cardiovasc. Med. 2021, 8, 789267. [Google Scholar] [CrossRef] [PubMed]
- Shishkova, V.N.; Martynov, A.I. Insulin resistance: Focus on the pathogenesis of cardiomyopathy. Cons. Medicum 2020, 22, 52–54. [Google Scholar] [CrossRef]
- Haemmerle, G.; Moustafa, T.; Woelkart, G.; Büttner, S.; Schmidt, A.; van de Weijer, T.; Hesselink, M.; Jaeger, D.; Kienesberger, P.C.; Zierler, K.; et al. ATGL-Mediated Fat Catabolism Regulates Cardiac Mitochondrial Function via PPAR-α and PGC-1. Nat. Med. 2011, 17, 1076–1085. [Google Scholar] [CrossRef]
- Mihalik, S.J.; Goodpaster, B.H.; Kelley, D.E.; Chace, D.H.; Vockley, J.; Toledo, F.G.S.; DeLany, J.P. Increased Levels of Plasma Acylcarnitines in Obesity and Type 2 Diabetes and Identification of a Marker of Glucolipotoxicity. Obesity 2010, 18, 1695–1700. [Google Scholar] [CrossRef]
- Elmariah, S.; Farrell, L.A.; Furman, D.; Lindman, B.R.; Shi, X.; Morningstar, J.E.; Rhee, E.P.; Gerszten, R.E. Association of Acylcarnitines With Left Ventricular Remodeling in Patients With Severe Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement. JAMA Cardiol. 2018, 3, 242–246. [Google Scholar] [CrossRef]
- Drapkina, O.M.; Shirobokikh, O.E. Role of Gut Microbiota in the Pathogenesis of Cardiovascular Diseases and Metabolic Syndrome. Ration. Pharmacother. Cardiol. 2018, 14, 567–574. [Google Scholar] [CrossRef]
- Xu, J.; Verbrugghe, A.; Lourenço, M.; Cools, A.; Liu, D.J.X.; Van de Wiele, T.; Marzorati, M.; Eeckhaut, V.; Van Immerseel, F.; Vanhaecke, L.; et al. The Response of Canine Faecal Microbiota to Increased Dietary Protein Is Influenced by Body Condition. BMC Vet. Res. 2017, 13, 374. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Wu, D.; Zeng, Y.; Wu, G.; Zheng, N.; Huang, W.; Li, Y.; Tao, X.; Zhu, W.; Sheng, L.; et al. The Microbial and Metabolic Signatures of Patients with Stable Coronary Artery Disease. Microbiol. Spectr. 2022, 10, e0246722. [Google Scholar] [CrossRef] [PubMed]
- Aguer, C.; McCoin, C.S.; Knotts, T.A.; Thrush, A.B.; Ono-Moore, K.; McPherson, R.; Dent, R.; Hwang, D.H.; Adams, S.H.; Harper, M.-E. Acylcarnitines: Potential Implications for Skeletal Muscle Insulin Resistance. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Havlenova, T.; Skaroupkova, P.; Miklovic, M.; Behounek, M.; Chmel, M.; Jarkovska, D.; Sviglerova, J.; Stengl, M.; Kolar, M.; Novotny, J.; et al. Right versus Left Ventricular Remodeling in Heart Failure Due to Chronic Volume Overload. Sci. Rep. 2021, 11, 17136. [Google Scholar] [CrossRef]
