HER2-Targeted Therapy—From Pathophysiology to Clinical Manifestation: A Narrative Review
Abstract
:1. Introduction
2. Pathophysiology of Trastuzumab-Induced Cardiotoxicity
2.1. Trastuzumab’s Action on Tumor Cells
2.2. NRG-1/ErbB Signaling Pathway’s Role in Heart Physiology
2.3. NRG-1/ErbB Signaling Pathway in Heart Failure Pathophysiology
2.4. Trastuzumab’s Effects on Cardiomyocytes
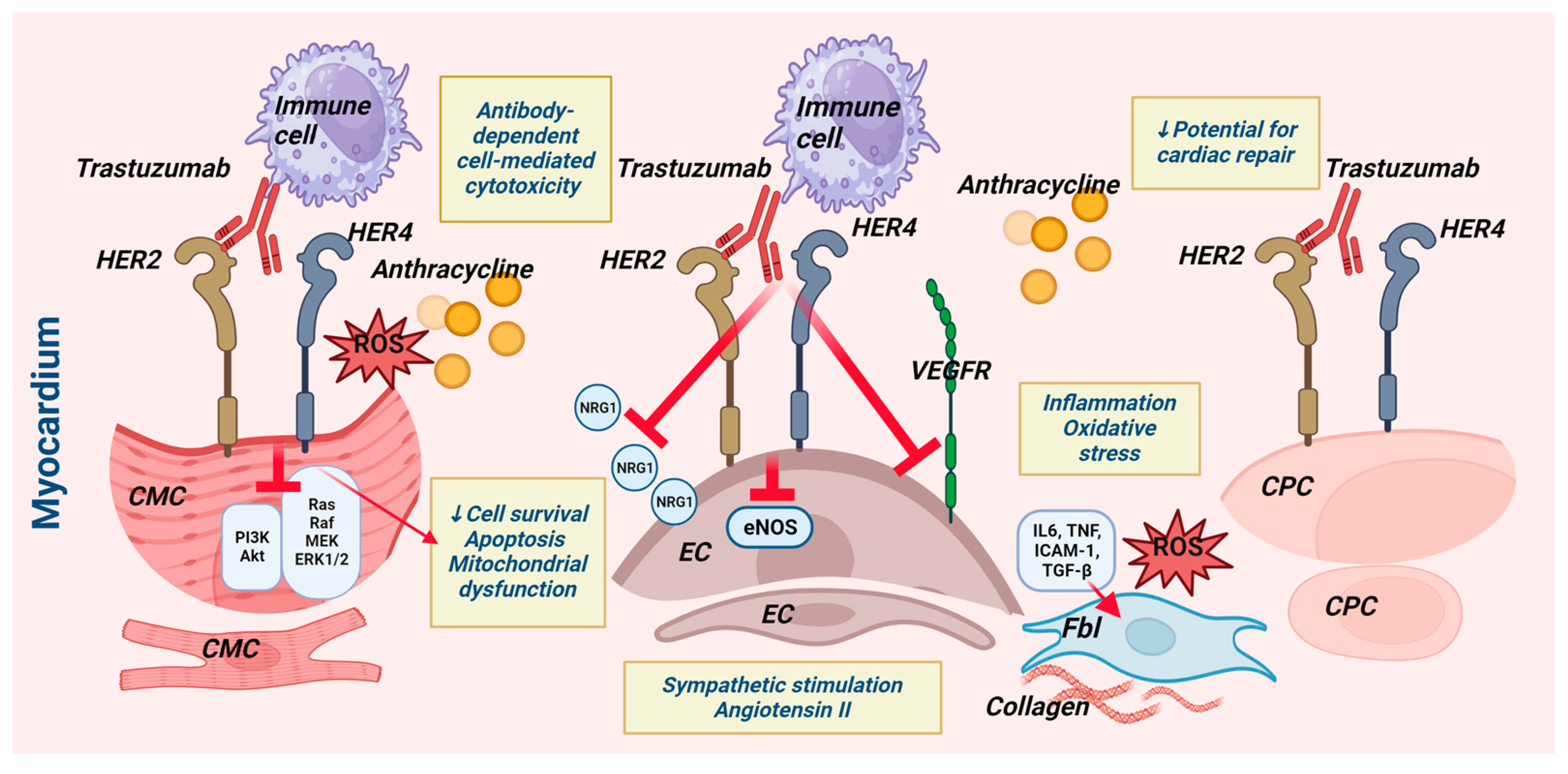
2.5. Effects of Trastuzumab on Other Heart Cell Types (Figure 1)
2.5.1. The Influence on Cardiac Progenitor Cells
2.5.2. Trastuzumab’s Effects on Endothelial Cells
2.5.3. Trastuzumab’s Effects on Cardiac Fibroblasts
2.5.4. Trastuzumab and Cells of the Inflammatory Response
2.6. Mechanisms of Trastuzumab-Induced Cardiotoxicity: A Summary
What Evidence Exists Regarding Structural Myocardial Damage Caused by Trastuzumab?
