Trigger and Substrate Mapping and Ablation for Ventricular Fibrillation in the Structurally Normal Heart
Abstract
1. Introduction/Background
2. Definitions and Epidemiology
3. Mechanisms of Ventricular Fibrillation
4. Catheter Ablation of VF
4.1. Rotor Ablation
4.2. J Wave Syndromes
4.3. Brugada Syndrome
4.4. Early-Repolarization Syndrome
4.5. Idiopathic Ventricular Fibrillation/Short-Coupled Ventricular Fibrillation
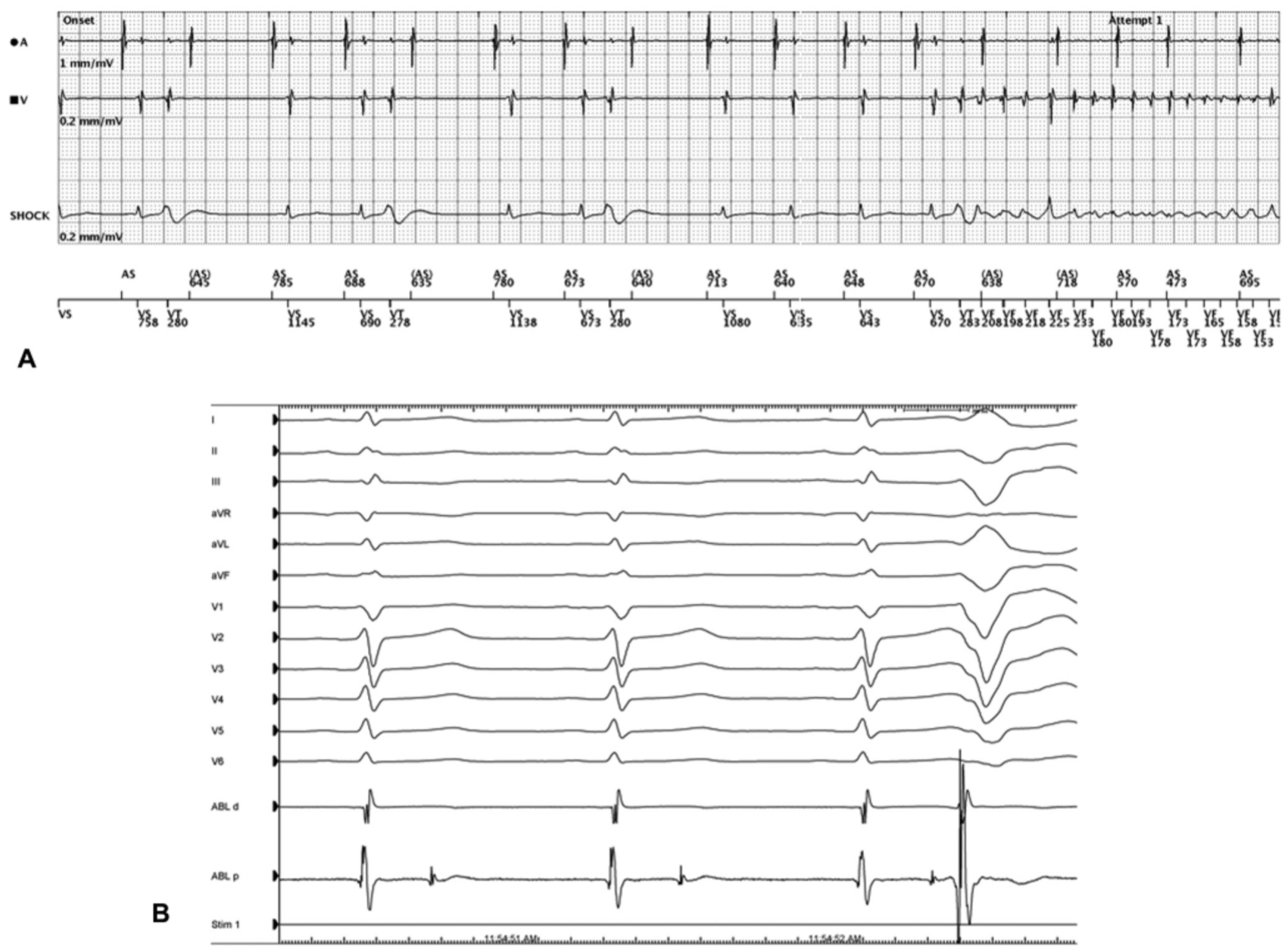
4.6. Purkinje Network Modification
4.7. Limitations of Catheter Ablation
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Chugh, S.S.; Kelly, K.L.; Titus, J.L. Sudden Cardiac Death With Apparently Normal Heart. Circulation 2020, 102, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Semsarian, C.; Ingles, J.; Wilde, A.A. Sudden cardiac death in the young: The molecular autopsy and a practical approach to surviving relatives. Eur. Heart J. 2015, 36, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.R.; Cheung, C.; Gerull, B.; Simpson, C.S.; Birnie, D.H.; Klein, G.J.; Champagne, J.; Healey, J.S.; Gibbs, K.; Talajic, M.; et al. Outcome of Apparently Unexplained Cardiac Arrest: Results From Investigation and Follow-Up of the Prospective Cardiac Arrest Survivors With Preserved Ejection Fraction Registry. Circ. Arrhythm. Electrophysiol. 2016, 9, e003619. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, V.; Bougouin, W.; Karam, N.; Dumas, F.; Sharifzadehgan, A.; Gandjbakhch, E.; Algalarrondo, V.; Narayanan, K.; Zhao, A.; Amet, D.; et al. Characteristics and clinical assessment of unexplained sudden cardiac arrest in the real-world setting: Focus on idiopathic ventricular fibrillation. Eur. Heart J. 2018, 39, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2018, 138, e272–e391. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef] [PubMed]
- Sears, S.F.; Hauf, J.D.; Kirian, K.; Hazelton, G.; Conti, J.B. Posttraumatic stress and the implantable cardioverter-defibrillator patient: What the electrophysiologist needs to know. Circ. Arrhythm. Electrophysiol. 2011, 4, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Kheiri, B.; Barbarawi, M.; Zayed, Y.; Hicks, M.; Osman, M.; Rashdan, L.; Kyi, H.H.; Bachuwa, G.; Hassan, M.; Stecker, E.C.; et al. Antiarrhythmic Drugs or Catheter Ablation in the Management of Ventricular Tachyarrhythmias in Patients With Implantable Cardioverter-Defibrillators: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Circ. Arrhythm. Electrophysiol. 2019, 12, e007600. [Google Scholar] [CrossRef]
- Brugada, P.; Brugada, J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: A distinct clinical and electrocardiographic syndrome. A multicenter report. J. Am. Coll. Cardiol. 1992, 20, 1391–1396. [Google Scholar] [CrossRef]
