Morphological and Functional Remodeling of the Ischemic Heart Correlates with Homocysteine Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Description and Clinical Characterization of the Patients
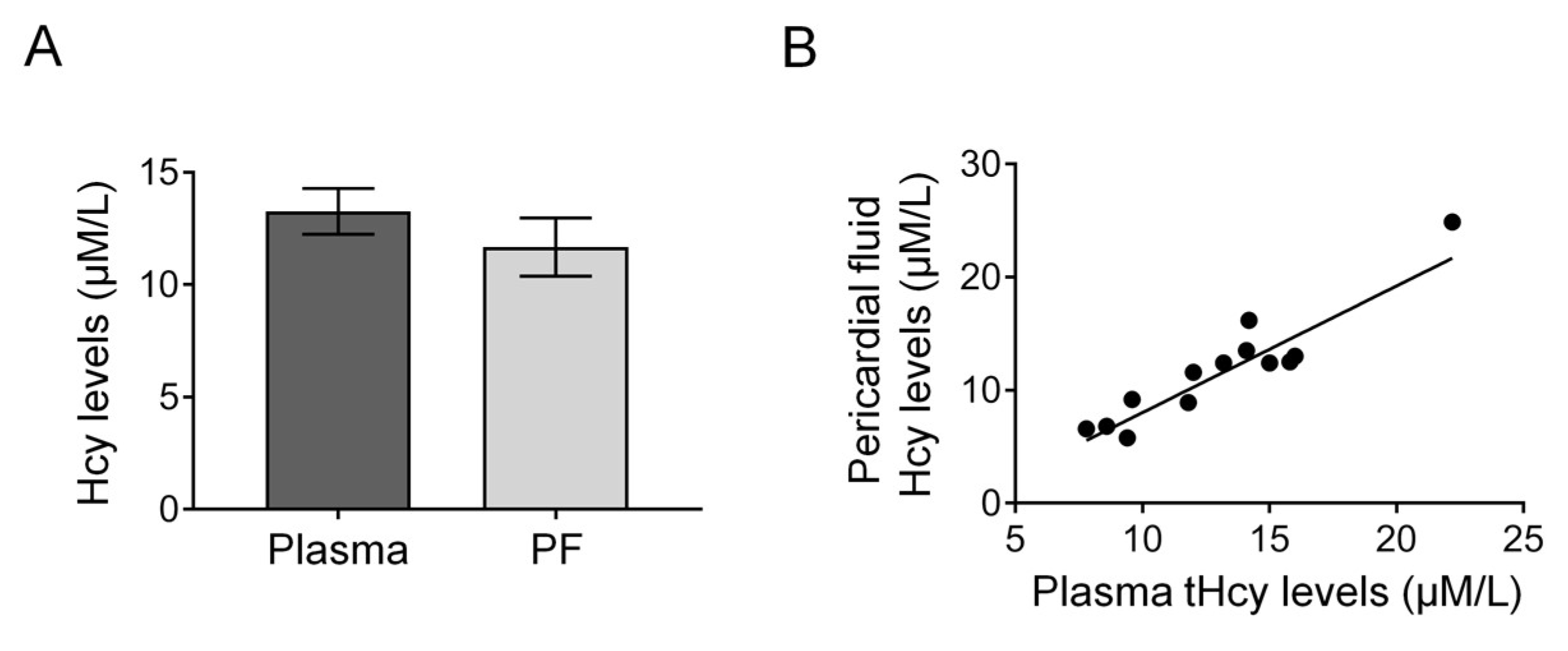
2.2. Measurements of tHcy and cTn-I
2.3. Echocardiography Measurements
2.4. Statistics and Calculations
3. Results
3.1. Characteristics of Patients
3.2. Echocardiographic Parameters of the Heart in CABG Patients
3.3. Correlation of Echocardiographic Parameters of the Heart with Homocysteine Levels in CABG Patients
3.4. Cardiac Troponin-I in Levels Are Increased in PF of CABG Patients
4. Discussion
4.1. Cardiac Ischemia, Remodeling and Homocysteine
4.2. Presence of Cardiac Hypoxia in the Patient Group Studied
4.3. Previous Findings with Homocysteine and the Heart
4.4. Pathomechanisms That May Contribute to Cardiac Remodeling and Contractile Dysfunction: Human Pericardial Fluid ADMA and Endothelin and Cardiac Ischemia
4.5. Hypoxia and Inflammation May Contribute to Cardiac Remodeling and Contractile Dysfunction
4.6. Clinical Importance of the Present Findings
4.7. Clinical Aspects Related to Medications
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nowbar, A.N.; Gitto, M.; Howard, J.P.; Francis, D.P.; Al-Lamee, R. Mortality from Ischemic Heart Disease. Circ. Cardiovasc. Qual Outcomes 2019, 12, e005375. [Google Scholar] [CrossRef] [PubMed]
- Sayols-Baixeras, S.; Lluís-Ganella, C.; Lucas, G.; Elosua, R. Pathogenesis of coronary artery disease: Focus on genetic risk factors and identification of genetic variants. Appl. Clin. Genet. 2014, 7, 15–32. [Google Scholar] [PubMed]
- Bhatt, A.S.; Ambrosy, A.P.; Velazquez, E.J. Adverse Remodeling and Reverse Remodeling After Myocardial Infarction. Curr. Cardiol. Rep. 2017, 19, 71. [Google Scholar] [CrossRef]
- Schirone, L.; Forte, M.; Palmerio, S.; Yee, D.; Nocella, C.; Angelini, F.; Pagano, F.; Schiavon, S.; Bordin, A.; Carrizzo, A.; et al. A Review of the Molecular Mechanisms Underlying the Development and Progres-sion of Cardiac Remodeling. Oxid. Med. Cell. Longev. 2017, 2017, 3920195. [Google Scholar] [CrossRef] [PubMed]
- Na, H.-M.; Cho, G.-Y.; Lee, J.M.; Cha, M.-J.; Yoon, Y.E.; Lee, S.-P.; Kim, H.-K.; Kim, Y.-J.; Sohn, D.-W. Echocardiographic Predictors for Left Ventricular Remodeling after Acute ST Elevation Myocardial Infarction with Low Risk Group: Speckle Tracking Analysis. J. Cardiovasc. Ultrasound 2016, 24, 128–134. [Google Scholar] [CrossRef]
- Nemeth, Z.; Cziraki, A.; Szabados, S.; Biri, B.; Keki, S.; Koller, A. Elevated Levels of Asymmetric Dimethylarginine (ADMA) in the Pericardial Fluid of Cardiac Patients Correlate with Cardiac Hypertrophy. PLoS ONE 2015, 10, e0135498. [Google Scholar] [CrossRef]