3. Trastuzumab-Induced Cardiotoxicity from a Clinical Perspective
3.1. Heart Failure and Left Ventricular Systolic Dysfunction: Large Randomized Controlled Trials’ Data
3.2. Heart Failure and Left Ventricular Systolic Dysfunction: Evidence from Real-World Clinical and Registry-Based Studies
3.3. Trastuzumab and Rhythmic Abnormalities
3.4. Right Ventricular Dysfunction
3.5. Heart Failure with Preserved Ejection Fraction
Diastolic Dysfunction
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strauss, E.; Herceptin—A Targeted Antibody Therapy for Breast Cancer. 2019 Lasker~DeBakey Clinical Medical Research Award. 2019. Available online: https://laskerfoundation.org/winners/herceptin-a-targeted-antibody-therapy-for-breast-cancer (accessed on 28 October 2023).
- Perez, E.A.; Romond, E.H.; Suman, V.J.; Jeong, J.H.; Sledge, G.; Geyer, C.E., Jr.; Martino, S.; Rastogi, P.; Gralow, J.; Swain, S.M.; et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 2014, 32, 3744–3752. [Google Scholar] [CrossRef]
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Castelo-Branco, L.; Gennari, A.; Harbeck, N.; Criscitiello, C.; Trapani, D.; on behalf of the Clinical Practice Guideline Author Group. ESMO Metastatic Breast Cancer Living Guidelines, v1.1 May 2023. Available online: https://www.esmo.org/living-guidelines/esmo-metastatic-breast-cancer-living-guideline. (accessed on 28 October 2023).
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Early Breast Cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Anjos, M.; Fontes-Oliveira, M.; Costa, V.M.; Santos, M.; Ferreira, R. An update of the molecular mechanisms underlying doxorubicin plus trastuzumab induced cardiotoxicity. Life Sci. 2021, 280, 119760. [Google Scholar] [CrossRef]
- Paulus, W.J.; Zile, M.R. From Systemic Inflammation to Myocardial Fibrosis: The Heart Failure with Preserved Ejection Fraction Paradigm Revisited. Circ. Res. 2021, 128, 1451–1467. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L.; Felker, G.M. Mechanisms and Models in Heart Failure: A Translational Approach. Circ. Res. 2021, 128, 1435–1450. [Google Scholar] [CrossRef]
- De Keulenaer, G.W.; Doggen, K.; Lemmens, K. The Vulnerability of the Heart as a Pluricellular Paracrine Organ. Circ. Res. 2010, 106, 35–46. [Google Scholar] [CrossRef]
- Sawyers, C.L. Herceptin: A First Assault on Oncogenes that Launched a Revolution. Cell 2019, 179, 8–12. [Google Scholar] [CrossRef]
- Lemmens, K.; Doggen, K.; De Keulenaer, G.W. Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: Implications for therapy of heart failure. Circulation 2007, 116, 954–960. [Google Scholar] [CrossRef]
- Ozcelik, C.; Erdmann, B.; Pilz, B.; Wettschureck, N.; Britsch, S.; Hübner, N.; Chien, K.R.; Birchmeier, C.; Garratt, A.N. Conditional mutation of the ErbB2 (HER2) receptor in cardiomyocytes leads to dilated cardiomyopathy. Proc. Natl. Acad. Sci. USA 2002, 99, 8880–8885. [Google Scholar] [CrossRef]
- García-Rivello, H.; Taranda, J.; Said, M.; Cabeza-Meckert, P.; Vila-Petroff, M.; Scaglione, J.; Ghio, S.; Chen, J.; Lai, C.; Laguens, R.P.; et al. Dilated cardiomyopathy in Erb-b4-deficient ventricular muscle. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, 58–63. [Google Scholar] [CrossRef]
- Perik, P.J.; de Vries, E.G.E.; Gietema, J.A.; van der Graaf, W.T.; Smilde, T.D.; Sleijfer, D.T.; van Veldhuisen, D.J. Serum HER2 levels are increased in patients with chronic heart failure. Eur. J. Heart Fail. 2007, 9, 173–177. [Google Scholar] [CrossRef]
- Feyen, E.; Ricke-Hoch, M.; Van Fraeyenhove, J.; Vermeulen, Z.; Scherr, M.; Dugaucquier, L.; Viereck, J.; Bruyns, T.; Thum, T.; Segers, V.F.M.; et al. ERBB4 and Multiple MicroRNAs That Target ERBB4 Participate in Pregnancy-Related Cardiomyopathy. Circ. Heart Fail. 2021, 14, e006898. [Google Scholar] [CrossRef] [PubMed]