- Mizusawa, Y.; Wilde, A.A. Brugada syndrome. Circ. Arrhythm. Electrophysiol. 2012, 5, 606–616. [Google Scholar] [CrossRef]
- Conte, G.; Sieira, J.; Ciconte, G.; de Asmundis, C.; Chierchia, G.B.; Baltogiannis, G.; Di Giovanni, G.; La Meir, M.; Wellens, F.; Czapla, J.; et al. Implantable cardioverter-defibrillator therapy in Brugada syndrome: A 20-year single-center experience. J. Am. Coll. Cardiol. 2015, 65, 879–888. [Google Scholar] [CrossRef]
- Priori, S.G.; Wilde, A.A.; Horie, M.; Cho, Y.; Behr, E.R.; Berul, C.; Blom, N.; Brugada, J.; Chiang, C.E.; Huikuri, H.; et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: Document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 2013, 10, 1932–1963. [Google Scholar] [CrossRef]
- Tikkanen, J.T.; Anttonen, O.; Junttila, M.J.; Aro, A.L.; Kerola, T.; Rissanen, H.A.; Reunanen, A.; Huikuri, H.V. Long-term outcome associated with early repolarization on electrocardiography. N. Engl. J. Med. 2009, 361, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Heng, S.J.; Clark, E.N.; Macfarlane, P.W. End QRS notching or slurring in the electrocardiogram: Influence on the definition of “early repolarization”. J. Am. Coll. Cardiol. 2012, 60, 947–948. [Google Scholar] [CrossRef] [PubMed]
- Shipley, R.; Hallaran, W. The four-lead electrocardiogram in two hundred normal men and women. Am. Heart J. 1936, 11, 325–345. [Google Scholar] [CrossRef]
- Bianco, M.; Bria, S.; Gianfelici, A.; Sanna, N.; Palmieri, V.; Zeppilli, P. Does early repolarization in the athlete have analogies with the Brugada syndrome? Eur. Heart J. 2001, 22, 504–510. [Google Scholar] [CrossRef]
- Haisaguerre, M.; Derval, N.; Sacher, F.; Jesel, L.; Deisenhofer, I.; de Roy, L.; Pasquié, J.L.; Nogami, A.; Babuty, D.; Yli-Mayry, S.; et al. Sudden Cardiac Arrest Associated with Early Repolarization. N. Engl. J. Med. 2008, 358, 2016–2023. [Google Scholar] [CrossRef]
- Rosso, R.; Kogan, E.; Belhassen, B.; Rozovski, U.; Scheinman, M.M.; Zeltser, D.; Halkin, A.; Steinvil, A.; Heller, K.; Glikson, M.; et al. J-point elevation in survivors of primary ventricular fibrillation and matched control subjects: Incidence and clinical significance. J. Am. Coll. Cardiol. 2008, 52, 1231–1238. [Google Scholar] [CrossRef]
- Derval, N.; Simpson, C.S.; Birnie, D.H.; Healey, J.S.; Chauhan, V.; Champagne, J.; Gardner, M.; Sanatani, S.; Yee, R.; Skanes, A.C.; et al. Prevalence and characteristics of early repolarization in the CASPER registry: Cardiac arrest survivors with preserved ejection fraction registry. J. Am. Coll. Cardiol. 2011, 58, 722–728. [Google Scholar] [CrossRef]