- Tremblay-Gravel, M.; Fortier, A.; Baron, C.; David, C.; Mehanna, P.; Ducharme, A.; Hussin, J.; Hu, Q.; Tardif, J.-C.; Des Rosiers, C.; et al. Long-Chain Acylcarnitines and Monounsaturated Fatty Acids Discriminate Heart Failure Patients According to Pulmonary Hypertension Status. Metabolites 2021, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Craig, D.; Ilkayeva, O.; Muehlbauer, M.; Kraus, W.E.; Newgard, C.B.; Shah, S.H.; Rajagopal, S. Plasma Acylcarnitines Are Associated with Pulmonary Hypertension. Pulm. Circ. 2017, 7, 211–218. [Google Scholar] [CrossRef]
- Dambrova, M.; Makrecka-Kuka, M.; Kuka, J.; Vilskersts, R.; Nordberg, D.; Attwood, M.M.; Smesny, S.; Sen, Z.D.; Guo, A.C.; Oler, E.; et al. Acylcarnitines: Nomenclature, Biomarkers, Therapeutic Potential, Drug Targets, and Clinical Trials. Pharmacol. Rev. 2022, 74, 506–551. [Google Scholar] [CrossRef]
- Johri, A.M.; Hétu, M.-F.; Heyland, D.K.; Herr, J.E.; Korol, J.; Froese, S.; Norman, P.A.; Day, A.G.; Matangi, M.F.; Michos, E.D.; et al. Progression of Atherosclerosis with Carnitine Supplementation: A Randomized Controlled Trial in the Metabolic Syndrome. Nutr. & Metab. 2022, 19, 26. [Google Scholar] [CrossRef]
- Song, X.; Qu, H.; Yang, Z.; Rong, J.; Cai, W.; Zhou, H. Efficacy and Safety of L-Carnitine Treatment for Chronic Heart Failure: A Meta-Analysis of Randomized Controlled Trials. Biomed. Res. Int. 2017, 2017, 6274854. [Google Scholar] [CrossRef] [PubMed]
- Alhasaniah, A.H. L-Carnitine: Nutrition, Pathology, and Health Benefits. Saudi J. Biol. Sci. 2023, 30, 103555. [Google Scholar] [CrossRef]

| Characteristics | CHF-CHD Group (n = 79) | CHD Group (n = 19) | p-Value |
|---|---|---|---|
| Gender, male | 56 (70.9%) | 10 (52.6%) | 0.13 |
| Age, years | 68.2 ± 7.2 | 66.5 ± 9 | 0.38 |
| BMI, kg/m2 | 30.7 [27.6; 34.6] | 30.6 ± 5.8 | 0.65 |
| NT-proBNP, pg/mL | 2767.5 [1392.4; 3562.5] | 140 [54; 176] | <0.05 |
| Characteristics | CHF-CHD Group (n = 79) | CHD Group (n = 19) | |||
|---|---|---|---|---|---|
| Number of Patients | % of Total | Number of Patients | % of Total | p-Value | |
| History of MI | 58 | 73 | 8 | 27.8 | 0.009 |
| Rhythm disturbances: AF/AF: Paroxysmal form Persistent form Permanent form Pacemaker rhythm | 49 14 15 20 4 | 62 18 19 25 5 | 5 5 - - - | 26.3 26.3 - - - | 0.005 |
| Hypertension | 79 | 100 | 19 | 100 | - |
| Stroke | 9 | 11.4 | 3 | 15.8 | 0.6 |
| CKD, eGFR <60 mL/min/1.73 m2 | 50 | 63.3 | 7 | 36.8 | 0.42 |
| Glucose disorders DM IGT | 38 20 | 48.1 25.3 | 9 2 | 47.4 10.5 | 0.25 |
| Dyslipidemia | 57 | 72.1 | 17 | 89.5 | 0.11 |
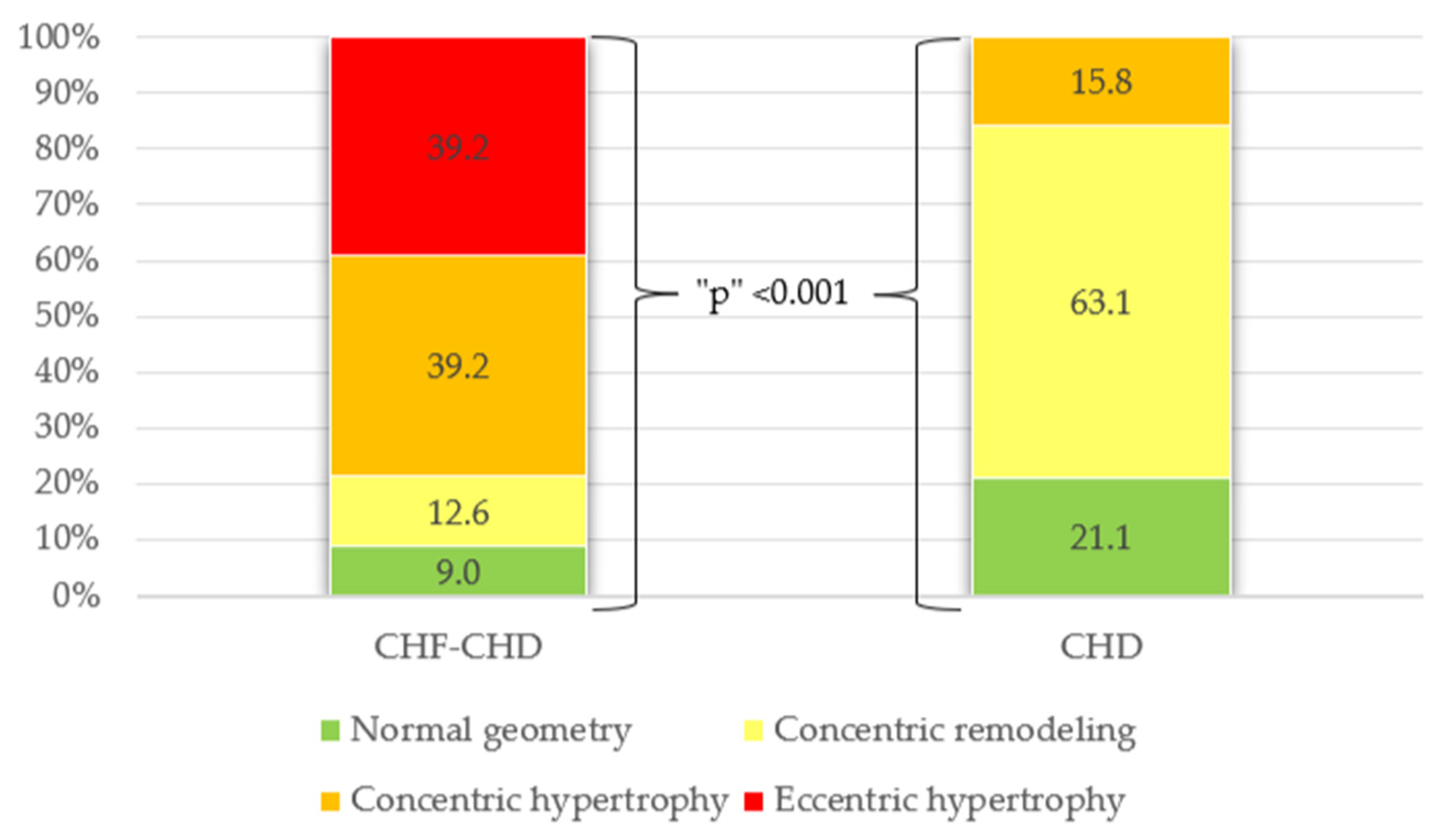
| Remodeling type according to LVMI/RWT: Normal geometry Concentric remodeling Concentric hypertrophy Eccentric hypertrophy | 7 10 31 31 | 9 12.6 39.2 39.2 | 4 12 3 - | 21.1 63.1 15.8 - | <0.001 |
| Heart Chamber | Parameters | Reference | CHF-CHD Group (n = 79) | CHD Group (n = 19) | p-Value |
|---|---|---|---|---|---|
| Left ventricle (LV) | EDD, mm | ≤58.4 (m), ≤52.2 (f) | 55 ± 7 | 47 ± 3.1 | <0.001 |