- Ky, B.; Kimmel, S.E.; Safa, R.N.; Putt, M.E.; Sweitzer, N.K.; Fang, J.C.; Sawyer, D.B.; Cappola, T.P. Neuregulin-1beta is associated with disease severity and adverse outcomes in chronic heart failure. Circulation 2009, 120, 310–317. [Google Scholar] [CrossRef] [PubMed]
- de Korte, M.A.; de Vries, E.G.; Lub-de Hooge, M.N.; Jager, P.L.; Gietema, J.A.; van der Graaf, W.T.; Sluiter, W.J.; van Veldhuisen, D.J.; Suter, T.M.; Sleijfer, D.T.; et al. 111Indium-trastuzumab visualises myocardial human epidermal growth factor receptor 2 expression shortly after anthracycline treatment but not during heart failure: A clue to uncover the mechanisms of trastuzumab-related cardiotoxicity. Eur. J. Cancer 2007, 43, 2046–2051. [Google Scholar] [CrossRef]
- Kurokawa, Y.K.; Shang, M.R.; Yin, R.T.; George, S.C. Modeling trastuzumab-related cardiotoxicity in vitro using human stem cell-derived cardiomyocytes. Toxicol. Lett. 2018, 285, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Kertmen, N.; Aksoy, S.; Uner, A.; Sargon, M.; Ozkayar, O.; Keskin, O.; Babacan, T.; Sarici, F.; Sendur, M.A.; Arik, Z.; et al. Which sequence best protects the heart against trastuzumab and anthracycline toxicity? An electron microscopy study in rats. Anticancer. Res. 2015, 35, 857–864. [Google Scholar] [PubMed]
- Laird-Fick, H.S.; Tokala, H.; Kandola, S.; Kehdi, M.; Pelosi, A.; Wang, L.; Grondahl, B. Early morphological changes in cardiac mitochondria after subcutaneous administration of trastuzumab in rabbits: Possible prevention with oral selenium supplementation. Cardiovasc. Pathol. 2020, 44, 10715. [Google Scholar] [CrossRef]
- Riccio, G.; Esposito, G.; Leoncini, E.; Contu, R.; Condorelli, G.; Chiariello, M.; Laccetti, P.; Hrelia, S.; D’Alessio, G.; De Lorenzo, C. Cardiotoxic effects, or lack thereof, of anti-ErbB2 immunoagents. FASEB J. 2009, 23, 3171–3178. [Google Scholar] [CrossRef]
- ElZarrad, M.K.; Mukhopadhyay, P.; Mohan, N.; Hao, E.; Dokmanovic, M.; Hirsch, D.S.; Shen, Y.; Pacher, P.; Wu, W.J. Trastuzumab alters the expression of genes essential for cardiac function and induces ultrastructural changes of cardiomyocytes in mice. PLoS ONE 2013, 8, e79543. [Google Scholar] [CrossRef]
- Altomare, C.; Lodrini, A.M.; Milano, G.; Biemmi, V.; Lazzarini, E.; Bolis, S.; Pernigoni, N.; Torre, E.; Arici, M.; Ferrandi, M.; et al. Structural and Electrophysiological Changes in a Model of Cardiotoxicity Induced by Anthracycline Combined with Trastuzumab. Front. Physiol. BI 2021, 12, 658790. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Yu, S.; Zhang, L.; Jiang, J.; Zhou, Q. Herceptin induces ferroptosis and mitochondrial dysfunction in H9c2 cells. Int. J. Mol. Med. 2022, 49, 17. [Google Scholar] [CrossRef] [PubMed]
- Necela, B.M.; Axenfeld, B.C.; Serie, D.J.; Kachergus, J.M.; Perez, E.A.; Thompson, E.A.; Norton, N. The antineoplastic drug, trastuzumab, dysregulates metabolism in iPSC-derived cardiomyocytes. Clin. Transl. Med. 2017, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Kitani, T.; Ong, S.G.; Lam, C.K.; Rhee, J.W.; Zhang, J.Z.; Oikonomopoulos, A.; Ma, N.; Tian, L.; Lee, J.; Telli, M.L.; et al. Human-Induced Pluripotent Stem Cell Model of Trastuzumab-Induced Cardiac Dysfunction in PatientsWith Breast Cancer. Circulation 2019, 139, 2451–2465. [Google Scholar] [CrossRef] [PubMed]
- Mohan, N.; Shen, Y.; Endo, Y.; ElZarrad, M.K.; Wu, W.J. Trastuzumab, but Not Pertuzumab, Dysregulates HER2 Signaling to Mediate Inhibition of Autophagy and Increase in Reactive Oxygen Species Production in Human Cardiomyocytes. Mol. Cancer Ther. 2016, 15, 1321–1331. [Google Scholar] [CrossRef]
- Savi, M.; Bocchi, L.; Rossi, S.; Frati, C.; Graiani, G.; Lagrasta, C.; Miragoli, M.; Di Pasquale, E.; Stirparo, G.G.; Mastrototaro, G.; et al. Antiarrhythmic effect of growth factor-supplemented cardiac progenitor cells in chronic infarcted heart. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, 1622–1648. [Google Scholar] [CrossRef] [PubMed]