- Sarkozy, A.; Chierchia, G.B.; Paparella, G.; Boussy, T.; De Asmundis, C.; Roos, M.; Henkens, S.; Kaufman, L.; Buyl, R.; Brugada, R.; et al. Inferior and lateral electrocardiographic repolarization abnormalities in Brugada syndrome. Circ. Arrhythm. Electrophysiol. 2009, 2, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Honarbakhsh, S.; Providencia, R.; Garcia-Hernandez, J.; Martin, C.A.; Hunter, R.J.; Lim, W.Y.; Kirkby, C.; Graham, A.J.; Sharifzadehgan, A.; Waldmann, V.; et al. A Primary Prevention Clinical Risk Score Model for Patients With Brugada Syndrome (BRUGADA-RISK). JACC Clin. Electrophysiol. 2021, 7, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Tulumen, E.; Schulze-Bahr, E.; Zumhagen, S.; Stallmeyer, B.; Seebohm, G.; Beckmann, B.M.; Kaab, S.; Rudic, B.; Liebe, V.; Wolpert, C.; et al. Early repolarization pattern: A marker of increased risk in patients with catecholaminergic polymorphic ventricular tachycardia. Europace 2016, 18, 1587–1592. [Google Scholar] [CrossRef]
- Tikkanen, J.T.; Junttila, M.J.; Anttonen, O.; Aro, A.L.; Luttinen, S.; Kerola, T.; Sager, S.J.; Rissanen, H.A.; Myerburg, R.J.; Reunanen, A.; et al. Early repolarization: Electrocardiographic phenotypes associated with favorable long-term outcome. Circulation 2011, 123, 2666–2673. [Google Scholar] [CrossRef] [PubMed]
- Conte, G.; Caputo, M.L.; Regoli, F.; Marcon, S.; Klersy, C.; Adjibodou, B.; Del Bufalo, A.; Moccetti, T.; Auricchio, A. True idiopathic ventricular fibrillation in out-of-hospital cardiac arrest survivors in the Swiss Canton Ticino: Prevalence, clinical features, and long-term follow-up. Europace 2017, 19, 259–266. [Google Scholar] [CrossRef]
- Conte, G.; Belhassen, B.; Lambiase, P.; Ciconte, G.; de Asmundis, C.; Arbelo, E.; Schaer, B.; Frontera, A.; Burri, H.; Calo, L.; et al. Out-of-hospital cardiac arrest due to idiopathic ventricular fibrillation in patients with normal electrocardiograms: Results from a multicentre long-term registry. Europace 2019, 21, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Blom, L.J.; Visser, M.; Christiaans, I.; Scholten, M.F.; Bootsma, M.; van den Berg, M.P.; Yap, S.C.; van der Heijden, J.F.; Doevendans, P.A.; Loh, P.; et al. Incidence and predictors of implantable cardioverter-defibrillator therapy and its complications in idiopathic ventricular fibrillation patients. Europace 2019, 21, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, C.; Davies, B.; Mellor, G.; Tadros, R.; Laksman, Z.W.; Roberts, J.D.; Green, M.; Alqarawi, W.; Angaran, P.; Healey, J.; et al. Short-coupled ventricular fibrillation represents a distinct phenotype among latent causes of unexplained cardiac arrest: A report from the CASPER registry. Eur. Heart J. 2021, 42, 2827–2838. [Google Scholar] [CrossRef]
- Leenhardt, A.; Glaser, E.; Burgurera, M.; Nurnberg, M.; Maison-Blanche, P.; Coumel, P. Short-Coupled Variant of Torsade de Pointes: A New Electrocardiographic Entity in the Spectrum of Idiopathic Ventricular Tachyarrhythmias. Circulation 1994, 89, 206–215. [Google Scholar] [CrossRef]