- Liu, C.-F.; Tang, W.H.W. Epigenetics in Cardiac Hypertrophy and Heart Failure. Jacc. Basic Transl. Sci. 2019, 4, 976–993. [Google Scholar] [CrossRef]
- Archer, C.R.; Robinson, E.; Drawnel, F.M.; Roderick, H.L. Endothelin-1 promotes hypertrophic remodelling of cardiac myocytes by activating sustained signalling and transcription downstream of endothelin type A receptors. Cell. Signal. 2017, 36, 240–254. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Z.; Gao, Y.; Liang, P.; Shan, Y.; He, J. Angiotensin II receptor blocker LCZ696 attenuates cardiac remodeling through the inhibition of the ERK signaling pathway in mice with pregnancy-associated cardiomyopathy. Cell Biosci. 2019, 9, 86. [Google Scholar] [CrossRef]
- Mandaviya, P.R.; Stolk, L.; Heil, S.G. Homocysteine and DNA methylation: A review of animal and human literature. Mol. Genet. Metab. 2014, 113, 243–252. [Google Scholar] [CrossRef]
- Kang, S.S.; Wong, P.W.K.; Malinow, M.R. Hyperhomocyst(e)inemia as a Risk Factor for Occlusive Vascular Disease. Annu. Rev. Nutr. 1992, 12, 279–298. [Google Scholar] [CrossRef]
- Julius, U.; Pietzsch, J.; Gromeier, S.; Schorr, H.; Herrmann, W. Homocysteine levels in patients treated with lipid apheresis: Effect of a vitamin therapy. Eur. J. Clin. Investig. 2001, 31, 667–671. [Google Scholar] [CrossRef]
- Kayadibi, H.; Sertoglu, E.; Uyanik, M. Plasma Total Homocysteine Levels in Diabetic Retinopathy. BioMed Res. Int. 2014, 2014, 758634. [Google Scholar] [CrossRef]
- Ueland, P.M.; Refsum, H.; Stabler, S.P.; Malinow, M.R.; Andersson, A.; Allen, R.H. Total homocysteine in plasma or serum: Methods and clinical applications. Clin. Chem. 1993, 39, 1764–1779. [Google Scholar] [CrossRef]
- Selhub, J.; Jacques, P.F.; Bostom, A.G.; D’Agostino, R.B.; Wilson, P.W.F.; Belanger, A.J.; O’Leary, D.H.; Wolf, P.A.; Schaefer, E.J.; Rosenberg, I.H. Association between Plasma Homocysteine Concentrations and Extracranial Carot-id-Artery Stenosis. N. Engl. J. Med. 1995, 332, 286–291. [Google Scholar] [CrossRef]
- Humphrey, L.L.; Fu, R.; Rogers, K.; Freeman, M.; Helfand, M. Homocysteine Level and Coronary Heart Disease Incidence: A Systematic Review and Me-ta-analysis. Mayo Clin. Proc. 2008, 83, 1203–1212. [Google Scholar] [CrossRef]
- Milani, R.V.; Lavie, C.J. Homocysteine: The Rubik’s Cube of Cardiovascular Risk Factors. Mayo Clin. Proc. 2008, 83, 1200–1202. [Google Scholar] [CrossRef]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef]
- Koller, A.; Szenasi, A.; Dornyei, G.; Kovacs, N.; Lelbach, A.; Kovacs, I. Coronary Microvascular and Cardiac Dysfunction Due to Homocysteine Pathometabolism; A Complex Therapeutic Design. Curr. Pharm. Des. 2018, 24, 2911–2920. [Google Scholar] [CrossRef]