- Barth, A.S.; Zhang, Y.; Li, T.; Smith, R.R.; Chimenti, I.; Terrovitis, I.; Davis, D.R.; Kizana, E.; Ho, A.S.; O’Rourke, B.; et al. Functional impairment of human resident cardiac stem cells by the cardiotoxic antineoplastic agent trastuzumab. Stem Cells Transl. Med. 2012, 1, 289–297. [Google Scholar] [CrossRef]
- Pinto, A.R.; Ilinykh, A.; Ivey, M.J.; Kuwabara, J.T.; D’Antoni, M.L.; Debuque, R.; Chandran, A.; Wang, L.; Arora, K.; Rosenthal, N.A.; et al. Revisiting Cardiac Cellular Composition. Circ. Res. 2016, 118, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.J.; Lerman, A. Endothelial dysfunction and cardiovascular disease. Glob. Cardiol. Sci. Pract. 2014, 2014, 291–308. [Google Scholar] [CrossRef]
- Zhang, A.; Shen, G.; Zhao, T.; Zhang, G.; Liu, J.; Song, L.; Wei, W.; Bing, L.; Wu, Z.; Wu, Q. Augmented inhibition of angiogenesis by combination of HER2 antibody chA21 and trastuzumab in human ovarian carcinoma xenograft. J. Ovarian Res. 2010, 3, 20. [Google Scholar] [CrossRef]
- Aird, W.C. Spatial and temporal dynamics of the endothelium. J. Thromb. Haemost. 2005, 3, 12392–12406. [Google Scholar] [CrossRef] [PubMed]
- Dugaucquier, L.; Feyen, E.; Mateiu, L.; Bruyns, T.A.M.; De Keulenaer, G.W.; Segers, V.F.M. The role of endothelial autocrine NRG1/ERBB4 signaling in cardiac remodeling. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Sandoo, A.; Kitas, G.D.; Carmichael, A.R. Endothelial Dysfunction as a Determinant of Trastuzumab-mediated Cardiotoxicity in Patients with Breast Cancer. Anticancer. Res. 2014, 34, 1147–1151. [Google Scholar] [PubMed]
- Hoffman, R.K.; Kim, B.-J.; Shah, P.D.; Carver, J.; Ky, B.; Ryeom, S. Damage to cardiac vasculature may be associated with breast cancer treatment-induced cardiotoxicity. Cardio-Oncology 2021, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Lyle, M.A.; Brozovich, F.V. HFpEF, a Disease of the Vasculature: A Closer Look at the Other Half. Mayo Clin. Proc. 2018, 93, 1305–1314. [Google Scholar] [CrossRef]
- Wilkinson, E.L.; Sidaway, J.E.; Cross, M.J. Cardiotoxic drugs Herceptin and doxorubicin inhibit cardiac microvascular endothelial cell barrier formation resulting in increased drug permeability. Biol. Open 2016, 5, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Coppola, C.; Riccio, G.; Barbieri, A.; Monti, M.G.; Piscopo, G.; Rea, D.; Arra, C.; Maurea, C.; De Lorenzo, C.; Maurea, N. Antineoplastic-related cardiotoxicity, morphofunctional aspects in a murine model: Contribution of the new tool 2D-speckle tracking. Onco Targets Ther. 2016, 9, 6785–6794. [Google Scholar] [CrossRef]
- Gambardella, J.; Trimarco, B.; Iaccarino, G.; Sorriento, D. Cardiac Nonmyocyte Cell Functions and Crosstalks in Response to Cardiotoxic Drugs. Oxid. Med. Cell Longev. 2017, 2017, 1089359. [Google Scholar] [CrossRef]
- Kabela, A.M.; Elkhoely, A.A. Targeting proinflammatory cytokines, oxidative stress, TGF-β1 and STAT-3 by rosuvastatin and ubiquinone to ameliorate trastuzumab cardiotoxicity. Biomed. Pharmacother. 2017, 93, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Ewer, M.S.; Vooletich, M.T.; Durand, J.B.; Woods, M.L.; Davis, J.R.; Valero, V.; Lenihan, D.J. Reversibility of trastuzumab-related cardiotoxicity: New insights based on clinical course and response to medical treatment. Clin. Oncol. 2005, 23, 7820–7826. [Google Scholar] [CrossRef] [PubMed]
- Banchs, J.A.; Ewer, M.S. Type I and Type II Cardiomyopathy Classifications Are Complete Nonsense: CON ACC Latest in Cardiology, Expert Analysis. 2018. Available online: https://www.acc.org/latest-in-cardiology/articles/2018/05/04/08/41/type-i-and-type-ii-cardiomyopathy-classifications-are-complete-nonsense-con (accessed on 28 October 2023).