| ESD, mm | ≤39.8 (m), ≤34.8 (f) | 44 ± 7.6 | 31 ± 3.6 | <0.001 | |
| EDV index, mL/m2 | <75 (m), <62 (f) | 69 [56; 86] | 47.9 ± 9 | <0.001 | |
| ESV, mL | <58 (m), <49 (f) | 92 [64; 117] | 40.2 ± 10.9 | <0.001 | |
| SV index, mL/min/m2 | >35 | 27.4 ± 7.6 | 26.9 [23.6; 30.9] | 0.25 | |
| Ejection fraction (Biplane), % | >52 (m), >54 (f) | 37 [31; 45] | 58 [55; 60] | <0.001 | |
| IVS, mm | ≤10 (m), ≤9 (f) | 12 [11; 13] | 10.9 ± 1.8 | 0.02 | |
| LVPW, mm | ≤10 (m), ≤9 (f) | 11 [10; 12] | 10.6 ± 1.4 | 0.24 | |
| LVMI, g/m2 | ≤95 (g), ≤115 (m) | 129.3 [108.4; 142.6] | 93.2 ± 15.7 | <0.001 | |
| Left atrium (LA) | mm | ≤40 (m), ≤38 (f) | 46 [44; 50] | 37.8 ± 5.6 | <0.001 |
| LA volume index, mL/m2 | ≤34 | 44 [38; 52] | 28.2 [23.2; 30.9] | <0.001 | |
| Right ventricle (RV) | Basal diameter, mm | <42 | 39 [36; 44] | 34.2 ± 5.6 | <0.001 |
| Free wall thickness, mm | <5 | 4 [4; 4] | 4 [3; 4] | 0.43 | |
| Right atrium (RA) | RA volume index, mL/m2 | <30 (m), <28 (f). | 33 [26; 41] | 22.2 [17.2; 24.9] | <0.001 |
| Aortic dimensions | Indexed size of Valsalva sinus, mm/m2 | ≤19 (g), ≤20 (f) | 16.5 ± 2 | 16.7 [14.5; 17.6] | 0.91 |
| Indexed proximal ascending aorta size, mm/m2 | ≤17 (g), ≤19 (f) | 15.9 ± 2.1 | 15.9 [13.8;17] | 0.65 | |
| Inferior vena cava (IVC) | mm | <21 | 21 ± 4 | 20 ± 2.2 | 0.33 |
| Pulmonary artery (PA) | PA size, mm | <25 | 24 [23; 25] | 23 [22; 23] | <0.001 |
| Estimated systolic pressure in LA, mm Hg. | <31 | 42 [31; 54] | 25 [22; 29] | <0.001 |
| Name of Acylcarnitines | Abbreviations | Class | CHF-CHD Group (N = 79) (Μm) | CHD Group (N = 19) (Μm) | p-Value | Normal Range (From HMDB Database) | Rates of LVEF < 50% in Patients with Deficit of Carnitine and Acs | Mean Value in Relation to The Limits of The Range: CHF-CHD Group; CHD Group | |
|---|---|---|---|---|---|---|---|---|---|
| Suggested Range (μM) | Reference | Number and % | |||||||
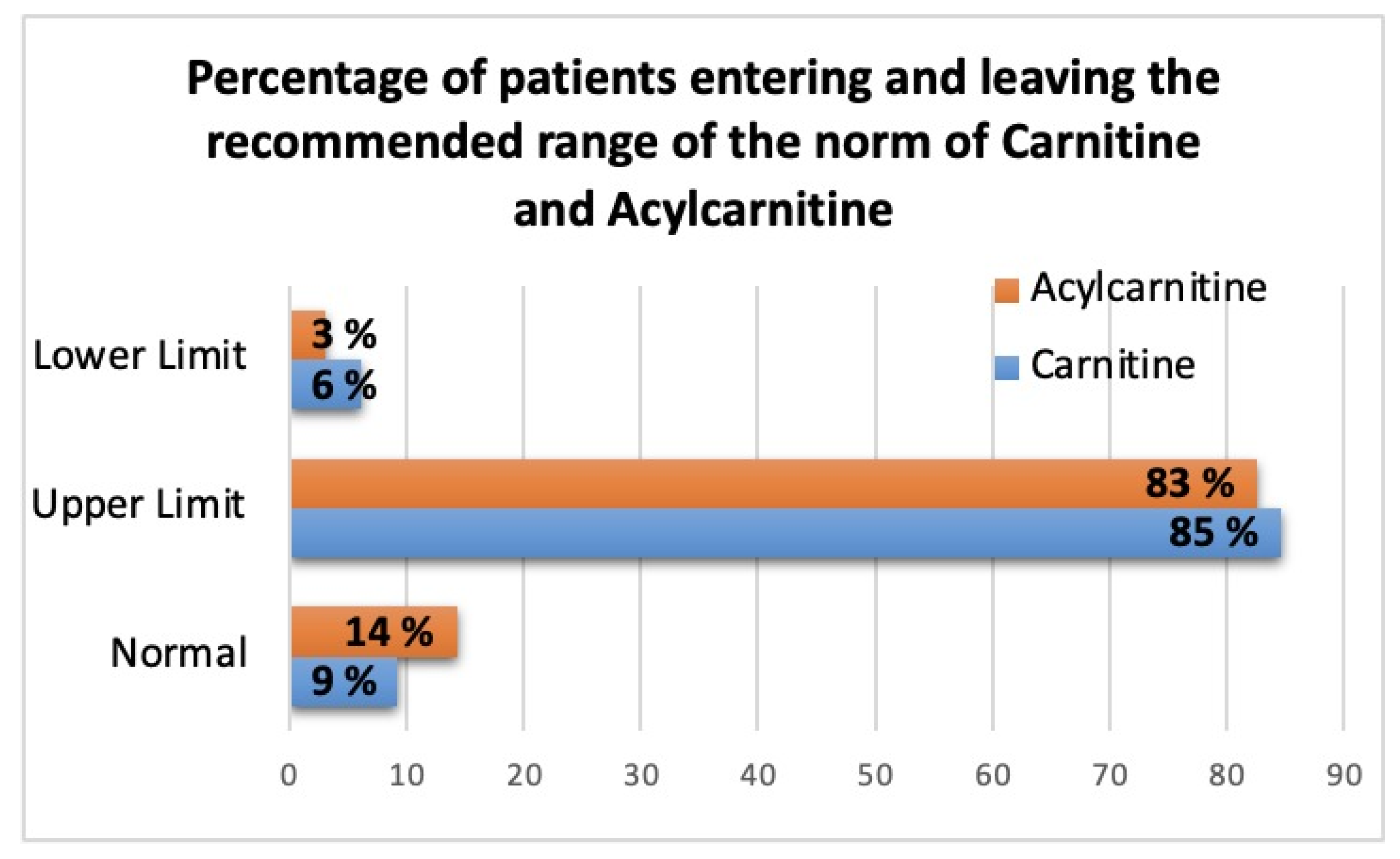
| Carnitine | C0 | Unesterified | 83.6 [48; 133] | 104.9 [67.2; 155.3] | 0.26 | 22.19–37.29 | http://www.ncbi.nlm.nih.gov/pubmed/21359215, accessed on 15 August 2023 | 5 (5.1%) | CHF-CHD: 2.2 times higher than the upper limit; CHD: 2.8 times higher than the upper limit |
| 25.4–54.1 | 15519880 | CHF-CHD: 1.5 times higher than the upper limit; CHD: 1.9 times higher than the upper limit | |||||||
| 32.8–43.6 | CHF-CHD: 1.9 times higher than the upper limit; CHD: 2.4 times higher than the upper limit | ||||||||
| 33.6–53.54 | http://www.ncbi.nlm.nih.gov/pubmed/26010610, accessed on 15 August 2023 | CHF-CHD: 1.6 times higher than the upper limit; CHD: 2 times higher than the upper limit | |||||||
| 26.08–52.58 | 26010610 | CHF-CHD: 1.05 times higher than the upper limit; CHD: 1.33 times higher than the upper limit | |||||||
