The VEST External Support for Saphenous Vein Grafts in Coronary Surgery: A Review of Randomized Clinical Trials
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Search Results
3.2. Preclinical Model Study
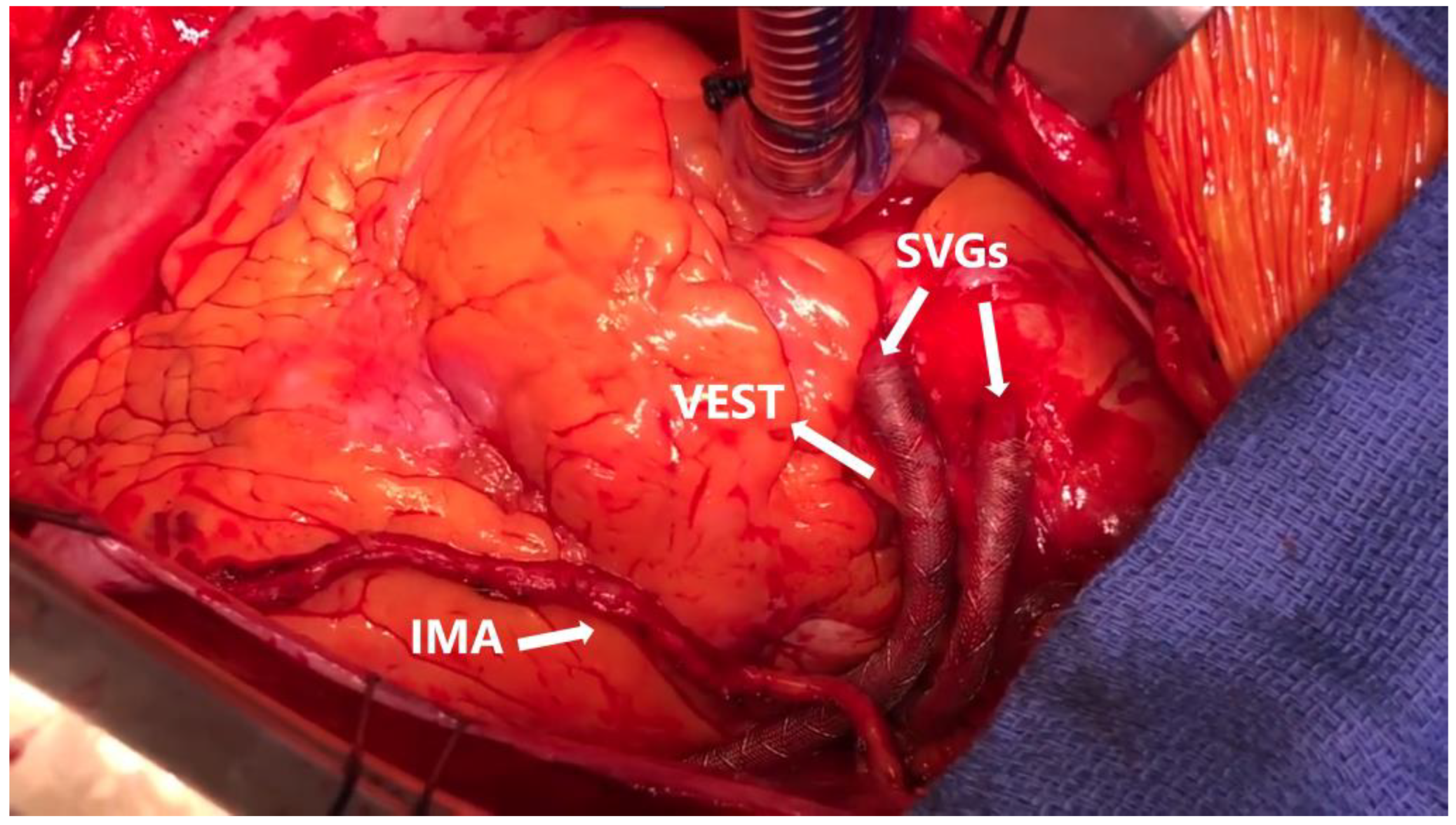
3.3. The VEST Studies
3.3.1. VEST I
3.3.2. VEST II
3.3.3. VEST III
3.3.4. VEST IV
3.3.5. US VEST
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, K.M.; Arghami, A.; Habib, R.; Daneshmand, M.A.; Parsons, N.; Elhalabi, Z.; Krohn, C.; Thourani, V.; Bowdish, M.E. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2022 Update on Outcomes and Research. Ann. Thorac. Surg. 2023, 115, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.; Zadina, K.; Moritz, T.; Ovitt, T.; Sethi, G.; Copeland, J.G.; Thottapurathu, L.; Krasnicka, B.; Ellis, N.; Anderson, R.J.; et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: Results from a Department of Veterans Affairs Cooperative Study. J. Am. Coll. Cardiol. 2004, 44, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Fitzgibbon, G.M.; Kafka, H.P.; Leach, A.J.; Keon, W.J.; Hooper, G.D.; Burton, J.R. Coronary bypass graft fate and patient outcome: Angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J. Am. Coll. Cardiol. 1996, 28, 616–626. [Google Scholar] [CrossRef]
- Xenogiannis, I.; Zenati, M.; Bhatt, D.L.; Rao, S.V.; Rodés-Cabau, J.; Goldman, S.; Shunk, K.A.; Mavromatis, K.; Banerjee, S.; Alaswad, K.; et al. Saphenous Vein Graft Failure: From Pathophysiology to Prevention and Treatment Strategies. Circulation 2021, 144, 728–745. [Google Scholar] [CrossRef]
- Caliskan, E.; de Souza, D.R.; Böning, A.; Liakopoulos, O.J.; Choi, Y.-H.; Pepper, J.; Gibson, C.M.; Perrault, L.P.; Wolf, R.K.; Kim, K.-B.; et al. Saphenous vein grafts in contemporary coronary artery bypass graft surgery. Nat. Rev. Cardiol. 2020, 17, 155–169. [Google Scholar] [CrossRef]
- Ben-Gal, Y.; Taggart, D.P.; Williams, M.R.; Orion, E.; Uretzky, G.; Shofti, R.; Banai, S.; Yosef, L.; Bolotin, G. Expandable external support device to improve Saphenous Vein Graft Patency after CABG. J. Cardiothorac. Surg. 2013, 8, 122. [Google Scholar] [CrossRef]
- Sandner, S.; Angleitner, P.; Laufer, G.; Zimpfer, D. External stent (VEST) for saphenous vein grafts in coronary artery bypass grafting. Multimed. Man. Cardiothorac. Surg. MMCTS 2019, 2019. [Google Scholar] [CrossRef]