- Witteles, R. Type I and Type II Cardiomyopathy Classifications Are Complete Nonsense: PRO. ACC Latest in Cardiology, Expert Analysis. 2018. Available online: https://www.acc.org/latest-in-cardiology/articles/2018/05/04/08/41/type-i-and-type-ii-cardiomyopathy-classifications-are-complete-nonsense-pro (accessed on 28 October 2023).
- Goldhar, H.A.; Yan, A.T.; Ko, D.T.; Earle, C.C.; Tomlinson, G.A.; Trudeau, M.E.; Krahn, M.D.; Krzyzanowska, M.K.; Pal, R.S.; Brezden-Masley, C.; et al. The Temporal Risk of Heart Failure Associated with Adjuvant Trastuzumab in Breast Cancer Patients: A Population Study. J. Natl. Cancer Inst. 2015, 108, djv301. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Long, J.B.; Hurria, A.; Owusu, C.; Steingart, R.M.; Gross, C.P. Incidence of Heart Failure or Cardiomyopathy After Adjuvant Trastuzumab Therapy for Breast Cancer. J. Am. Coll. Cardiol. 2012, 60, 2504–2512. [Google Scholar] [CrossRef]
- Leong, D.P.; Cosman, T.; Alhussein, M.M.; Kumar Tyagi, N.; Karampatos, S.; Barron, C.C.; Wright, D.; Tandon, V.; Magloire, P.; Joseph, P.; et al. Safety of Continuing Trastuzumab Despite Mild Cardiotoxicity: A Phase I Trial. JACC CardioOncol 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. ESC Scientific Document Group. ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Fallah-Rad, N.; Lytwyn, M.; Fang, T.; Kirkpatrick, I.; Jassal, D.S. Delayed contrast enhancement cardiac magnetic resonance imaging in trastuzumab induced cardiomyopathy. J. Cardiovasc. Magn. Reson. 2008, 10, 5. [Google Scholar] [CrossRef]
- Wadhwa, D.; Fallah-Rad, N.; Grenier, D.; Krahn, M.; Fang, T.; Ahmadie, R.; Walker, J.R.; Lister, D.; Arora, R.C.; Barac, I.; et al. Trastuzumab mediated cardiotoxicity in the setting of adjuvant chemotherapy for breast cancer: A retrospective study. Breast Cancer Res. Treat. 2009, 117, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Amir, E.; Bedard, P.; Crean, A.; Paul, N.; Nguyen, E.T.; Wintersperger, B.J. Regional myocardial edema detected by T2 mapping is a feature of cardiotoxicity in breast cancer patients receiving sequential therapy with anthracyclines and trastuzumab. J. Cardiovasc. Magn. Reson. 2014, 16, P273. [Google Scholar] [CrossRef]
- Modi, K.; Joppa, S.; Chen, K.A.; Athwal, P.S.S.; Okasha, O.; Velangi, P.S.; Hooks, M.; Nijjar, P.S.; Blaes, A.H.; Shenoy, C. Myocardial damage assessed by late gadolinium enhancement on cardiovascular magnetic resonance imaging in cancer patients treated with anthracyclines and/or trastuzumab. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 427–434. [Google Scholar] [CrossRef]
- Seidman, A.; Hudis, C.; Pierri, M.K.; Shak, S.; Paton, V.; Ashby, M.; Murphy, M.; Stewart, S.J.; Keefe, D. Cardiac dysfunction in the trastuzumab clinical trials experience. J. Clin. Oncol. 2002, 20, 1215–1221. [Google Scholar] [CrossRef]
- Tan-Chiu, E.; Yothers, G.; Romond, E.; Geyer, C.E., Jr.; Ewer, M.; Keefe, D.; Shannon, R.P.; Swain, S.M.; Brown, A.; Fehrenbacher, L.; et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J. Clin. Oncol. 2005, 23, 7811–7819. [Google Scholar] [CrossRef]
