The Heart–Brain Interplay in Multiple Sclerosis from Pathophysiology to Clinical Practice: A Narrative Review
Abstract
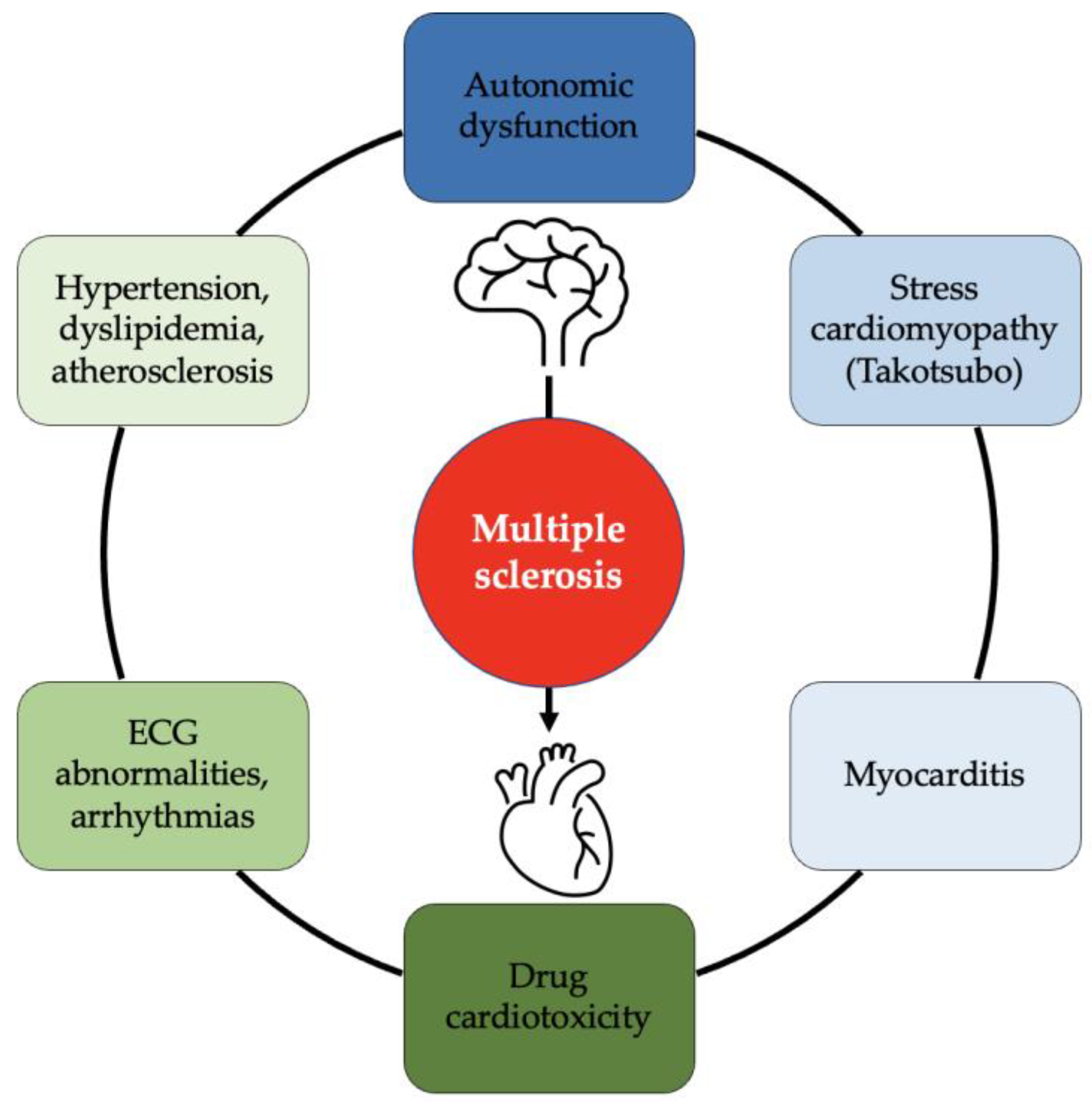
1. Introduction
1.1. Autonomous Nervous System
1.2. ANS in MS Patients
2. Heart Health in MS Patients
2.1. Cardiovascular Risk in MS Patients
2.2. Cardiac Diseases in MS Patients
3. Inflammation in MS and Heart
3.1. Pathophysiology of Inflammation
3.2. MS and Myocarditis
4. MS Therapy and Heart Consequences of Chronic Therapy
5. Discussion and Clinical Recommendations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MS | Multiple Sclerosis |
| CNS | Central Nervous System |
| BBB | Blood Brain Barrier |
| QoL | Quality Of Life |
| ANS | Autonomic Nervous System |
| CIS | Clinically Isolated Syndrome |
| HRV | Heart Rate Variability |
| EDSS | Expanded Disability Status Scale |
| HF | Heart Failure |
| SPMS | Secondary Progressive Multiple Sclerosis |
| DCM | Dilated Cardiomyopathy |
| NMOSD | Neuromyelitis Optica Spectrum Disorder |
| EBV | Epstein–Barr Virus |
| RRMS | Relapsing-Remitting Multiple Sclerosis |
| DMT | Disease-modifying Treatments |
| PMS | Progressive Multiple Sclerosis |
| S1P | Sphingosine 1-Phosphate |
| S1PR | Sphingosine 1-Phosphate Receptor |
References
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis. Prim. 2018, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Patterson, K.R.; Bar-Or, A. Reassessing B cell contributions in multiple sclerosis. Nat. Immunol. 2018, 19, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Kutzelnigg, A.; Lucchinetti, C.F.; Stadelmann, C.; Brück, W.; Rauschka, H.; Bergmann, M.; Schmidbauer, M.; Parisi, J.E.; Lassmann, H. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 2005, 128, 2705–2712. [Google Scholar] [CrossRef]
- Kingwell, E.; van der Kop, M.; Zhao, Y.; Shirani, A.; Zhu, F.; Oger, J.; Tremlett, H. Relative mortality and survival in multiple sclerosis: Findings from British Columbia, Canada. J. Neurol. Neurosurg. Psychiatry 2012, 83, 61–66. [Google Scholar] [CrossRef]
- Marrie, R.A.; Elliott, L.; Marriott, J.; Cossoy, M.; Blanchard, J.; Leung, S.; Yu, N. Effect of comorbidity on mortality inmultiple sclerosis. Neurology 2015, 85, 240–247. [Google Scholar] [CrossRef]
- Marrie, R.A.; Cohen, J.; Stuve, O.; Trojano, M.; Sorensen, P.S.; Reingold, S.; Cutter, G.; Reider, N. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: Overview. Mult. Scler. J. 2015, 21, 263–281. [Google Scholar] [CrossRef]
- Ragonese, P.; Aridon, P.; Mazzola, M.A.; Callari, G.; Palmeri, B.; Famoso, G.; Terruso, V.; Salemi, G.; D’Amelio, M.; Savettieri, G. Multiple sclerosis survival: A population-based study in Sicily. Eur. J. Neurol. 2010, 17, 391–397. [Google Scholar] [CrossRef]
- Manouchehrinia, A.; Tanasescu, R.; Tench, C.R.; Constantinescu, C.S. Mortality in multiple sclerosis: Meta-analysis of standardised mortality ratios. J. Neurol. Neurosurg. Psychiatry 2016, 87, 324–331. [Google Scholar] [CrossRef]
- Imrich, R.; Vlcek, M.; Penesova, A.; Radikova, Z.; Havranova, A.; Sivakova, M.; Siarnik, P.; Kollar, B.; Sokolov, T.; Turcani, P.; et al. Cardiac autonomic function in patients with early multiple sclerosis. Clin. Auton. Res. 2021, 31, 553–562. [Google Scholar] [CrossRef]
- Marrie, R.A.; Reider, N.; Cohen, J.; Stuve, O.; Trojano, M.; Cutter, G.; Reingold, S.; Sorensen, P.S. A systematic review of the incidence and prevalence of cardiac, cerebrovascular, and peripheral vascular disease in multiple sclerosis. Mult. Scler. J. 2015, 21, 318–331. [Google Scholar] [CrossRef]
- Marrie, R.A. Comorbidity in multiple sclerosis: Implications for patient care. Nat. Rev. Neurol. 2017, 13, 375–382. [Google Scholar] [CrossRef]
- Fawzy, A.M.; Lip, G.Y.H. Cardiovascular disease prevention: Risk factor modification at the heart of the matter. Lancet Reg. Health West. Pac. 2021, 17, 100291. [Google Scholar] [CrossRef] [PubMed]