- Belhassen, B.; Viskin, S. Idiopathic ventricular tachycardia and fibrillation. J. Cardiovasc. Electrophysiol. 1993, 4, 356–368. [Google Scholar] [CrossRef]
- Belhassen, B.; Glick, A.; Viskin, S. Excellent long-term reproducibility of the electrophysiologic efficacy of quinidine in patients with idiopathic ventricular fibrillation and Brugada syndrome. Pacing Clin. Electrophysiol. 2009, 32, 294–301. [Google Scholar] [CrossRef]
- Haissaguerre, M.; Cheniti, G.; Escande, W.; Zhao, A.; Hocini, M.; Bernus, O. Idiopathic ventricular fibrillation with repetitive activity inducible within the distal Purkinje system. Heart Rhythm 2019, 16, 1268–1272. [Google Scholar] [CrossRef] [PubMed]
- Haissaguerre, M.; Hocini, M.; Cheniti, G.; Duchateau, J.; Sacher, F.; Puyo, S.; Cochet, H.; Takigawa, M.; Denis, A.; Martin, R.; et al. Localized Structural Alterations Underlying a Subset of Unexplained Sudden Cardiac Death. Circ. Arrhythm. Electrophysiol. 2018, 11, e006120. [Google Scholar] [CrossRef]
- Mellor, G.; Steinberg, C.; Laksman, Z.; Deyell, M.W. Short-Coupled Ventricular Fibrillation. J. Cardiovasc. Electrophysiol. 2016, 27, 1236–1237. [Google Scholar] [CrossRef]
- Anderson, R.D.; Kumar, S.; Kalman, J.M.; Sanders, P.; Sacher, F.; Hocini, M.; Jais, P.; Haisaguerre, M.; Lee, G. Catheter Ablation of Ventricular Fibrillation. Heart Lung Circ. 2019, 28, 110–122. [Google Scholar] [CrossRef]
- Haissaguerre, M.; Duchateau, J.; Dubois, R.; Hocini, M.; Cheniti, G.; Sacher, F.; Lavergne, T.; Probst, V.; Surget, E.; Vigmond, E.; et al. Idiopathic Ventricular Fibrillation: Role of Purkinje System and Microstructural Myocardial Abnormalities. JACC Clin. Electrophysiol. 2020, 6, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Nash, M.P.; Mourad, A.; Clayton, R.H.; Sutton, P.M.; Bradley, C.P.; Hayward, M.; Paterson, D.J.; Taggart, P. Evidence for multiple mechanisms in human ventricular fibrillation. Circulation 2006, 114, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Moe, G.K.; Abildskov, J.A. Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am. Heart J. 1959, 58, 59–70. [Google Scholar] [CrossRef]
- Garfinkel, A.; Kim, Y.; Voroshilovsky, O.; Qu, Z.; Kil, J.R.; Lee, M.; Karagueuzian, H.S.; Weiss, J.N.; Chen, P. Preventing ventricular fibrillation by flattening cardiac restitution. Proc. Natl. Acad. Sci. USA 2000, 97, 6061–6066. [Google Scholar] [CrossRef]
- Jalife, J. Ventricular Fibrillation: Mechanisms of initiation and maintenance. Annu. Rev. Physiol. 2000, 62, 25–50. [Google Scholar] [CrossRef] [PubMed]