- Perez, E.A.; Suman, V.J.; Davidson, N.E.; Sledge, G.W.; Kaufman, P.A.; Hudis, C.A.; Martino, S.; Gralow, J.R.; Dakhil, S.R.; Ingle, J.N.; et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J. Clin. Oncol. 2008, 26, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- de Azambuja, E.; Procter, M.J.; van Veldhuisen, D.J.; Agbor-Tarh, D.; Metzger-Filho, O.; Steinseifer, J.; Untch, M.; Smith, I.E.; Gianni, L.; Baselga, J.; et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin Adjuvant trial (BIG 1-01). J. Clin. Oncol. 2014, 32, 2159–2165. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Breast Cancer International Research Group. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.D.; Blackwell, K.L.; Lawrence, J.; Pippen, J.E., Jr.; Roe, M.T.; Wood, F.; Paton, V.; Holmgren, E.; Mahaffey, K. WIndependent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: A combined review of cardiac data from the National Surgical Adjuvant breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J. Clin. Oncol. 2010, 28, 3416–3421. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xu, T.; Li, Y.; Liang, C.; Chen, J.; Lu, Y.; Wu, Z.; Wu, S. Risk of cardiac dysfunction with trastuzumab in breast cancer patients: A meta-analysis. Cancer Treat. Rev. 2011, 37, 312–320. [Google Scholar] [CrossRef]
- Chavez-MacGregor, M.; Zhang, N.; Buchholz, T.A.; Zhang, Y.; Niu, J.; Elting, L.; Smith, B.D.; Hortobagyi, G.N.; Giordano, S.H. Trastuzumab-Related Cardiotoxicity Among Older Patients with Breast Cancer. J. Clin. Oncol. 2013, 31, 4222–4228. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Abdel-Qadir, H.; Fischer, H.D.; Camacho, X.; Amir, E.; Austin, P.C.; Lee, D.S. Breast Cancer Therapy–Related Cardiac Dysfunction in Adult Women Treated in Routine Clinical Practice: A Population-Based Cohort Study. J. Clin. Oncol. 2016, 34, 2239–2246. [Google Scholar] [CrossRef]
- Bowles, E.J.; Wellman, R.; Feigelson, H.S.; Onitilo, A.A.; Freedman, A.N.; Delate, T.; Allen, L.A.; Nekhlyudov, L.; Goddard, K.A.; Davis, R.L.; et al. Pharmacovigilance Study Team. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: A retrospective cohort study. J. Natl. Cancer Inst. 2012, 104, 1293–1305. [Google Scholar] [CrossRef]
- Guglin, M.; Hartlage, G.; Reynolds, C.; Chen, R.; Patel, V. Trastuzumab-induced cardiomyopathy: Not as benign as it looks? A retrospective study. J. Card. Fail. 2009, 15, 651–657. [Google Scholar] [CrossRef]
- Tarantini, L.; Cioffi, G.; Gori, S.; Tuccia, F.; Boccardi, L.; Bovelli, D.; Lestuzzi, C.; Maurea, N.; Oliva, S.; Russo, G.; et al. Italian Cardio-Oncologic Network. Trastuzumab adjuvant chemotherapy and cardiotoxicity in real-world women with breast cancer. J. Card. Fail. 2012, 18, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Siri-Angkul, N.; Chattipakorn, S.C.; Chattipakorn, N. The mechanistic insights of the arrhythmogenic effect of trastuzumab. Biomed. Pharmacother. 2021, 139, 111620. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.; Soloperto, R.; Casciano, O.; Esposito, R.; Lembo, M.; Canonico, M.; Arpino, G.; Giuliano, M.; De Placido, S.; Esposito, G. Right ventricular dysfunction parallels left ventricular functional involvement in women with breast cancer experiencing subclinical cardiotoxicity. Eur. Heart J. Cardiovasc. Imaging 2021, 22 (Suppl. S1), jeaa356.187. [Google Scholar] [CrossRef]
- Calleja, A.; Poulin, F.; Khorolsky, C.; Shariat, M.; Bedard, P.L.; Amir, E.; Rakowski, H.; McDonald, M.; Delgado, D.; Thavendiranathan, P. Right Ventricular Dysfunction in Patients Experiencing Cardiotoxicity during Breast Cancer Therapy. J. Oncol. 2015, 2015, 609194. [Google Scholar] [CrossRef] [PubMed]
- Bendahou, H.; Abouriche, A.; Maaroufi, A.; Ettagmouti, Y.; Zahri, S.; Ammouri, Z.; Belkouchia, S.; Rochd, E.; Ejjebli, S.; Benmalek, R.; et al. Evaluation of right ventricular dysfunction concomitant to left ventricular dysfunction in cardiotoxicity induced by trastuzumab in patients followed for HER2+ breast cancer: Experience of the first cardio-oncology unit in Morocco. Arch. Cardiovasc. Dis. Suppl. 2023, 15, 168. [Google Scholar] [CrossRef]