- Roifman, I.; Beck, P.L.; Anderson, T.J.; Eisenberg, M.J.; Genest, J. Chronic inflammatory diseases and cardiovascular risk: A systematic review. Can. J. Cardiol. 2011, 27, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Smolen, A.J. Morphology of synapses in the autonomic nervous system. J. Electron Microsc. Tech. 1988, 10, 187–204. [Google Scholar] [CrossRef]
- Davis, M. The Role of the Amygdala in Fear and Anxiety. Annu. Rev. Neurosci. 1992, 15, 353–375. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, M.G.; Benarroch, E.E. Central Control of Autonomic Function and Involvement in Neurodegenerative Disorders, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 117, pp. 45–57. [Google Scholar] [CrossRef]
- Magnano, A.R.; Holleran, S.; Ramakrishnan, R.; A Reiffel, J.; Bloomfield, D.M. Autonomic nervous system influences on qt interval in normal subjects. J. Am. Coll. Cardiol. 2002, 39, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Flachenecker, P.; Reiners, K.; Krauser, M.; Wolf, A.; Toyka, K.V. Autonomic dysfunction in multiple sclerosis is related to disease activity and progression of disability. Mult. Scler. J. 2001, 7, 327–334. [Google Scholar] [CrossRef]
- Armour, J.A.; Murphy, D.A.; Yuan, B.-X.; MacDonald, S.; Hopkins, D.A. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat. Rec. 1997, 247, 289–298. [Google Scholar] [CrossRef]
- Armour, J.A. Functional anatomy of intrathoracic neurons innervating the atria and ventricles. Hear. Rhythm. 2010, 7, 994–996. [Google Scholar] [CrossRef]
- Habek, M. Immune and autonomic nervous system interactions in multiple sclerosis: Clinical implications. Clin. Auton. Res. 2019, 29, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Sirbu, C.A.; Mezei, R.-M.; Falup-Pecurariu, C.; Bratu, O.G.; Sirbu, A.M.; Ghinescu, M.C.; Radu, F.I. Autonomic dysfunctions in multiple sclerosis: Challenges of clinical practice (Review). Exp. Ther. Med. 2020, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Vita, G.; Fazio, M.C.; Milone, S.; Blandino, A.; Salvi, L.; Messina, C. Cardiovascular autonomic dysfunction in multiple sclerosis is likely related to brainstem lesions. J. Neurol. Sci. 1993, 120, 82–86. [Google Scholar] [CrossRef]
- Habek, M.; Crnošija, L.; Lovrić, M.; Junaković, A.; Skorić, M.K.; Adamec, I. Sympathetic cardiovascular and sudomotor functions are frequently affected in early multiple sclerosis. Clin. Auton. Res. 2016, 26, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Saari, A.; Tolonen, U.; Pääkkö, E.; Suominen, K.; Pyhtinen, J.; Sotaniemi, K.; Myllylä, V. Cardiovascular autonomic dysfunction correlates with brain MRI lesion load in MS. Clin. Neurophysiol. 2004, 115, 1473–1478. [Google Scholar] [CrossRef]
- De Seze, J.; Stojkovic, T.; Gauvrit, J.-Y.; Devos, D.; Ayachi, M.; Cassim, F.; Michel, T.S.; Pruvo, J.-P.; Guieu, J.-D.; Vermersch, P. Autonomic dysfunction in multiple sclerosis: Cervical spinal cord atrophy correlates. J. Neurol. 2001, 248, 297–303. [Google Scholar] [CrossRef]
- Mikkola, A.; Ojanen, A.; Hartikainen, J.E.K.; Remes, A.M.; Simula, S. The impact of multiple sclerosis onset symptom on cardiac repolarization. Brain Behav. 2017, 7, e00742. [Google Scholar] [CrossRef] [PubMed]
- de Seze, J.; Stojkovic, T.; Gauvrit, J.Y.; Saint Michel, T.; Ayachi, M.; Pruvo, J.P.; Vermersch, P. Cardiac repolarization abnormalities in multiple sclerosis: Spinal cord MRI correlates. Muscle Nerve. 2000, 23, 1284–1286. [Google Scholar] [CrossRef]
- Habek, M.; Skorić, M.K.; Crnošija, L.; Gabelić, T.; Barun, B.; Adamec, I. Postural Orthostatic Tachycardia Predicts Early Conversion to Multiple Sclerosis after Clinically Isolated Syndrome. Eur. Neurol. 2017, 77, 253–257. [Google Scholar] [CrossRef]
- Shah, A.J.; Wittbrodt, M.T.; Bremner, J.D.; Vaccarino, V. Cardiovascular pathophysiology from the cardioneural perspective and its clinical applications. Trends Cardiovasc. Med. 2022, 32, 172–177. [Google Scholar] [CrossRef]