- Joseph, J.; Joseph, L.; Shekhawat, N.S.; Devi, S.; Wang, J.; Melchert, R.B.; Hauer-Jensen, M.; Kennedy, R.H. Hyperhomocysteinemia leads to pathological ventricular hypertrophy in normotensive rats. Am. J. Physiol. Circ. Physiol. 2003, 285, H679–H686. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Lee, K.L.; Jones, R.H.; Al-Khalidi, H.R.; Hill, J.A.; Panza, J.A.; Michler, R.E.; Bonow, R.O.; Doenst, T.; Petrie, M.C.; et al. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. New Engl. J. Med. 2016, 374, 1511–1520. [Google Scholar]
- Ottani, F.; Galvani, M.; Nicolini, F.A.; Ferrini, D.; Pozzati, A.; Di Pasquale, G.; Jaffe, A.S. Elevated cardiac troponin levels predict the risk of adverse outcome in patients with acute coronary syndromes. Am. Hear. J. 2000, 140, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.V.; Anand, A.; Sandoval, Y.; Lee, K.K.; Smith, S.W.; Adamson, P.D.; Chapman, A.R.; Langdon, T.; Sandeman, D.; Vaswani, A.; et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: A cohort study. Lancet 2015, 386, 2481–2488. [Google Scholar] [CrossRef]
- Hessel, M.H.M.; Atsma, D.E.; van der Valk, E.J.M.; Bax, W.H.; Schalij, M.J.; van der Laarse, A. Release of cardiac troponin I from viable cardiomyocytes is mediated by in-tegrin stimulation. Pflugers Arch. 2008, 455, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Storti, S.; Prontera, C.; Parri, M.S.; Iervasi, A.; Vittorini, S.; Emdin, M.; Zucchelli, G.C.; Longombardo, G.; Migliorini, P.; Clerico, A. Evaluation of the analytical performance of the advanced method for cardiac troponin I for the AxSYM platform: Comparison with the old method and the Access system. Clin. Chem. Lab. Med. 2006, 44, 1022–1029. [Google Scholar] [CrossRef]
- Lonati, S.; Novembrino, C.; Ippolito, S.; Accinni, R.; Galli, C.; Troonen, H.; Campolo, J.; Della Noce, C.; Lunghi, G.; Bamonti Catena, F. Analytical performance and method comparison study of the total homocysteine fluorescence polarization immunoassay (FPIA) on the AxSYM analyzer. Clin. Chem Lab. Med. 2004, 42, 228–234. [Google Scholar] [CrossRef]
- Otterstad, J.E. Measuring left ventricular volume and ejection fraction with the biplane Simpson’s method. Heart 2002, 88, 559. [Google Scholar] [CrossRef]
- Devereux, R.B.; Alonso, D.R.; Lutas, E.M.; Gottlieb, G.J.; Campo, E.; Sachs, I.; Reichek, N. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am. J. Cardiol. 1986, 57, 450–458. [Google Scholar] [CrossRef]
- Yeh, J.-K.; Chen, C.-C.; Hsieh, M.-J.; Tsai, M.-L.; Yang, C.-H.; Chen, D.-Y.; Chang, S.-H.; Wang, C.-Y.; Lee, C.-H.; Hsieh, I.-C. Impact of Homocysteine Level on Long-term Cardiovascular Outcomes in Patients after Coronary Artery Stenting. J. Atheroscler. Thromb. 2017, 24, 696–705. [Google Scholar] [CrossRef]