- Grover, S.; Leong, D.P.; Chakrabarty, A.; Joerg, L.; Kotasek, D.; Cheong, K.; Joshi, R.; Joseph, M.X.; DePasquale, C.; Koczwara, B.; et al. Left and right ventricular effects of anthracycline and trastuzumab chemotherapy: A prospective study using novel cardiac imaging and biochemical markers. Int. J. Cardiol. 2013, 168, 5465–5467. [Google Scholar] [CrossRef]
- Barthur, A.; Brezden-Masley, C.; Connelly, K.A.; Dhir, V.; Chan, K.K.; Haq, R.; Kirpalani, A.; Barfett, J.J.; Jimenez-Juan, L.; Karur, G.R.; et al. Longitudinal assessment of right ventricular structure and function by cardiovascular magnetic resonance in breast cancer patients treated with trastuzumab: A prospective observational study. J. Cardiovasc. Magn. Reson. 2017, 19, 44. [Google Scholar] [CrossRef]
- Bayar, N.; Küçükseymen, S.; Göktaş, S.; Arslan, Ş. Right ventricle failure associated wıth trastuzumab. Ther. Adv. Drug Saf. 2015, 6, 98–102. [Google Scholar] [CrossRef]
- Kılıçaslan, B.; Özdoğan, Ö.; Demir Pişkin, G.; Kahya Eren, N.; Dursun, H. Echocardiographic signs of right ventricle changes after Trastuzumab treatment in breast cancer patients with erb-2 overexpression. Anatol. J. Cardiol. 2015, 15, 143–148. [Google Scholar] [CrossRef]
- Theetha Kariyanna, P.; Kumar, A.; Jayarangaiah, A.; Shetty, M.; Chowdhury, Y.; Das, S.; Jayarangaiah, A. Chemotherapy induced right ventricular cardiomyopathy; a systematic review and meta-analysis. Front. Cardiovasc. Med. 2023, 10, 1103941. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 4, 4–131. [Google Scholar] [CrossRef]
- Bonow, R.O.; Bacharach, S.L.; Green, M.V.; Kent, K.M.; Rosing, D.M.; Lipson, L.C.; Leon, M.B.; Epstein, S.E. Impaired left ventricular diastolic filling in patients with coronary artery disease: Assessment with radionuclide angiography. Circulation 1981, 64, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Inouye, I.; Massie, B.; Loge, D.; Topic, N.; Silverstein, D.; Simpson, P.; Tubau, J. Abnormal left ventricular filling: An early finding in mild to moderate systemic hypertension. Am. J. Cardiol. 1984, 53, 120–126. [Google Scholar] [CrossRef]
- Cao, L.; Cai, G.; Chang, C.; Miao, A.Y.; Yu, X.L.; Yang, Z.Z.; Ma, J.L.; Zhang, Q.; Wu, J.; Guo, X.M.; et al. Diastolic Dysfunction Occurs Early in HER2-Positive Breast Cancer Patients Treated Concurrently with Radiation Therapy and Trastuzumab. Oncologist 2015, 20, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Takeshita, K.; Murotani, K.; Mitsuma, A.; Hayashi, H.; Tsunoda, N.; Kikumori, T.; Murohara, T.; Ando, Y. Assessment of left ventricular diastolic function during trastuzumab treatment in patients with HER2-positive breast cancer. Breast Cancer 2017, 24, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Nadouri, D.; Osler, E.; Johnson, C.; Dent, S.; Dwivedi, G. Diastolic dysfunction can precede systolic dysfunction on MUGA in cancer patients receiving trastuzumab-based therapy. Nucl. Med. Commun. 2019, 40, 22–29. [Google Scholar] [CrossRef]
- Upshaw, J.N.; Finkelman, B.; Hubbard, R.A.; Smith, A.M.; Narayan, H.K.; Arndt, L.; Domchek, S.; DeMichele, A.; Fox, K.; Shah, P. Comprehensive Assessment of Changes in Left Ventricular Diastolic Function with Contemporary Breast Cancer Therapy. JACC Cardiovasc. Imaging 2020, 13 Pt 2, 198–210. [Google Scholar] [CrossRef]
- Chang, W.T.; Chen, P.W.; Lin, H.W.; Lin, S.H.; Li, Y.H. Risks of trastuzumab-related cardiotoxicity in breast cancer patients in Taiwan. ESC Heart Fail. 2021, 8, 5149–5158. [Google Scholar] [CrossRef]