- Racosta, J.M.; Kimpinski, K. Autonomic dysfunction, immune regulation, and multiple sclerosis. Clin. Auton. Res. 2016, 26, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Sandroni, P.; Benarroch, E.E.; Low, P.A. Pharmacological dissection of components of the Valsalva maneuver in adrenergic failure. J. Appl. Physiol. 1991, 71, 1563–1567. [Google Scholar] [CrossRef] [PubMed]
- Flachenecker, P.; Wolf, A.; Krauser, M.; Hartung, H.-P.; Reiners, K. Cardiovascular autonomic dysfunction in multiple sclerosis: Correlation with orthostatic intolerance. J. Neurol. 1999, 246, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Autonomic nervous system and neuroimmune interactions. Neurology 2019, 92, 377–385. [Google Scholar] [CrossRef]
- Sternberg, E.M. Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 2006, 6, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Palladino, R.; Marrie, R.A.; Majeed, A.; Chataway, J. Evaluating the Risk of Macrovascular Events and Mortality Among People With Multiple Sclerosis in England. JAMA Neurol. 2020, 77, 820–828. [Google Scholar] [CrossRef]
- Rapp, D.; Michels, S.; Schöpe, J.; Schwingshackl, L.; Tumani, H.; Senel, M. Associations between multiple sclerosis and incidence of heart diseases: Systematic review and meta-analysis of observational studies. Mult. Scler. Relat. Disord. 2021, 56, 103279. [Google Scholar] [CrossRef]
- Bezzini, D.; Gualdani, E.; Razzanelli, M.; Battaglia, M.A.; Cortese, R.; Francesconi, P.; Ulivelli, M. Prevalence of chronic comorbidities in people with multiple sclerosis: Descriptive study based on administrative data in Tuscany (Central Italy). Neurol Sci. 2022, 43, 6407–6414. [Google Scholar] [CrossRef]
- Tettey, P.; Simpson, J.S.; Taylor, B.; Blizzard, L.; Ponsonby, A.-L.; Dwyer, T.; Kostner, K.; Van Der Mei, I. An adverse lipid profile is associated with disability and progression in disability, in people with MS. Mult. Scler. J. 2014, 20, 1737–1744. [Google Scholar] [CrossRef]
- Healy, B.C.; Ali, E.N.; Guttmann, C.; Chitnis, T.; Glanz, B.I.; Buckle, G.; Houtchens, M.; Stazzone, L.; Moodie, J.; Berger, A.M.; et al. Smoking and disease progression in multiple sclerosis. Arch. Neurol. 2009, 66, 858–864. [Google Scholar] [CrossRef]
- Christiansen, C.F.; Christensen, S.; Farkas, D.K.; Miret, M.; Sørensen, H.T.; Pedersen, L. Risk of arterial cardiovascular diseases in patients with multiple sclerosis: A population-based cohort study. Neuroepidemiology 2010, 35, 267–274. [Google Scholar] [CrossRef]
- Amatya, B.; Khan, F.; Galea, M. Rehabilitation for people with multiple sclerosis: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2019, 1, CD012732. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.; Wens, I.; Keytsman, C.; Eijnde, B.O.; Dendale, P. Is long-term exercise intervention effective to improve cardiac autonomic control during exercise in subjects with multiple sclerosis? A randomized controlled trial–PubMed. Eur. J. Phys. Rehabil. Med. 2015, 51, 223–231. [Google Scholar] [PubMed]
- Senaratne, M.P.; Carroll, D.; Warren, K.G.; Kappagoda, T. Evidence for cardiovascular autonomic nerve dysfunction in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 1984, 47, 947–952. [Google Scholar] [CrossRef]
- Hale, L.A.; Nukada, H.; Du Plessis, L.J.; Peebles, K.C. Clinical screening of autonomic dysfunction in multiple sclerosis. Physiother Res Int. 2009, 14, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.; Wens, I.; Dendale, P.; Eijnde, B.O. Exercise-onset heart rate increase is slowed in multiple sclerosis patients: Does a disturbed cardiac autonomic control affect exercise tolerance? Neurorehabilitation 2013, 33, 139–146. [Google Scholar] [CrossRef]
- Hansen, d.; Wens, I.; Keytsman, C.; Eijnde, O.; Dendale, P. Is long-term exercise intervention effective to improve cardiac autonomic control during exercisein subjects with multiple sclerosis? A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2015, 51, 223–231. [Google Scholar] [PubMed]