- Taggart, D.P.; Ben Gal, Y.; Lees, B.; Patel, N.; Webb, C.; Rehman, S.M.; Desouza, A.; Yadav, R.; De Robertis, F.; Dalby, M.; et al. A Randomized Trial of External Stenting for Saphenous Vein Grafts in Coronary Artery Bypass Grafting. Ann. Thorac. Surg. 2015, 99, 2039–2045. [Google Scholar] [CrossRef]
- Taggart, D.P.; Gavrilov, Y.; Krasopoulos, G.; Rajakaruna, C.; Zacharias, J.; De Silva, R.; Channon, K.M.; Gehrig, T.; Donovan, T.J.; Friedrich, I.; et al. External stenting and disease progression in saphenous vein grafts two years after coronary artery bypass grafting: A multicenter randomized trial. J. Thorac. Cardiovasc. Surg. 2022, 164, 1532–1541.e2. [Google Scholar] [CrossRef]
- Taggart, D.P.; Webb, C.M.; Desouza, A.; Yadav, R.; Channon, K.M.; De Robertis, F.; Di Mario, C. Long-term performance of an external stent for saphenous vein grafts: The VEST IV trial. J. Cardiothorac. Surg. 2018, 13, 117. [Google Scholar] [CrossRef]
- Goldstein, D.J.; Puskas, J.D.; Alexander, J.H.; Chang, H.L.; Gammie, J.S.; Marks, M.E.; Iribarne, A.; Vengrenyuk, Y.; Raymond, S.; Taylor, B.S.; et al. External Support for Saphenous Vein Grafts in Coronary Artery Bypass Surgery: A Randomized Clinical Trial. JAMA Cardiol. 2022, 7, 808–816. [Google Scholar] [CrossRef]
- Taggart, D.P.; Amin, S.; Djordjevic, J.; Oikonomou, E.K.; Thomas, S.; Kampoli, A.-M.; Sabharwal, N.; Antoniades, C.; Krasopoulos, G. A prospective study of external stenting of saphenous vein grafts to the right coronary artery: The VEST II study. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2017, 51, 952–958. [Google Scholar] [CrossRef]
- Lopes, R.D.; Hafley, G.E.; Allen, K.B.; Ferguson, T.B.; Peterson, E.D.; Harrington, R.A.; Mehta, R.H.; Gibson, C.M.; Mack, M.J.; Kouchoukos, N.T.; et al. Endoscopic versus Open Vein-Graft Harvesting in Coronary-Artery Bypass Surgery. N. Engl. J. Med. 2009, 361, 235–244. [Google Scholar] [CrossRef]
- Gemelli, M.; Gallo, M.; Addonizio, M.; Pahwa, S.; Van den Eynde, J.; Trivedi, J.; Slaughter, M.S.; Gerosa, G. Venous External Support in Coronary Artery Bypass Surgery: A Systematic Review and Meta-Analysis. Curr. Probl. Cardiol. 2023, 48, 101687. [Google Scholar] [CrossRef] [PubMed]
- Soletti, G.J.; Dimagli, A.; Perezgrovas Olaria, R.; Harik, L.; Alzghari, T.; Cancelli, G.; Castagnini, S.; Lau, C.; Girardi, L.N.; Gaudino, M. External stenting for saphenous vein grafts in coronary surgery: A systematic review and meta-analysis. J. Clin. Med. 2023, 53, e14046. [Google Scholar]