| 19.0–65.0 | http://metabolomicscentre.ca/, accessed on 15 August 2023 | CHF-CHD: 1.3 times higher than the upper limit; CHD: 1.6 times higher than the upper limit | |||||||
| 28.3–42.3 | http://www.ncbi.nlm.nih.gov/pubmed/28278231, accessed on 15 August 2023 | CHF-CHD: 2 times higher than the upper limit; CHD: 2.5 times higher than the upper limit | |||||||
| 34.1–57.3 | 21359215 | CHF-CHD: 1.4 times higher than the upper limit; CHD: 1.8 times higher than the upper limit | |||||||
| Acetylcarnitine | C2 | Short-chain | 13.43 [9.1; 20.48] | 11.93 [7.5; 19.7] | 0.21 | 3.33–7.63 | 21359215 | 12 (12.2%) | CHF-CHD: 1.7 times higher than the upper limit; CHD: 1.56 times higher than the upper limit |
| 3.00–12.5 | http://metabolomicscentre.ca/, accessed on 15 August 2023 | CHF-CHD: 10.7 times higher than the upper limit; CHD: Close to the upper limit | |||||||
| 5.6–6.8 (male) | http://www.ncbi.nlm.nih.gov/pubmed/12905800, accessed on 15 August 2023 | CHF-CHD: 2 times higher than the upper limit; CHD: 1.7 times higher than the upper limit | |||||||
| 5.0–6.4 (female) | 12905800 | CHF-CHD: 2.1 times higher than the upper limit; CHD: 1.9 times higher than the upper limit | |||||||
| 4.30–8.82 | 28278231 | CHF-CHD: 1.5 times higher than the upper limit; CHD: 1.4 times higher than the upper limit | |||||||
| Propionylcarnitine | C3 | Short-chain | 0.6 [0.4; 0.79] | 0.63 ± 0.27 | 0.96 | 0.26–0.46 | 21359215 | 20 (20.4%) | CHF-CHD: 1.3 times higher than the upper limit; CHD: 1.4 times higher than the upper limit |
| 0.379–0.421 | 16425363 | CHF-CHD: 1.3 times higher than the upper limit; CHD: 1.4 times higher than the upper limit | |||||||
| 0.15–0.7 | http://metabolomicscentre.ca/, accessed on 15 August 2023 | CHF-CHD: Close to the upper limit; CHD: Close to the upper limit | |||||||
| 0.30–0.58 | 26010610 | CHF-CHD: Close to the upper limit; CHD: Close to the upper limit | |||||||
| 0.24–0.44 | http://www.ncbi.nlm.nih.gov/pubmed/28278231, accessed on 15 August 2023 | CHF-CHD: 1.4 times higher than the upper limit; CHD: 1.4 times higher than the upper limit | |||||||
| Butyrylcarnitine | C4 | Short-chain | 0.175 [0.12; 0.22] | 0.12 [0.11; 0.18] | 0.036 | 0.10–0.42 | 21359215 | 12 (12.2%) | CHF-CHD: Close to the lower limit; CHD: Close to the lower limit |
| 0.10–0.45 | http://metabolomicscentre.ca/, accessed on 15 August 2023 | CHF-CHD: Close to the lower limit; CHD: Close to the lower limit | |||||||
| 0.254–0.28 | http://www.ncbi.nlm.nih.gov/pubmed/16425363, accessed on 15 August 2023 | CHF-CHD: 1.5 times lower than the lower limit; CHD: 2.1 times lower than the lower limit | |||||||
| Tiglylcarnitine | C5:1 | Short-chain | 0.013 [0.01; 0.02] | 0.012 ± 0.005 | 0.43 | 0.04–0.06 | 21359215 | 75 (76.5%) | CHF-CHD: 3.1 times lower than the lower limit; CHD: 3.3 times lower than the lower limit |