- Wens, I.; Eijnde, B.O.; Hansen, D. Muscular, cardiac, ventilatory and metabolic dysfunction in patients with multiple sclerosis: Implications for screening, clinical care and endurance and resistance exercise therapy, a scoping review. J. Neurol. Sci. 2016, 367, 107–121. [Google Scholar] [CrossRef]
- Chatterjee, S. ECG Changes in Subarachnoid Haemorrhage: A Synopsis. Neth. Heart J. 2011, 19, 31–34. [Google Scholar] [CrossRef]
- Mauck, H.; Hockman, C.H. Central nervous system mechanisms mediating cardiac rate and rhythm. Am. Heart J. 1967, 74, 96–109. [Google Scholar] [CrossRef]
- Sörös, P.; Hachinski, V. Cardiovascular and neurological causes of sudden death after ischaemic stroke. Lancet Neurol. 2012, 11, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.A.; Parra, A.; Shimbo, D.; Fernandez, A.; Schmidt, J.M.; Peter, P.; Claassen, J.; Wartenberg, K.E.; Rincon, F.; Badjatia, N.; et al. Cardiac arrhythmias after subarachnoid hemorrhage: Risk factors and impact on outcome. Cerebrovasc. Dis. 2008, 26, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Chagnac, Y.; Martinovits, G.; Tadmor, R.; Goldhammer, Y. Paroxysmal atrial fibrillation associated with an attack of multiple sclerosis. Postgrad. Med. J. 1986, 62, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Uriel, N.; Kaluski, E.; Hendler, A.; Leitman, M.; Vered, Z. Cardiogenic shock in a young female with multiple sclerosis. Resuscitation 2006, 70, 153–157. [Google Scholar] [CrossRef]
- Padley, J.R.; Feneley, M.P.; Hayward, C.S.; Markus, R. Neurocardiogenic pulmonary oedema: Initial presentation of multiple sclerosis. Heart Lung Circ. 2012, 21, 853–855. [Google Scholar] [CrossRef]
- Dell’Aquila, A.; Sciatti, E.; Vizzardi, E.; Metra, M. The brain-heart connection: A multiple sclerosis relapse presenting as Takotsubo Syndrome. A case report and literature review. Monaldi Arch. Chest Dis. 2020, 90, 1153. [Google Scholar] [CrossRef]
- Vizzardi, E.; Dâaloia, A.; Zanini, G.; Fiorina, C.; Chiari, E.; Nodari, S.; Cas, L.D. Tako-tsubo-like left ventricular dysfunction: Transient left ventricular apical ballooning syndrome. Int. J. Clin. Pract. 2010, 64, 67–74. [Google Scholar] [CrossRef]
- Abraham, J.; Mudd, J.O.; Kapur, N.; Klein, K.; Champion, H.C.; Wittstein, I.S. Stress Cardiomyopathy After Intravenous Administration of Catecholamines and Beta-Receptor Agonists. J. Am. Coll. Cardiol. 2009, 53, 1320–1325. [Google Scholar] [CrossRef]
- Ghadri, J.-R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International expert consensus document on takotsubo syndrome (Part I): Clinical characteristics, diagnostic criteria, and pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef]
- Vizzardi, E.; Bonadei, I.; Piovanelli, B.; Bugatti, S.; D′Aloia, A. Biventricular Tako-Tsubo cardiomyopathy: Usefulness of 2D speckle tracking strain echocardiography. J. Clin. Ultrasound 2014, 42, 121–124. [Google Scholar] [CrossRef]
- Midaglia, L.; Mariño, J.M.J.; Garriga, J.S.; Rovira, A.; Vidal-Jordana, A.; A López-Pérez, M.; E Marzo-Sola, M.; Escribano, F.L.; Montalban, X. An uncommon first manifestation of multiple sclerosis: Tako-Tsubo cardiomyopathy. Mult. Scler. J. 2016, 22, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Bayer, A.D.; Cahill, J.F.; Rizvi, S.A. Multiple sclerosis relapse presenting as an acute cardiomyopathy. Mult. Scler. Relat. Disord. 2019, 27, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Prestipino, E.; Squitieri, M.; Razzolini, L.; Pastò, L.; Forleo, P.; Amato, M.P. A case of Takotsubo syndrome during a multiple sclerosis brainstem relapse. Mult. Scler. Relat. Disord. 2018, 24, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.J.; Zipes, D.P. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circulation Research. 2014, 114, 1004–1021. [Google Scholar] [CrossRef]
- Siscovick, D.S.; Raghunathan, T.; Rautaharju, P.; Psaty, B.M.; Cobb, L.A.; Wagner, E.H. Clinically silent electrocardiographic abnormalities and risk of primary cardiac arrest among hypertensive patients. Circulation 1996, 94, 1329–1333. [Google Scholar] [CrossRef]