- Haissaguerre, M.; Shoda, M.; Jais, P.; Nogami, A.; Shah, D.C.; Kautzner, J.; Arentz, T.; Kalushe, D.; Lamaison, D.; Griffith, M.; et al. Mapping and ablation of idiopathic ventricular fibrillation. Circulation 2002, 106, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Nogami, A.; Sugiyasu, A.; Kubota, S.; Kato, K. Mapping and ablation of idiopathic ventricular fibrillation from the Purkinje system. Heart Rhythm 2005, 2, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Nademanee, K.; Veerakul, G.; Chandanamattha, P.; Chaothawee, L.; Ariyachaipanich, A.; Jirasirirojanakorn, K.; Likittanasombat, K.; Bhuripanyo, K.; Ngarmukos, T. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation 2011, 123, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Nademanee, K.; Haissaguerre, M.; Hocini, M.; Nogami, A.; Cheniti, G.; Duchateau, J.; Behr, E.R.; Saba, M.; Bokan, R.; Lou, Q.; et al. Mapping and Ablation of Ventricular Fibrillation Associated With Early Repolarization Syndrome. Circulation 2019, 140, 1477–1490. [Google Scholar] [CrossRef] [PubMed]
- Nogami, A. Mapping and ablating ventricular premature contractions that trigger ventricular fibrillation: Trigger elimination and substrate modification. J. Cardiovasc. Electrophysiol. 2015, 26, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Haissaguerre, M.; Extramiana, F.; Hocini, M.; Cauchemez, B.; Jais, P.; Cabrera, J.A.; Farre, J.; Leenhardt, A.; Sanders, P.; Scavee, C.; et al. Mapping and ablation of ventricular fibrillation associated with long-QT and Brugada syndromes. Circulation 2003, 108, 925–928. [Google Scholar] [CrossRef]
- Pereira, H.; Niederer, S.; Rinaldi, C.A. Electrocardiographic imaging for cardiac arrhythmias and resynchronization therapy. Europace 2020, 22, 1447–1462. [Google Scholar] [CrossRef]
- Krummen, D.E.; Hayase, J.; Morris, D.J.; Ho, J.; Smetak, M.R.; Clopton, P.; Rappel, W.J.; Narayan, S.M. Rotor stability separates sustained ventricular fibrillation from self-terminating episodes in humans. J. Am. Coll. Cardiol. 2014, 63, 2712–2721. [Google Scholar] [CrossRef]
- Mahida, S.; Derval, N.; Sacher, F.; Leenhardt, A.; Deisenhofer, I.; Babuty, D.; Schlapfer, J.; de Roy, L.; Frank, R.; Yli-Mayry, S.; et al. Role of electrophysiological studies in predicting risk of ventricular arrhythmia in early repolarization syndrome. J. Am. Coll. Cardiol. 2015, 65, 151–159. [Google Scholar] [CrossRef]
- Krummen, D.E.; Hayase, J.; Vampola, S.P.; Ho, G.; Schricker, A.A.; Lalani, G.G.; Baykaner, T.; Coe, T.M.; Clopton, P.; Rappel, W.J.; et al. Modifying Ventricular Fibrillation by Targeted Rotor Substrate Ablation: Proof-of-Concept from Experimental Studies to Clinical VF. J. Cardiovasc. Electrophysiol. 2015, 26, 1117–1126. [Google Scholar] [CrossRef]
- Bray, M.A.; Wikswo, J.P. Considerations in phase plane analysis for nonstationary reentrant cardiac behavior. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2002, 65, 051902. [Google Scholar] [CrossRef]