- Yu, A.F.; Flynn, J.R.; Moskowitz, C.S.; Scott, J.M.; Oeffinger, K.C.; Dang, C.T.; Liu, J.E.; Jones, L.W.; Steingart, R.M. Long-term Cardiopulmonary Consequences of Treatment-Induced Cardiotoxicity in Survivors of ERBB2-Positive Breast Cancer. JAMA Cardiol. 2020, 5, 309–317. [Google Scholar] [CrossRef]
- van der Linde, D.; van Hagen, I.; Veen, K.; Zuetenhorst, H.; van Dalen, B. Global longitudinal strain: An early marker for cardiotoxicity in patients treated for breast cancer. Neth. Heart J. 2023, 31, 103–108. [Google Scholar] [CrossRef]
- Negishi, T.; Thavendiranathan, P.; Penicka, M.; Lemieux, J.; Murbraech, K.; Miyazaki, S.; Shirazi, M.; Santoro, C.; Cho, G.Y.; Popescu, B.A.; et al. Cardioprotection Using Strain-Guided Management of Potentially Cardiotoxic Cancer Therapy: 3-Year Results of the SUCCOUR Trial. JACC Cardiovasc. Imaging 2023, 16, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Critical Reanalysis of the Mechanisms Underlying the Cardiorenal Benefits of SGLT2 Inhibitors and Reaffirmation of the Nutrient Deprivation Signaling/Autophagy Hypothesis. Circulation 2022, 146, 1383–1405. [Google Scholar] [CrossRef]
- Min, J.; Wu, L.; Liu, Y.; Song, G.; Deng, Q.; Jin, W.; Yu, W.; Abudureyimu, M.; Pei, Z.; Ren, J. Empagliflozin attenuates trastuzumab-induced cardiotoxicity through suppression of DNA damage and ferroptosis. Life Sci. 2023, 312, 121207. [Google Scholar] [CrossRef] [PubMed]
| STUDY | GROUPS | HF III–IV NYHA CLASS | HF | ASYMPTOMATIC DECREASE IN LVEF |
|---|---|---|---|---|
| RANDOMIZED CONTROLLED TRIALS | ||||
| NSABP B-31 (Tan-Chiu E, 2005) [55] |
| HF III–IV NYHA class + CV death
| CREC criteria decreased LVEF
| |
| NCCTG N9831 (Perez EA, 2008) [56] |
| HF III–IV NYHA class + CV death
| CREC criteria decreased LVEF
| |
| BIG 1-01—Extended 8-year HERA study Early-stage BC (Evandro de Azambuja, 2014) [57] |
|
| Decreased LVEF
| |
| BCIRG-006—Early-stage BC n = 3222 (Slamon D, 2011) [58] |
|
| Decreased LVEF
| |
| Meta-analysis—10 RCTs on BC (Chen T, 2011) [60] |
|
| Decreased LVEF
| |
| OBSERVATIONAL REAL-WORLD AND REGISTER-BASED TRIALS | ||||
| (Chen J, 2012) [47] n = 45 537, mean age 76.2 (67 to 94) years |
| HF + cardiomyopathy 1st and 3rd years
| ||
| (Chavez-MacGregor M, 2013) [61] n = 9525, mean age 71 years |
| HF
| ||
| (Thavendiranathan P, 2016) [62] n = 18,540, mean age 54 years N = 18,540, follow-up 3 years |
| HF + CV death
| ||
| (Bowles EJ & Team, 2012) [63] n = 12,500, mean age 60 years |
| HF + Cardiomyopathy 1st and 5th years
| ||
| (Guglin M, 2009) [64] n = 118, mean age 51.2 years | Trastuzumab 93% of patients—anthracycline-based therapy | HFrEF + HFpEF 11 (9.3%) |
| |
| (Tarantini L, 2012) [65] n = 499, mean age 55 ± 11 years | Trastuzumab 87% of patients—anthracycline-based therapy | HF II NYHA class 3% |
| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slavcheva, S.E.; Angelov, A. HER2-Targeted Therapy—From Pathophysiology to Clinical Manifestation: A Narrative Review. J. Cardiovasc. Dev. Dis. 2023, 10, 489. https://doi.org/10.3390/jcdd10120489
Slavcheva SE, Angelov A. HER2-Targeted Therapy—From Pathophysiology to Clinical Manifestation: A Narrative Review. Journal of Cardiovascular Development and Disease. 2023; 10(12):489. https://doi.org/10.3390/jcdd10120489
Chicago/Turabian StyleSlavcheva, Svetoslava Elefterova, and Atanas Angelov. 2023. "HER2-Targeted Therapy—From Pathophysiology to Clinical Manifestation: A Narrative Review" Journal of Cardiovascular Development and Disease 10, no. 12: 489. https://doi.org/10.3390/jcdd10120489