- Porthan, K.; Viitasalo, M.; Jula, A.; Reunanen, A.; Rapola, J.; Väänänen, H.; Nieminen, M.S.; Toivonen, L.; Salomaa, V.; Oikarinen, L. Predictive value of electrocardiographic QT interval and T-wave morphology parameters for all-cause and cardiovascular mortality in a general population sample. Heart Rhythm. 2009, 6, 1202–1208.e1. [Google Scholar] [CrossRef]
- Frohman, E.M.; Racke, M.K.; Raine, C.S. Multiple Sclerosis–The Plaque and Its Pathogenesis. N. Engl. J. Med. 2006, 354, 942–955. [Google Scholar] [CrossRef]
- Lassmann, H.; van Horssen, J.; Mahad, D. Progressive multiple sclerosis: Pathology and pathogenesis. Nat. Rev. Neurol. 2012, 8, 647–656. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Maisch, B.; Richter, A.; Sandmöller, A.; Portig, I.; Pankuweit, S. Inflammatory Dilated Cardiomyopathy (DCMI). Herz 2005, 30, 535–544. [Google Scholar] [CrossRef]
- Martino, G.; Furlan, R.; Brambilla, E.; Bergami, A.; Ruffini, F.; Gironi, M.; Poliani, P.L.; Grimaldi, L.M.; Comi, G. Cytokines and immunity in multiple sclerosis: The dual signal hypothesis. J. Neuroimmunol. 2000, 109, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, D.; Stafford, K.A.; Sung, Y.K. Update on coxsackievirus B3 myocarditis. Curr. Opin. Rheumatol. 2012, 24, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Yu, M.; Lin, Q.-W.; Cao, A.-L.; Yu, X.; Dong, J.-H.; Wang, J.-P.; Zhang, J.-H.; Wang, M.; Guo, H.-P.; et al. Th17 Cells Contribute to Viral Replication in Coxsackievirus B3-Induced Acute Viral Myocarditis. J. Immunol. 2010, 185, 4004–4010. [Google Scholar] [CrossRef]
- Noutsias, M.; Rohde, M.; Göldner, K.; Block, A.; Blunert, K.; Hemaidan, L.; Hummel, M.; Blohm, J.-H.; Lassner, D.; Kühl, U.; et al. Expression of functional T-cell markers and T-cell receptor Vbeta repertoire in endomyocardial biopsies from patients presenting with acute myocarditis and dilated cardiomyopathy. Eur. J. Heart Fail. 2011, 13, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Fuse, K.; Kodama, M.; Aizawa, Y.; Yamaura, M.; Tanabe, Y.; Takahashi, K.; Sakai, K.; Miida, T.; Oda, H.; Higuma, N. Th1/Th2 Balance Alteration in the Clinical Course of a Patient With Acute Viral Myocarditis. Jpn. Circ. J. 2001, 65, 1082–1084. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Bar-Or, A.; Comi, G.; Giovannoni, G.; Hartung, H.-P.; Hemmer, B.; Lublin, F.; Montalban, X.; Rammohan, K.W.; Selmaj, K.; et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N. Engl. J. Med. 2017, 376, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Waters, P.; Woodhall, M.; O’Connor, K.C.; Reindl, M.; Lang, B.; Sato, D.K.; Juryńczyk, M.; Tackley, G.; Rocha, J.; Takahashi, T.; et al. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol. Neuroinflamm. 2015, 2, e89. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Mahon, N.; Baig, M.K.; Tona, F.; Murphy, R.T.; Elliott, P.M.; McKenna, W.J. Prospective Familial Assessment in Dilated Cardiomyopathy. Circulation 2007, 115, 76–83. [Google Scholar] [CrossRef]
- Caforio, A.L.; Grazzini, M.; Mann, J.M.; Keeling, P.J.; Bottazzo, G.F.; McKenna, W.J.; Schiaffino, S. Identification of alpha- and beta-cardiac myosin heavy chain isoforms as major autoantigens in dilated cardiomyopathy. Circulation 1992, 85, 1734–1742. [Google Scholar] [CrossRef]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Pender, M.P.; A Csurhes, P.; Burrows, J.M.; Burrows, S.R. Defective T-cell control of Epstein–Barr virus infection in multiple sclerosis. Clin. Transl. Immunol. 2017, 6, e147. [Google Scholar] [CrossRef]
- VRicigliano, V.A.G.; Handel, A.E.; Sandve, G.K.; Annibali, V.; Ristori, G.; Mechelli, R.; Cader, M.Z.; Salvetti, M. EBNA2 Binds to Genomic Intervals Associated with Multiple Sclerosis and Overlaps with Vitamin D Receptor Occupancy. PLoS ONE 2015, 10, e0119605. [Google Scholar] [CrossRef]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef]