- Narayan, S.M.; Krummen, D.E.; Rappel, W.J. Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation. J. Cardiovasc. Electrophysiol. 2012, 23, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Haissaguerre, M.; Nademanee, K.; Hocini, M.; Cheniti, G.; Duchateau, J.; Frontera, A.; Sacher, F.; Derval, N.; Denis, A.; Pambrun, T.; et al. Depolarization versus repolarization abnormality underlying inferolateral J-wave syndromes: New concepts in sudden cardiac death with apparently normal hearts. Heart Rhythm 2019, 16, 781–790. [Google Scholar] [CrossRef]
- Antzelevitch, C.; Di Diego, J.M. J wave syndromes: What’s new? Trends Cardiovasc. Med. 2022, 32, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Nagase, S.; Morita, H.; Ito, H. Left ventricular epicardial electrogram recordings in idiopathic ventricular fibrillation with inferior and lateral early repolarization. Heart Rhythm 2014, 11, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Krittayaphong, R.; Veerakulb, G.; Nademaneec, K.; Kangkagated, C. Heart rate variability in patients with Brugada syndrome in Thailand. Eur. Heart J. 2003, 24, 1771–1778. [Google Scholar] [CrossRef]
- Postema, P.G.; van Dessel, P.F.; de Bakker, J.M.; Dekker, L.R.; Linnenbank, A.C.; Hoogendijk, M.G.; Coronel, R.; Tijssen, J.G.; Wilde, A.A.; Tan, H.L. Slow and discontinuous conduction conspire in Brugada syndrome: A right ventricular mapping and stimulation study. Circ. Arrhythm. Electrophysiol. 2008, 1, 379–386. [Google Scholar] [CrossRef]
- Gepstein, L.; Hayam, G.; Ben-Haim, S. A novel method for nonfluoroscopic catheter-based electroanatomical mapping of the heart. In vitro and in vivo accuracy results. Circulation 1997, 95, 1611–1622. [Google Scholar] [CrossRef]
- Nademanee, K.; Raju, H.; de Noronha, S.V.; Papadakis, M.; Robinson, L.; Rothery, S.; Makita, N.; Kowase, S.; Boonmee, N.; Vitayakritsirikul, V.; et al. Fibrosis, Connexin-43, and Conduction Abnormalities in the Brugada Syndrome. J. Am. Coll. Cardiol. 2015, 66, 1976–1986. [Google Scholar] [CrossRef]
- Chung, F.P.; Raharjo, S.B.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Tuan, T.C.; Chao, T.F.; Liao, J.N.; Lin, C.Y.; et al. A novel method to enhance phenotype, epicardial functional substrates, and ventricular tachyarrhythmias in Brugada syndrome. Heart Rhythm 2017, 14, 508–517. [Google Scholar] [CrossRef]
- Talib, A.K.; Takagi, M.; Shimane, A.; Nakano, M.; Hayashi, T.; Okajima, K.; Kentaro, M.; Fukada, K.; Kowase, S.; Kurosaki, K.; et al. Efficacy of Endocardial Ablation of Drug-Resistant Ventricular Fibrillation in Brugada Syndrome: Long-Term Outcome. Circ. Arrhythm. Electrophysiol. 2018, 11, e005631. [Google Scholar] [CrossRef] [PubMed]
- Nademanee, K.; Hocini, M.; Haissaguerre, M. Epicardial substrate ablation for Brugada syndrome. Heart Rhythm 2017, 14, 457–461. [Google Scholar] [CrossRef]