| 0.04–0.08 | Molecular You | CHF-CHD: 3.1 times lower than the lower limit; CHD: 3.3 times higher than the upper limit | |||||||
| Isovalerylcarnitine | C5 | Short-chain | 0.07 [0.05; 0.1] | 0.07 [0.06; 0.08] | 0.98 | 0.128–0.148 | 16425363 | 65 (66.3%) | CHF-CHD: 1.8 times lower than the lower limit; CHD: 1.8 times higher than the upper limit |
| Hydroxyisovalerylcarnitine | C5-OH | Short-chain | 0.005 [0.003; 0.007] | 0.004 [0.003; 0.005] | 0.09 | < 0.51 | 15505778 | 0 (0%) | CHF-CHD and CHD: Normal level |
| Glutarylcarnitine | C5-DC | Short-chain | 0.1 [0.06; 0.14] | 0.1 [0.08; 0.12] | 1.00 | 0.015–0.04 | Molecular You | 74 (75.5%) | CHF-CHD and CHD: In 2.5 times higher than the upper limit |
| Hexanoylcarnitine | C6 | Medium-chain | 0.06 [0.04; 0.07] | 0.047 [0.04; 0.06] | 0.11 | 0.074–0.086 | 16425363 | 60 (61.2%) | CHF-CHD: 1.23 times lower than the lower limit; CHD: 1.6 times lower than the lower limit |
| 0.02–0.13 | Molecular You | CHF-CHD: Close to mean of the range; CHD: Close to mean of the range | |||||||
| 0.04–0.08 | 28278231 | CHF-CHD: Close to mean of the range; CHD: Close to the lower limit | |||||||
| Adipoylcarnitine | C6-DC | Medium-chain | 0.015 [0.01; 0.02] | 0.019 [0.01; 0.03] | 0.19 | - | - | - | - |
| Octenoylcarnitine | C8:1 | Medium-chain | 0.023 [0.016; 0.03] | 0.017 [0.01; 0.04] | 0.17 | 0.05–0.35 | http://www.ncbi.nlm.nih.gov/pubmed/21359215, accessed on 15 August 2023 | 74 (75.5%) | CHF-CHD: 1.23 times lower than the lower limit; CHD: 3 times lower than the lower limit |
| Octanoylcarnitine | C8 | Medium-chain | 0.166 [0.12; 0.2] | 0.15 [0.11; 0.19] | 0.27 | 0.15–0.31 | http://www.ncbi.nlm.nih.gov/pubmed/21359215, accessed on 15 August 2023 | 32 (32.6%) | CHF-CHD: Close to the lower limit; CHD: Close to the lower limit |
| 0.112–0.13 | 16425363 | CHF-CHD: 1.3 times higher than the upper limit; CHD: 1.2 times higher than the upper limit | |||||||
| 0.17–0.5 | http://metabolomicscentre.ca/, accessed on 15 August 2023 | CHF-CHD: Close to the lower limit; CHD: 1.13 times lower than the lower limit | |||||||
| 0.15–0.27 | http://www.ncbi.nlm.nih.gov/pubmed/26010610, accessed on 15 August 2023 | CHF-CHD: Close to the lower limit; CHD: Close to the lower limit | |||||||
| 0.11–0.25 | http://www.ncbi.nlm.nih.gov/pubmed/28278231, accessed on 15 August 2023 | CHF-CHD: Close to the lower limit; CHD: Close to the lower limit | |||||||
| 0.17–0.5 | http://metabolomicscentre.ca/, accessed on 15 August 2023 | CHF-CHD: Close to the lower limit; CHD: Close to the lower limit | |||||||
| Decanoylcarnitine | C10 | Medium-chain | 0.27 [0.2; 0.4] | 0.27 ± 0.13 | 0.52 | 0.15–0.37 | 21359215 | 10 (10.2%) | CHF-CHD: Close to mean of the range; CHD: Close to mean of the range |