- Bagi, Z.; Csekö, C.; Tóth, E.; Koller, A. Oxidative stress-induced dysregulation of arteriolar wall shear stress and blood pressure in hyperhomocysteinemia is prevented by chronic vitamin C treatment. Am. J. Physiol. Circ. Physiol. 2003, 285, H2277–H2283. [Google Scholar] [CrossRef]
- Sundström, J.; Sullivan, L.; Selhub, J.; Benjamin, E.J.; D’Agostino, R.B.; Jacques, P.F.; Rosenberg, I.H.; Levy, D.; Wilson, P.W.; Vasan, R.S. Relations of plasma homocysteine to left ventricular structure and function: The Framingham Heart Study. Eur. Hear. J. 2004, 25, 523–530. [Google Scholar] [CrossRef]
- Chen, F.; Guo, Y.-H.; Gao, W.; Feng, X.-H.; Chen, L.; Tang, C.-S. [Effect of hyperhomocysteinemia on cardiac remodeling in rats]. Beijing Da Xue Xue Bao Yi Xue Ban = J. Peking Univ. Heal. Sci. 2006, 38, 179–183. [Google Scholar]
- Ungvari, Z.; Csiszar, A.; Edwards, J.G.; Kaminski, P.M.; Wolin, M.S.; Kaley, G.; Koller, A. Increased superoxide production in coronary arteries in hyperhomocysteinemia: Role of tumor necrosis factor-alpha, NAD(P)H oxidase, and inducible nitric oxide synthase. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 418–424. [Google Scholar] [CrossRef]
- Nemeth, Z.; Cziraki, A.; Szabados, S.; Horvath, I.; Koller, A. Pericardial fluid of cardiac patients elicits arterial constriction: Role of endothelin-1. Can. J. Physiol. Pharmacol. 2015, 93, 779–785. [Google Scholar] [CrossRef]
- Veresh, Z.; Debreczeni, B.; Hamar, J.; Kaminski, P.M.; Wolin, M.S.; Koller, A. Asymmetric Dimethylarginine Reduces Nitric Oxide Donor-Mediated Dilation of Arterioles by Activating the Vascular Renin-Angiotensin System and Reactive Oxygen Species. J. Vasc. Res. 2012, 49, 363–372. [Google Scholar] [CrossRef]
- Cziraki, A.; Lenkey, Z.; Sulyok, E.; Szokodi, I.; Koller, A. L-Arginine-Nitric Oxide-Asymmetric Dimethylarginine Path-way and the Coronary Circulation: Translation of Basic Science Results to Clinical Practice. Front. Pharmacol. 2020, 11, 569914. [Google Scholar] [CrossRef]
- Cohn, J.N.; Ferrari, R.; Sharpe, N.; on Behalf of an International Forum on Cardiac Remodeling. Cardiac remodeling—concepts and clinical implications: A consensus paper from an inter-national forum on cardiac remodeling. J. Am. Coll. Cardiol. 2000, 35, 569–582. [Google Scholar] [CrossRef]
- Czubryt, M.P. Cardiac Fibroblast to Myofibroblast Phenotype Conversion—An Unexploited Therapeutic Target. J. Cardiovasc. Dev. Dis. 2019, 6, 28. [Google Scholar] [CrossRef]
- Bagchi, R.A.; Roche, P.; Aroutiounova, N.; Espira, L.; Abrenica, B.; Schweitzer, R.; Czubryt, M.P. The transcription factor scleraxis is a critical regulator of cardiac fibroblast phenotype. BMC Biol. 2016, 14, 21. [Google Scholar] [CrossRef]