- Lee, G.S.; Fremes, S.E.; Tam, D.Y. Commentary: Can the Venous Graft External SupporT (VEST) trials bypass surrogate outcomes? J. Thorac. Cardiovasc. Surg. 2022. [Google Scholar] [CrossRef]
- Goldstein, D.J. Device profile of the VEST for external support of SVG Coronary artery bypass grafting: Historical development, current status, and future directions. Expert Rev. Med. Devices 2021, 18, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.; Webb, C.M.; Chong, C.F.; Moat, N.E. Radial Artery Versus Saphenous Vein Patency (RSVP) Trial Investigators Radial artery versus saphenous vein patency randomized trial: Five-year angiographic follow-up. Circulation 2008, 117, 2859–2864. [Google Scholar] [CrossRef]
- Gaudino, M.; Rahouma, M.; Abouarab, A.; Leonard, J.; Kamel, M.; Di Franco, A.; Demetres, M.; Tam, D.Y.; Tranbaugh, R.; Girardi, L.N.; et al. Radial artery versus saphenous vein as the second conduit for coronary artery bypass surgery: A meta-analysis. J. Thorac. Cardiovasc. Surg. 2019, 157, 1819–1825.e10. [Google Scholar] [CrossRef]
- Cancelli, G.; Audisio, K.; Chadow, D.; Soletti, G.J.; Gaudino, M. The evidence for radial artery grafting: When and when not? JTCVS Tech. 2021, 10, 114–119. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Y.; Wu, Z.; Liu, Z.; Zheng, J. Mid-term and long-term outcomes of endoscopic versus open vein harvesting for coronary artery bypass: A systematic review and meta-analysis. Int. J. Surg. Lond. Engl. 2019, 72, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Sandner, S.E.; Donovan, T.J.; Edelstein, S.; Puskas, J.D.; Angleitner, P.; Krasopoulos, G.; Channon, K.; Gehrig, T.; Rajakaruna, C.; Ladyshenskij, L.; et al. Effects of the harvesting technique and external stenting on progression of vein graft disease 2 years after coronary artery bypass. Eur. J. Cardiothorac. Surg. 2022, 62, ezac045. [Google Scholar] [CrossRef] [PubMed]
- FitzGibbon, G.M.; Leach, A.J.; Keon, W.J.; Burton, J.R.; Kafka, H.P. Coronary bypass graft fate. Angiographic study of 1,179 vein grafts early, one year, and five years after operation. J. Thorac. Cardiovasc. Surg. 1986, 91, 773–778. [Google Scholar] [CrossRef] [PubMed]
- FitzGibbon, G.M.; Leach, A.J.; Kafka, H.P.; Keon, W.J. Coronary bypass graft fate: Long-term angiographic study. J. Am. Coll. Cardiol. 1991, 17, 1075–1080. [Google Scholar] [CrossRef]
- Palanisamy, V.; Kurian, V.M.; Sethuratnam, R. In the VEST trial: Are we missed to address the pathology incurred by the external stent? J. Cardiothorac. Surg. 2021, 16, 257. [Google Scholar] [CrossRef]