| 0.132–0.15 | 16425363 | CHF-CHD: 1.8 times higher than the upper limit; CHD:In 1.8 times higher than the upper limit | |||||||
| 0.18–0.44 | http://www.ncbi.nlm.nih.gov/pubmed/28278231, accessed on 15 August 2023 | CHF-CHD: Close to mean of the range; CHD: Close to mean of the range | |||||||
| 0.16–0.55 | http://metabolomicscentre.ca/, accessed on 15 August 2023 | CHF-CHD: Close to mean of the range; CHD: Close to mean of the range | |||||||
| 0.14–0.32 | http://www.ncbi.nlm.nih.gov/pubmed/26010610, accessed on 15 August 2023 | CHF-CHD: Close to the upper limit; CHD: Close to the upper limit | |||||||
| Decenoylcarnitine | C10:1 | Medium-chain | 0.20 [0.15; 0.3] | 0.17 [0.12; 0.19] | 0.05 | 0.12–0.4 | http://metabolomicscentre.ca/, accessed on 15 August 2023 | 8 (8.2%) | CHF-CHD: Close to the lower limit; CHD: Close to the lower limit |
| Decadienoylcarnitine | C10:2 | Medium-chain | 0.01 [0.01; 0.02] | 0.009 [0.007; 0.014] | 0.01 | - | - | - | - |
| Dodecanoylcarnitine | C12 | Medium-chain | 0.08 [0.05; 0.1] | 0.06 [0.047; 0.07] | 0.03 | 0.048–0.056 | http://www.ncbi.nlm.nih.gov/pubmed/16425363, accessed on 15 August 2023 | 64 (65.3%) | CHF-CHD: 1.4 times higher than the upper limit; CHD: Close to the upper limit |
| 0.07–0.13 | 21359215 | CHF-CHD: Close to the lower limit; CHD: Close to the lower limit | |||||||
| 0.057–0.19 | Molecular You | CHF-CHD: Close to the lower limit; CHD: Close to the lower limit | |||||||
| 0.048–0.056 | 16425363 | CHF-CHD: 1.4 times higher than the upper limit; CHD: Close to the upper limit | |||||||
| 0.057–0.19 | Molecular You | CHF-CHD: Close to the lower limit; CHD: Close to the lower limit | |||||||
| 0.005–0.069 | 9034211 | CHF-CHD: 1.2 times higher than the upper limit; CHD: Close to the upper limit | |||||||
| Dodecenoylcarnitine | C12:1 | Medium-chain | 0.04 [0.03; 0.06] | 0.032 [0.027; 0.045] | 0.009 | 0.1–0.4 | Molecular You | 5 (5.1%) | CHF-CHD: 2.5 times lower than the lower limit; CHD: 3.1 times lower than the lower limit |
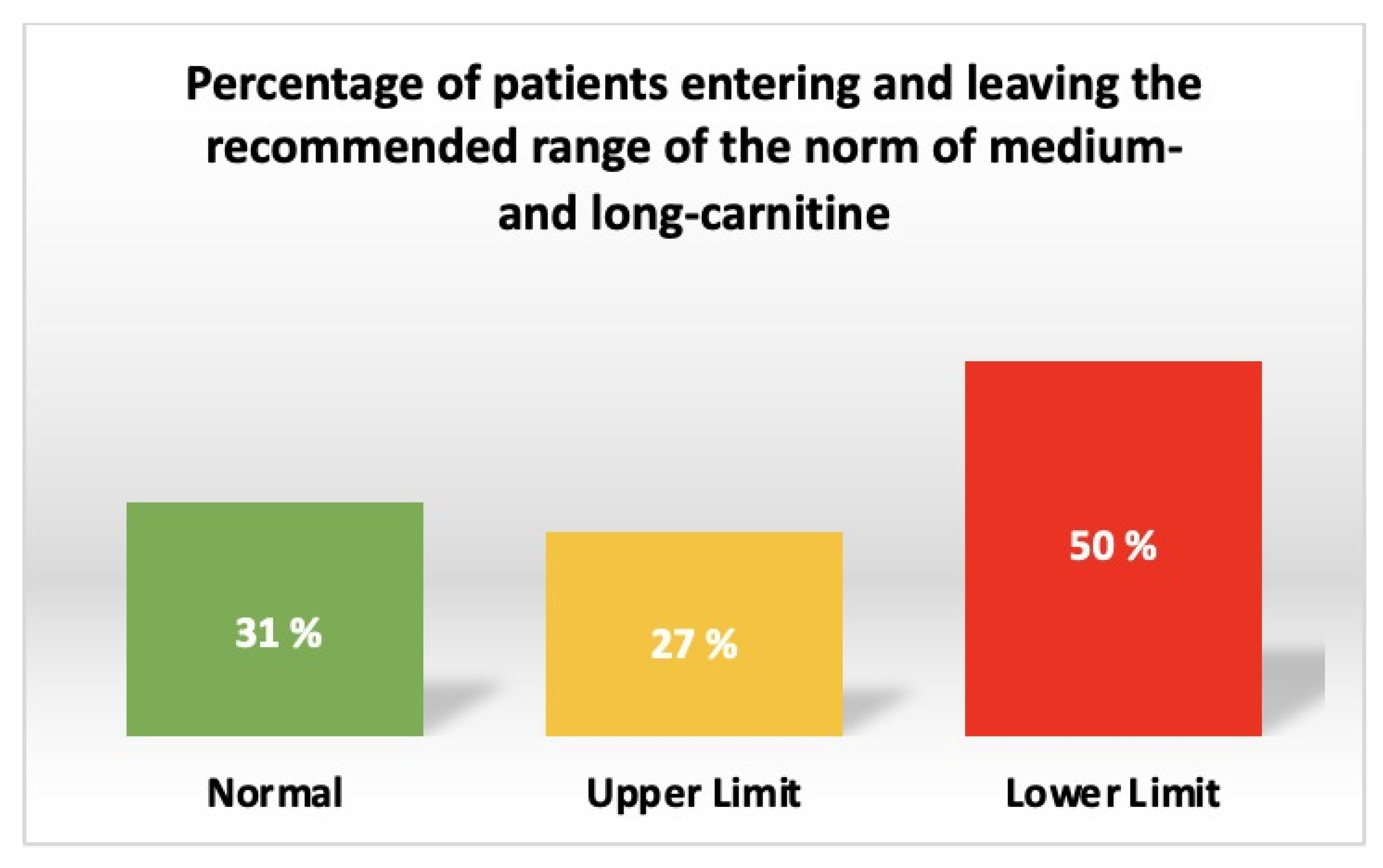
| Tetradecanoylcarnitine | C14 | Long-chain | 0.03 [0.02; 0.04] | 0.02 ± 0.007 | 0.01 | 0.03–0.05 | http://www.ncbi.nlm.nih.gov/pubmed/21359215, accessed on 15 August 2023 | 40 (40.8%) | CHF-CHD: Close to the lower limit; CHD: 1.5 times lower than the lower limit |
| Tetradecenoylcarnitine | C14:1 | Long-chain | 0.053 [0.04; 0.08] | 0.036 [0.024; 0.045] | 0.001 | 0.03–0.09 | 21359215 | 11 (11.2%) | CHF-CHD: Close to mean of the range; CHD: Close to the lower limit |
| 0.02–0.24 | Molecular You | 3 (3%) | CHF-CHD: Close to the lower limit; CHD: Close to the lower limit | ||||||