- Brugada, J.; Pappone, C.; Berruezo, A.; Vicedomini, G.; Manguso, F.; Ciconte, G.; Giannelli, L.; Santinelli, V. Brugada Syndrome Phenotype Elimination by Epicardial Substrate Ablation. Circ. Arrhythm. Electrophysiol. 2015, 8, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Pappone, C.; Ciconte, G.; Manguso, F.; Vicedomini, G.; Mecarocci, V.; Conti, M.; Giannelli, L.; Pozzi, P.; Borrelli, V.; Menicanti, L.; et al. Assessing the Malignant Ventricular Arrhythmic Substrate in Patients With Brugada Syndrome. J. Am. Coll. Cardiol. 2018, 71, 1631–1646. [Google Scholar] [CrossRef]
- Kamakura, T.; Cochet, H.; Juhoor, M.; Nakatani, Y.; Ramirez, F.D.; Andre, C.; Nakashima, T.; Krisai, P.; Takagi, T.; Tixier, R.; et al. Role of endocardial ablation in eliminating an epicardial arrhythmogenic substrate in patients with Brugada syndrome. Heart Rhythm 2021, 18, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Kotake, Y.; Barua, S.; Kazi, S.; Virk, S.; Bhaskaran, A.; Campbell, T.; Bennett, R.G.; Kumar, S. Efficacy and safety of catheter ablation for Brugada syndrome: An updated systematic review. Clin. Res. Cardiol. 2022. [Google Scholar] [CrossRef]
- Boukens, B.J.; Potse, M.; Coronel, R. Fibrosis and Conduction Abnormalities as Basis for Overlap of Brugada Syndrome and Early Repolarization Syndrome. Int. J. Mol. Sci. 2021, 22, 1570. [Google Scholar] [CrossRef]
- Nakagawa, H.; Beckman, K.J.; McClelland, J.H.; Wang, X.; Arruda, M.; Santoro, C.; Hazlitt, A.; Abdalla, I.; Singh, A.; Gossinger, H.; et al. Radiofrequency Catheter Ablation of Idiopathic Left Ventricular Tachycardia Guided by a Purkinje Potential. Circulation 1993, 88, 2607–2617. [Google Scholar] [CrossRef] [PubMed]
- Knecht, S.; Sacher, F.; Wright, M.; Hocini, M.; Nogami, A.; Arentz, T.; Petit, B.; Franck, R.; De Chillou, C.; Lamaison, D.; et al. Long-term follow-up of idiopathic ventricular fibrillation ablation: A multicenter study. J. Am. Coll. Cardiol. 2009, 54, 522–528. [Google Scholar] [CrossRef]
- Morady, F.; Kadish, A.H.; DiCarlo, L.; Kou, W.H.; Winston, S.; deBuitlier, M.; Calkins, H.; Rosenheck, S.; Sousa, J. Long-term results of catheter ablation of idiopathic right ventricular tachycardia. Circulation 1990, 82, 2093–2099. [Google Scholar] [CrossRef] [PubMed]
- Buxton, A.E.; Waxman, H.L.; Marchlinski, F.E.; Simson, M.B.; Cassidy, D.; Josephson, M.E. Right ventricular tachycardia: Clinical and electrophysiologic characteristics. Circulation 1983, 68, 917–927. [Google Scholar] [CrossRef]