- Whitney, D.G.; Kahn, E.M.; Estes, J.W. Valvular occlusion of the arterialized saphenous vein. Am. Surg. 1976, 42, 879–887. [Google Scholar]

| VEST I | VEST II | VEST III | VEST IV | US VEST | |
|---|---|---|---|---|---|
| Author, year | Taggart, 2015 | Taggart, 2016 | Taggart, 2022 | Taggart, 2018 | Goldstein, 2022 |
| RCT | Yes | No | Yes | Yes | Yes |
| Multi-center | Yes | No | Yes | Yes | Yes |
| Number of patients | 30 | 30 | 184 | 30 | 224 |
| Multi-vessel coronaropathy | Yes | Yes | Yes | Yes | Yes |
| Follow-up (months) | 12 | 3–6 | 24 | 54 | 12 |
| Patency VEST-SVGs (%) | 70.0 | 86.2 | 78.3 | 70.0 | 76.7 |
| Patency control-SVGs (%) | 82.1 | 100 | 82.2 | 77.0 | 77.8 |
| VEST reduced IH | Yes | - | Yes | Yes | No |
| VEST reduced IMT | No | - | Yes | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soletti, G.J.; Dell’Aquila, M.; Harik, L.; Cancelli, G.; Alzghari, T.; Perezgrovas-Olaria, R.; Dimagli, A.; An, K.R.; Leith, J.; Rossi, C.S.; et al. The VEST External Support for Saphenous Vein Grafts in Coronary Surgery: A Review of Randomized Clinical Trials. J. Cardiovasc. Dev. Dis. 2023, 10, 453. https://doi.org/10.3390/jcdd10110453
Soletti GJ, Dell’Aquila M, Harik L, Cancelli G, Alzghari T, Perezgrovas-Olaria R, Dimagli A, An KR, Leith J, Rossi CS, et al. The VEST External Support for Saphenous Vein Grafts in Coronary Surgery: A Review of Randomized Clinical Trials. Journal of Cardiovascular Development and Disease. 2023; 10(11):453. https://doi.org/10.3390/jcdd10110453
Chicago/Turabian StyleSoletti, Giovanni Jr., Michele Dell’Aquila, Lamia Harik, Gianmarco Cancelli, Talal Alzghari, Roberto Perezgrovas-Olaria, Arnaldo Dimagli, Kevin R. An, Jordan Leith, Camilla Sofia Rossi, and et al. 2023. "The VEST External Support for Saphenous Vein Grafts in Coronary Surgery: A Review of Randomized Clinical Trials" Journal of Cardiovascular Development and Disease 10, no. 11: 453. https://doi.org/10.3390/jcdd10110453
APA StyleSoletti, G. J., Dell’Aquila, M., Harik, L., Cancelli, G., Alzghari, T., Perezgrovas-Olaria, R., Dimagli, A., An, K. R., Leith, J., Rossi, C. S., Barile, C. F., Demetres, M., Lau, C., Girardi, L. N., & Gaudino, M. (2023). The VEST External Support for Saphenous Vein Grafts in Coronary Surgery: A Review of Randomized Clinical Trials. Journal of Cardiovascular Development and Disease, 10(11), 453. https://doi.org/10.3390/jcdd10110453