| Tetradecadienoylcarnitine | C14:2 | Long-chain | 0.029 [0.02; 0.04] | 0.018 [0.01; 0.02] | <0.001 | - | - | - | - |
| Hydroxytetradecanoylcarnitine | C14-OH | Long-chain | 0.0005 [0.0003; 0.001] | 0.0004 [0.0003; 0.0005] | 0.18 | 0.015–0.03 | Molecular You | 79 (80.6%) | CHF-CHD: 30 times lower than the lower limit; CHD: 37.5 times lower than the lower limit |
| Palmitoylcarnitine | C16 | Long-chain | 0.11 [0.09; 0.13] | 0.095 ± 0.02 | 0.04 | 0.107–1.119 | http://www.ncbi.nlm.nih.gov/pubmed/16425363, accessed on 15 August 2023 | 38 (38.7%) | CHF-CHD: Close to the lower limit; CHD: 1.13 times lower than the lower limit |
| Hexadecenoylcarnitine | C16:1 | Long-chain | 0.023 [0.02; 0.03] | 0.018 ± 0.008 | 0.002 | 0.01–0.06 | https://molecularyou.com/, accessed on 15 August 2023 | 3 (3%) | CHF-CHD: Close to the lower limit; CHD: Close to the lower limit |
| 0.02–0.04 | http://www.ncbi.nlm.nih.gov/pubmed/21359215, accessed on 15 August 2023 | CHF-CHD: Close to the lower limit; CHD: Close to the lower limit | |||||||
| Hydroxyhexadecenoylcarnitine | C16:1-OH | Long-chain | 0.0017 [0.0007; 0.003] | 0.001 [0.0006; 0.002] | 0.33 | - | - | - | - |
| Hydroxyhexadecanoylcarnitine | C16-OH | Long-chain | 0.042 [0.03; 0.05] | 0.038 [0.034; 0.06] | 0.76 | 0.005–0.02 | Molecular You | 0 (0%) | CHF-CHD: 2.1 times higher than the upper limit; CHD: 1.9 times higher than the upper limit |
| Stearoylcarnitine | C18 | Long-chain | 0.03 [0.02; 0.04] | 0.03 ± 0.009 | 0.91 | 0.03–0.05 | 21359215 | 37 (37.7%) | CHF-CHD: Close to the lower limit; CHD: Close to the lower limit |
| Oleoylcarnitine | C18:1 | Long-chain | 0.073 [0.06; 0.09] | 0.055 ± 0.017 | 0.001 | 0.04–0.21 | Molecular You | 9 (9.2%) | CHF-CHD: Close to the lower limit; CHD: Close to the lower limit |
| Hydroxyoctadecenoylcarnitine | C18:1-OH | Long-chain | 0.0003 [0.0002; 0.0006] | 0.0003 [0.0001; 0.0005] | 0.49 | 0.000–0.023 | Molecular You | 0 (0%) | CHF-CHD: Close to the lower limit; CHD: Close to the lower limit |
| Linoleoylcarnitine | C18:2 | Long-chain | 0.043 [0.03; 0.06] | 0.03 ± 0.01 | <0.001 | 0.03–0.09 | 21359215 | 20 (20.4%) | CHF-CHD: Close to the lower limit; CHD: Close to the lower limit |
| Hydroxyoctadecanoylcarnitine | C18-OH | Long-chain | 0.0007 [0.0006; 0.001] | 0.0008 ± 0.0004 | 0.38 | - | - | - | - |
| Title | Demographic Characteristics | CHD | HF | Changes in Acylcarnitines |
|---|---|---|---|---|
| Karagiannidis, E. (2022). [19] | N = 316 patients, mean age 67 ± 11 years old; 70.3% male | + | − | Increasing levels of ceramide ratio C24:1/C24:0, acylcarnitine ratio C4/C18:2. |
| Deda, O. (2022). [15] | N = 958 serum samples | + | − | Elevation of short-chain acylcarnitine C2, C4, C5 and C6 levels. Long-chain acylcarnitines C16, C18:1, and C18:2 were higher in Stable angina compared to STEMI. Ratio C4/C18:2 is useful for the prediction of CAD severity |