- Kanisicak, O.; Khalil, H.; Ivey, M.J.; Karch, J.; Maliken, B.D.; Correll, R.N.; Brody, M.J.; Lin, S.-C.J.; Aronow, B.J.; Tallquist, M.D.; et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. 2016, 7, 12260. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y. TGF-β in fibrosis by acting as a conductor for contractile properties of myofibroblasts. Cell. Biosci. 2019, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.E.; Hagood, J.S. Epigenetic Regulation of Myofibroblast Phenotypes in Fibrosis. Curr. Pathobiol. Rep. 2018, 6, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Bode-Böger, S.M.; Scalera, F.; Ignarro, L.J. The l-arginine paradox: Importance of the l-arginine/asymmetrical dimethylarginine ra-tio. Pharmacol. Ther. 2007, 114, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Suematsu, N.; Ojaimi, C.; Kinugawa, S.; Wang, Z.; Xu, X.; Koller, A.; Recchia, F.A.; Hintze, T.H. Hyperhomocysteinemia Alters Cardiac Substrate Metabolism by Impairing Nitric Oxide Bioavailability Through Oxidative Stress. Circulation 2007, 115, 255–262. [Google Scholar] [CrossRef]
- Okuyan, E.; Uslu, A.; Çakar, M.A.; Sahin, I.; Önür, I.; Enhos, A.; Biter, H.A.; Çetin, Ş.; Dinçkal, M.H. Homocysteine Levels in Patients with Heart Failure with Preserved Ejection Fraction. Cardiology 2010, 117, 21–27. [Google Scholar] [CrossRef]
- Gupta, A.; Moustapha, A.; Jacobsen, D.W.; Goormastic, M.; Tuzcu, E.M.; Hobbs, R.; Young, J.; James, K.; McCarthy, P.; van Lente, F.; et al. High Homocysteine, Low Folate, and Low Vitamin B6 Concentrations: Prevalent Risk Factors for Vascular Disease in Heart Transplant Recipients. Transplantation 1998, 65, 544–550. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Fu, Y.N.; Xiao, H.; Ma, X.W.; Jiang, S.Y.; Xu, M.; Zhang, Y.Y. Metformin attenuates pressure overload-induced cardiac hy-pertrophy via AMPK activation. Acta Pharmacol. Sin. 2011, 32, 879–887. [Google Scholar] [CrossRef]
- Li, J.; Minćzuk, K.; Massey, J.C.; Howell, N.L.; Roy, R.J.; Paul, S.; Patrie, J.T.; Kramer, C.M.; Epstein, F.H.; Carey, R.M.; et al. Metformin Improves Cardiac Metabolism and Function, and Prevents Left Ventricular Hypertrophy in Spontaneously Hypertensive Rats. J. Am. Hear. Assoc. 2020, 9, e015154. [Google Scholar] [CrossRef]
- Mohan, M.; Al-Talabany, S.; McKinnie, A.; Mordi, I.R.; Singh, J.S.S.; Gandy, S.J.; Baig, F.; Hussain, M.S.; Bhalraam, U.; Khan, F.; et al. A randomized controlled trial of met-formin on left ventricular hypertrophy in patients with coronary artery disease without diabetes: The MET-REMODEL trial. Eur. Heart J. 2019, 40, 3409–3417. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Li, Q.; Ren, K.; Sun, X.; Li, J. Metformin Treatment and Homocysteine: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2016, 8, 798. [Google Scholar] [CrossRef]