- Richter, J.; Quintanilla-Martinez, L.; Bienemann, K.; Zeus, T.; Germing, U.; Sander, O.; Kandolf, R.; Häussinger, D.; Klingel, K. An unusual presentation of a common infection. Infection 2013, 41, 565–569. [Google Scholar] [CrossRef]
- Chimenti, C.; Verardo, R.; Grande, C.; Francone, M.; Frustaci, A. Infarct-like myocarditis with coronary vasculitis and aneurysm formation caused by Epstein–Barr virus infection. ESC Heart Fail. 2020, 7, 938–941. [Google Scholar] [CrossRef]
- E Martinez, N.; Sato, F.; Kawai, E.; Omura, S.; Chervenak, R.P.; Tsunoda, I. Regulatory T cells and Th17 cells in viral infections: Implications for multiple sclerosis and myocarditis. Futur. Virol. 2012, 7, 593–608. [Google Scholar] [CrossRef]
- Myers, J.M.; Cooper, L.T.; Kem, D.C.; Stavrakis, S.; Kosanke, S.D.; Shevach, E.M.; Fairweather, D.; Stoner, J.A.; Cox, C.J.; Cunningham, M.W. Cardiac myosin-Th17 responses promote heart failure in human myocarditis. J. Clin. Investig. 2016, 1, 85851. [Google Scholar] [CrossRef] [PubMed]
- Venken, K.; Hellings, N.; Thewissen, M.; Somers, V.; Hensen, K.; Rummens, J.-L.; Medaer, R.; Hupperts, R.; Stinissen, P. Compromised CD4+ CD25highregulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology 2008, 123, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Sala, S.; Peretto, G.; Gramegna, M.; Palmisano, A.; Villatore, A.; Vignale, D.; De Cobelli, F.; Tresoldi, M.; Cappelletti, A.M.; Basso, C.; et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur. Heart J. 2020, 41, 1861–1862. [Google Scholar] [CrossRef]
- Peretto, G.; Villatore, A.; Rizzo, S.; Esposito, A.; De Luca, G.; Palmisano, A.; Vignale, D.; Cappelletti, A.; Tresoldi, M.; Campochiaro, C.; et al. The Spectrum of COVID-19-Associated Myocarditis: A Patient-Tailored Multidisciplinary Approach. J. Clin. Med. 2021, 10, 1974. [Google Scholar] [CrossRef]
- Kaufmann, C.C.; Villatore, A.; Heugl, M.; Kvakan, H.; Zweiker, D.; Sala, S.; Mazzone, P.; Huber, K.; Peretto, G. Cardiac inflammation associated with COVID-19 mRNA vaccination in patients with and without previous myocarditis. Minerva Cardioangiol. 2023, 71. [Google Scholar] [CrossRef]
- Moore, L.; Ghannam, M.; Manousakis, G. A first presentation of multiple sclerosis with concurrent COVID-19 infection. Eneurologicalsci 2021, 22, 100299. [Google Scholar] [CrossRef] [PubMed]
- Palao, M.; Fernández-Díaz, E.; Gracia-Gil, J.; Romero-Sánchez, C.; Díaz-Maroto, I.; Segura, T. Multiple sclerosis following SARS-CoV-2 infection. Mult. Scler. Relat. Disord. 2020, 45, 102377. [Google Scholar] [CrossRef] [PubMed]
- Yavari, F.; Raji, S.; Moradi, F.; Saeidi, M. Demyelinating Changes Alike to Multiple Sclerosis: A Case Report of Rare Manifestations of COVID-19. Case Rep. Neurol. Med. 2020, 2020, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Bai, W.Z.; Hashikawa, T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020, 92, 552–555. [Google Scholar] [CrossRef]
- Bellucci, G.; Rinaldi, V.; Buscarinu, M.C.; Reniè, R.; Bigi, R.; Pellicciari, G.; Morena, E.; Romano, C.; Marrone, A.; Mechelli, R.; et al. Multiple Sclerosis and SARS-CoV-2: Has the Interplay Started? Front. Immunol. 2021, 12, 755333. [Google Scholar] [CrossRef]
- Toljan, K.; Amin, M.; Kunchok, A.; Ontaneda, D. New diagnosis of multiple sclerosis in the setting of mRNA COVID-19 vaccine exposure. J. Neuroimmunol. 2022, 362, 577785. [Google Scholar] [CrossRef]
- Mincu, R.I.; Magda, S.L.; Mihaila, S.; Florescu, M.; Mihalcea, D.J.; Velcea, A.; Chiru, A.; Tiu, C.; Popescu, B.O.; Cinteza, M.; et al. Impaired Cardiac Function in Patients with Multiple Sclerosis by Comparison with Normal Subjects. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Akgul, F.; Mclek, I.; Duman, T.; Seyfeli, E.; Seydaliyeva, T.; Yalcin, F. Subclinical left ventricular dysfunction in multiple sclerosis. Acta Neurol. Scand. 2006, 114, 114–118. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Poh, K.-K.; Mega, J.L.; Shepard, J.-A.O.; Stone, J.R.; Frosch, M.P. Case 36-2007. N. Engl. J. Med. 2007, 357, 2167–2178. [Google Scholar] [CrossRef]