- Noda, T.; Shimizu, W.; Taguchi, A.; Aiba, T.; Satomi, K.; Suyama, K.; Kurita, T.; Aihara, N.; Kamakura, S. Malignant entity of idiopathic ventricular fibrillation and polymorphic ventricular tachycardia initiated by premature extrasystoles originating from the right ventricular outflow tract. J. Am. Coll. Cardiol. 2005, 46, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Van Herendael, H.; Zado, E.S.; Haqqani, H.; Tschabrunn, C.M.; Callans, D.J.; Frankel, D.S.; Lin, D.; Garcia, F.; Hutchinson, M.D.; Riley, M.; et al. Catheter ablation of ventricular fibrillation: Importance of left ventricular outflow tract and papillary muscle triggers. Heart Rhythm 2014, 11, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.; Seo, J.; Kim, H.; Kim, M.; Choi, J.Y.; Kang, D.; Oh, S. Visualization of left ventricular Purkinje fiber distribution using widefield optical coherence microscopy. Int. J. Clin. Exp. Pathol. 2020, 13, 3013–3020. [Google Scholar]
- Berenfeld, O.; Jalife, J. Purkinje-muscle reentry as a mechanism of polymorphic ventricular arrhythmias in a 3-dimensional model of the ventricles. Circ. Res. 1998, 82, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Haissaguerre, M.; Cheniti, G.; Hocini, M.; Sacher, F.; Ramirez, F.D.; Cochet, H.; Bear, L.; Tixier, R.; Duchateau, J.; Walton, R.; et al. Purkinje network and myocardial substrate at the onset of human ventricular fibrillation: Implications for catheter ablation. Eur. Heart J. 2022, 43, 1234–1247. [Google Scholar] [CrossRef]
- Deyell, M.W.; Krahn, A.D.; Goldberger, J.J. Sudden cardiac death risk stratification. Circ. Res. 2015, 116, 1907–1918. [Google Scholar] [CrossRef]
- Sapp, J.L.; Bar-Tal, M.; Howes, A.J.; Toma, J.E.; El-Damaty, A.; Warren, J.W.; MacInnis, P.J.; Zhou, S.; Horacek, B.M. Real-Time Localization of Ventricular Tachycardia Origin From the 12-Lead Electrocardiogram. JACC Clin. Electrophysiol. 2017, 3, 687–699. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christie, S.; Idris, S.; Bennett, R.G.; Deyell, M.W.; Roston, T.; Laksman, Z. Trigger and Substrate Mapping and Ablation for Ventricular Fibrillation in the Structurally Normal Heart. J. Cardiovasc. Dev. Dis. 2023, 10, 200. https://doi.org/10.3390/jcdd10050200
Christie S, Idris S, Bennett RG, Deyell MW, Roston T, Laksman Z. Trigger and Substrate Mapping and Ablation for Ventricular Fibrillation in the Structurally Normal Heart. Journal of Cardiovascular Development and Disease. 2023; 10(5):200. https://doi.org/10.3390/jcdd10050200
Chicago/Turabian StyleChristie, Simon, Sami Idris, Richard G. Bennett, Marc W. Deyell, Thomas Roston, and Zachary Laksman. 2023. "Trigger and Substrate Mapping and Ablation for Ventricular Fibrillation in the Structurally Normal Heart" Journal of Cardiovascular Development and Disease 10, no. 5: 200. https://doi.org/10.3390/jcdd10050200
APA StyleChristie, S., Idris, S., Bennett, R. G., Deyell, M. W., Roston, T., & Laksman, Z. (2023). Trigger and Substrate Mapping and Ablation for Ventricular Fibrillation in the Structurally Normal Heart. Journal of Cardiovascular Development and Disease, 10(5), 200. https://doi.org/10.3390/jcdd10050200