| Gander, J. (2021). [20] | N = 116, mean age 70.8 ± 8.7 years old; 65% male | + | − | Circulating medium- and long-chain acylcarnitines, especially C6:0, C8:0, C8:1, C12:1, C14:1, C16:0, C16:1, C18:1, and C20:4, were found to be elevated in CAD patients. |
| Chen, W.S. (2020). [21] | N = 79 HF patients hospitalized because of acute decompensation with a left ventricular ejection fraction (LVEF) < 40%; mean age 61.5 ± 13.0 years old; 64.6% male | − | + | Nine acylcarnitines could discriminate the IMP group from the NIMP group, including three long-chain (C18:1, C16, and C16:1) and six short-chain acylcarnitines (C5, C5-OH, C4, C4:1-DC, C3, and C2). |
| Selvaraj, S. (2022). [22] | N = 234 DEFINE-HF patients, mean age 62 ± 11 years old; 75% male | − | + | Changes in LCAC/dicarboxylated LCAC were positively associated with change in NT-proBNP, whereas changes in proline and histidine (factor 10) were negatively associated with changes in NT-proBNP. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belenkov, Y.N.; Ageev, A.A.; Kozhevnikova, M.V.; Khabarova, N.V.; Krivova, A.V.; Korobkova, E.O.; Popova, L.V.; Emelyanov, A.V.; Appolonova, S.A.; Moskaleva, N.E.; et al. Relationship of Acylcarnitines to Myocardial Ischemic Remodeling and Clinical Manifestations in Chronic Heart Failure. J. Cardiovasc. Dev. Dis. 2023, 10, 438. https://doi.org/10.3390/jcdd10100438
Belenkov YN, Ageev AA, Kozhevnikova MV, Khabarova NV, Krivova AV, Korobkova EO, Popova LV, Emelyanov AV, Appolonova SA, Moskaleva NE, et al. Relationship of Acylcarnitines to Myocardial Ischemic Remodeling and Clinical Manifestations in Chronic Heart Failure. Journal of Cardiovascular Development and Disease. 2023; 10(10):438. https://doi.org/10.3390/jcdd10100438
Chicago/Turabian StyleBelenkov, Yuri N., Anton A. Ageev, Maria V. Kozhevnikova, Natalia V. Khabarova, Anastasia V. Krivova, Ekaterina O. Korobkova, Ludmila V. Popova, Alexey V. Emelyanov, Svetlana A. Appolonova, Natalia E. Moskaleva, and et al. 2023. "Relationship of Acylcarnitines to Myocardial Ischemic Remodeling and Clinical Manifestations in Chronic Heart Failure" Journal of Cardiovascular Development and Disease 10, no. 10: 438. https://doi.org/10.3390/jcdd10100438
APA StyleBelenkov, Y. N., Ageev, A. A., Kozhevnikova, M. V., Khabarova, N. V., Krivova, A. V., Korobkova, E. O., Popova, L. V., Emelyanov, A. V., Appolonova, S. A., Moskaleva, N. E., Shestakova, K. M., & Privalova, E. V. (2023). Relationship of Acylcarnitines to Myocardial Ischemic Remodeling and Clinical Manifestations in Chronic Heart Failure. Journal of Cardiovascular Development and Disease, 10(10), 438. https://doi.org/10.3390/jcdd10100438