- Ghei, M. Case 36-2007: A woman with rash, fever, and hypotension. N. Engl. J. Med. 2008, 358, 1405–1407. [Google Scholar] [CrossRef] [PubMed]
- Descamps, V.; Joly, P.; Musette, P. Case 36-2007: A woman with rash, fever, and hypotension. N. Engl. J. Med. 2008, 358, 1406. [Google Scholar]
- Fett, J.D. Case 36-2007: A woman with rash, fever, and hypotension. N. Engl. J. Med. 2008, 358, 1405–1406. [Google Scholar]
- Scolding, N.J.; Ali, H.; Sheppard, M.; Simon, A.R. Alemtuzumab and Fatal Myocarditis. Neurol. Clin. Pract. 2021, 11, e46–e47. [Google Scholar] [CrossRef] [PubMed]
- Diarra, A.; Gantois, G.; Lazrek, M.; Verdier, B.; Elsermans, V.; Zephir, H.; Longère, B.; Gkizas, X.; Goeminne, C.; Lemesle, G.; et al. Fatal Enterovirus-related Myocarditis in a Patient with Devic’s Syndrome Treated with Rituximab. Card. Fail. Rev. 2021, 7, e09. [Google Scholar] [CrossRef]
- Cosgrove, J.; Alli, S.; Ramadan, H.; Ford, H.L. Myocarditis and diffuse skeletal muscle oedema: New features of neuromyelitis optica spectrum disorder? A case report. Mult. Scler. J. 2014, 20, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Giovannoni, G.; Hunter, S.F. Sphingosine 1-phosphate Receptor Modulator Therapy for Multiple Sclerosis: Differential Downstream Receptor Signalling and Clinical Profile Effects. Drugs 2021, 81, 207–231. [Google Scholar] [CrossRef]
- Hartung, H.-P.; Gonsette, R.; Konig, N.; Kwiecinski, H.; Guseo, A.; Morrissey, S.P.; Krapf, H.; Zwingers, T. Mitoxantrone in progressive multiple sclerosis: A placebo-controlled, double-blind, randomised, multicentre trial. Lancet 2002, 360, 2018–2025. [Google Scholar] [CrossRef]
- Hamzehloo, A.; Etemadifar, M. Mitoxantrone-induced cardiotoxicity in patients with multiple sclerosis. Arch Iran Med. 2006, 9, 111–114. [Google Scholar]
- Najafian, J.; Nasri, A.; Etemadifar, M.; Salehzadeh, F. Late cardiotoxicity in MS patients treated with mitoxantrone. Int. J. Prev. Med. 2019, 10, 211. [Google Scholar] [CrossRef]
- Vasheghani-Farahani, A.; Sahraian, M.A.; Darabi, L.; Aghsaie, A.; Minagar, A. Incidence of various cardiac arrhythmias and conduction disturbances due to high dose intravenous methylprednisolone in patients with multiple sclerosis. J. Neurol. Sci. 2011, 309, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Camm, J.; Hla, T.; Bakshi, R.; Brinkmann, V. Cardiac and vascular effects of fingolimod: Mechanistic basis and clinical implications. Am. Hear. J. 2014, 168, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, D.R.; Rammohan, K.W.; Hawker, K.; Fox, E. Fingolimod: A review of its mode of action in the context of its efficacy and safety profile in relapsing forms of multiple sclerosis. Expert Rev. Neurother. 2016, 16, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, P.S.; Berger, J.R. Delayed fingolimod-associated asystole. Mult. Scler. J. 2011, 17, 1387–1389. [Google Scholar] [CrossRef]
- Brænne, I.; Zeng, L.; Willenborg, C.; Tragante, V.; Kessler, T.; CARDIoGRAM Consortium; CARDIoGRAMplusC4D Consortium; Willer, C.J.; Laakso, M.; Wallentin, L.; et al. Genomic correlates of glatiramer acetate adverse cardiovascular effects lead to a novel locus mediating coronary risk. PLoS ONE 2017, 12, e0182999. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.E.; Olsson, T.P.; Wolinsky, J.S.; Comi, G.; Kappos, L.; Hu, X.; Xu, X.; Lublin, A.L.; Truffinet, P.; Chavin, J.; et al. Long-term safety and efficacy of teriflunomide in patients with relapsing multiple sclerosis: Results from the TOWER extension study. Mult. Scler. Relat. Disord. 2020, 46, 102438. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, Y.; Xiao, Z.; Xu, L.; Wang, P.; Ma, Q. Protective effect of dimethyl fumarate on oxidative damage and signaling in cardiomyocytes. Mol. Med. Rep. 2020, 22, 2783–2790. [Google Scholar] [CrossRef]
- Killestein, J.; Van Oosten, B. Emerging safety issues in alemtuzumab-treated MS patients. Mult. Scler. J. 2019, 25, 1206–1208. [Google Scholar] [CrossRef]