- Aung, N.; Sanghvi, M.M.; Piechnik, S.K.; Neubauer, S.; Munroe, P.B.; Petersen, S.E. The Effect of Blood Lipids on the Left Ventricle: A Mendelian Randomization Study. J. Am. Coll. Cardiol. 2020, 76, 2477–2488. [Google Scholar] [CrossRef]
- Liao, J.K. Statin therapy for cardiac hypertrophy and heart failure. J. Investig. Med. 2004, 52, 248–253. [Google Scholar] [CrossRef]
- Wile, D.J.; Toth, C. Association of Metformin, Elevated Homocysteine, and Methylmalonic Acid Levels and Clinically Wors-ened Diabetic Peripheral Neuropathy. Diabetes Care 2010, 33, 156–161. [Google Scholar] [CrossRef]





| Pre-Operative Data | |
|---|---|
| Age (year) | 62.1 ± 2.1 |
| Sex (male/female) | 7/7 |
| Hypertension | 6 |
| Echocardiographic indices of LVH | 7 |
| Diabetes mellitus | 6 |
| Previous acute myocardial infarction | 2 |
| Pre-operative medication | |
| Beta-blocker | 13 |
| Ca-channel blocker | 2 |
| ACE-inhibitor | 5 |
| Diuretic | 1 |
| Aspirin | 12 |
| Antiplatelet medication (Clopidogrel) | 6 |
| Anti-diabetic | 6 |
| Statin | 13 |
| Anti-arrhythmic | 2 |
| Echocardiographic Parameter | CABG tHcy < 12 μM/L | CABG tHcy > 12 μM/L | p |
|---|---|---|---|
| LVED (mm) | 45.83 ± 1.47 | 52.00 ± 1.88 | p < 0.05 |
| LVES (mm) | 28.17 ± 1.17 | 34.75 ± 2.35 | p < 0.05 |
| LA (cm2) | 14.71 ± 0.68 | 17.12 ± 0.65 | p < 0.05 |
| LVEF (%) | 60.67 ± 1.63 | 53.63 ± 3.46 | p < 0.05 |
| PW (mm) | 11.50 ± 0.5 | 12.00 ± 0.27 | N.S. |
| cLVM (g) | 189.96 ± 17.34 | 256.14 ± 14.12 | p < 0.05 |
| IVS (mm) | 11.17 ± 0.4 | 12.50 ± 0.57 | N.S. |
| RVOT EDA (cm2) | 27.17 ± 1.74 | 30.00 ± 1.41 | p < 0.05 |
| RA (cm2) | 14.05 ± 0.81 | 14.90 ± 0.93 | N.S. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cziraki, A.; Nemeth, Z.; Szabados, S.; Nagy, T.; Szántó, M.; Nyakas, C.; Koller, A. Morphological and Functional Remodeling of the Ischemic Heart Correlates with Homocysteine Levels. J. Cardiovasc. Dev. Dis. 2023, 10, 122. https://doi.org/10.3390/jcdd10030122
Cziraki A, Nemeth Z, Szabados S, Nagy T, Szántó M, Nyakas C, Koller A. Morphological and Functional Remodeling of the Ischemic Heart Correlates with Homocysteine Levels. Journal of Cardiovascular Development and Disease. 2023; 10(3):122. https://doi.org/10.3390/jcdd10030122
Chicago/Turabian StyleCziraki, Attila, Zoltan Nemeth, Sandor Szabados, Tamas Nagy, Márk Szántó, Csaba Nyakas, and Akos Koller. 2023. "Morphological and Functional Remodeling of the Ischemic Heart Correlates with Homocysteine Levels" Journal of Cardiovascular Development and Disease 10, no. 3: 122. https://doi.org/10.3390/jcdd10030122
APA StyleCziraki, A., Nemeth, Z., Szabados, S., Nagy, T., Szántó, M., Nyakas, C., & Koller, A. (2023). Morphological and Functional Remodeling of the Ischemic Heart Correlates with Homocysteine Levels. Journal of Cardiovascular Development and Disease, 10(3), 122. https://doi.org/10.3390/jcdd10030122