- Al-Yafeai, Z.; Carvajal-González, A.; Abduljabar, H.; Arvas, M.; Patel, S.; Patel, N. Novel multiple sclerosis agents-associated cardiotoxicity: A real-world pharmacovigilance study. Int. J. Cardiol. 2022, 362, 153–157. [Google Scholar] [CrossRef]
| Treatment | Type | Cardiac Effects (Incidence %) | Preventive Measures | Ref. |
|---|---|---|---|---|
| High dose corticosteroids | Anti-inflammatory and immunosuppressive | Arrhythmia, in particular sinus tachycardia (41.9%). In particular, women, smokers, elevated BMI, and other autonomic dysfunction | From observation and administration of pharmacological treatments based on ECG results up to temporary cardiac pacing. High-risk groups might benefit from cardiac Holter monitoring 12 h after the steroid pulse | [112] |
| Glatiramer acetate | s.c. synthetic polypeptides: shift of immune response | Hypertension, increased risk of coronary artery disease and myocardial infarction (rare), post-injection transient chest pain | Monitoring | [116] |
| Mitoxantrone | Anthracycline drug: ROS production, DNA intercalation | Early and late-left ventricular dysfunction (2%–4%) | Use of minimal effective dose and appropriate screening of cardiac function | [111] |
| IFN family | Recombinant protein | No evidence of cardiac effect | None | |
| Teriflunomide | Oral pyrimidine synthesis inhibitor | Hypertension (5.6%) | Monitoring | [117] |
| Dimethyl fumarate | Oral NRF2 agonist | Improves left ventricular functioning, promotes reparative phenotype on cardiac myofibroblasts and macrophages | None | [118] |
| Fingolimod | Oral S1P inhibitor | Symptomatic bradycardia (1%), delay in atrioventricular conduction (0.2–3%) | Monitoring for 6 h after the first dose. Concomitant use of heart rate-lowering drugs is not recommended | [113] |
| Alemtuzumab | i.v. monoclonal anti-CD52 antibody | Several autoimmune phenomena | Use when other DMT are contraindicated or ineffective | [120] |
| Cladribine | Oral purine analogue | No evidence of cardiac effect | None | |
| Ocrelizumab | i.v. monoclonal anti-CD20 antibody | Cardiotoxic events, coronary artery disease (0.76%), cardiac failure (0.28%), and atrial fibrillation (0.23%) | Obtain baseline electrocardiography and echocardiogram (need for cardiovascular risk stratification) | [120] |
| Ofatumumab | i.v. monoclonal anti-CD20 antibody | Cardiotoxic events, coronary artery disease (1.2%), cardiac failure (0.55%), and atrial fibrillation (1.07%) | Obtain baseline electrocardiography and echocardiogram (need for cardiovascular risk stratification) | [120] |
| Natalizumab | i.v. monoclonal anti-VLA4 antibody | No evidence of cardiac effect | None |
|
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavarella, M.; Villatore, A.; Rocca, M.A.; Peretto, G.; Filippi, M. The Heart–Brain Interplay in Multiple Sclerosis from Pathophysiology to Clinical Practice: A Narrative Review. J. Cardiovasc. Dev. Dis. 2023, 10, 153. https://doi.org/10.3390/jcdd10040153
Zavarella M, Villatore A, Rocca MA, Peretto G, Filippi M. The Heart–Brain Interplay in Multiple Sclerosis from Pathophysiology to Clinical Practice: A Narrative Review. Journal of Cardiovascular Development and Disease. 2023; 10(4):153. https://doi.org/10.3390/jcdd10040153
Chicago/Turabian StyleZavarella, Matteo, Andrea Villatore, Maria Assunta Rocca, Giovanni Peretto, and Massimo Filippi. 2023. "The Heart–Brain Interplay in Multiple Sclerosis from Pathophysiology to Clinical Practice: A Narrative Review" Journal of Cardiovascular Development and Disease 10, no. 4: 153. https://doi.org/10.3390/jcdd10040153
APA StyleZavarella, M., Villatore, A., Rocca, M. A., Peretto, G., & Filippi, M. (2023). The Heart–Brain Interplay in Multiple Sclerosis from Pathophysiology to Clinical Practice: A Narrative Review. Journal of Cardiovascular Development and Disease, 10(4), 153. https://doi.org/10.3390/jcdd10040153










