Cardiomyocyte-Specific Wt1 Is Involved in Cardiac Metabolism and Response to Damage
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Models
2.2. Neonatal Cardiomyocyte Isolation and Culture
2.3. Wt1 Silencing via Lentiviral shRNA Particles in Cultured Cardiomyocytes
2.4. RNA Isolation, Reverse Transcription, and qRT-PCR of Neonatal Cardiomyocytes
2.5. Protein Extraction, Quantification, and Western Blot
2.6. Flow Cytometry
2.7. Proliferation Assay in Neonatal Cardiomyocytes
2.8. Cell Viability Assay in Cultured Neonatal Cardiomyocytes
2.9. Laminin Immunostaining and Confocal Microscopy
2.10. Doxorubicin Treatment
2.11. Electrocardiography (ECG)
2.12. Proteomics
2.13. Fibrosis and Image Analysis
2.14. Statistics
3. Results
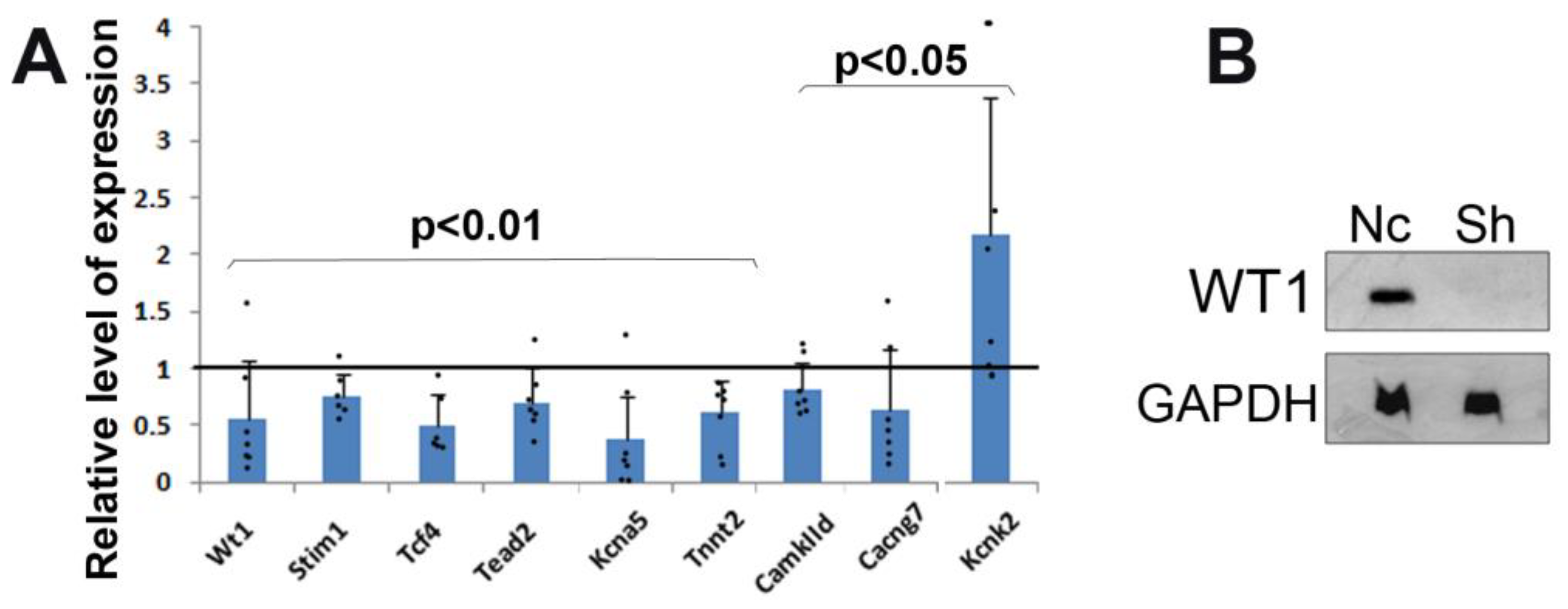
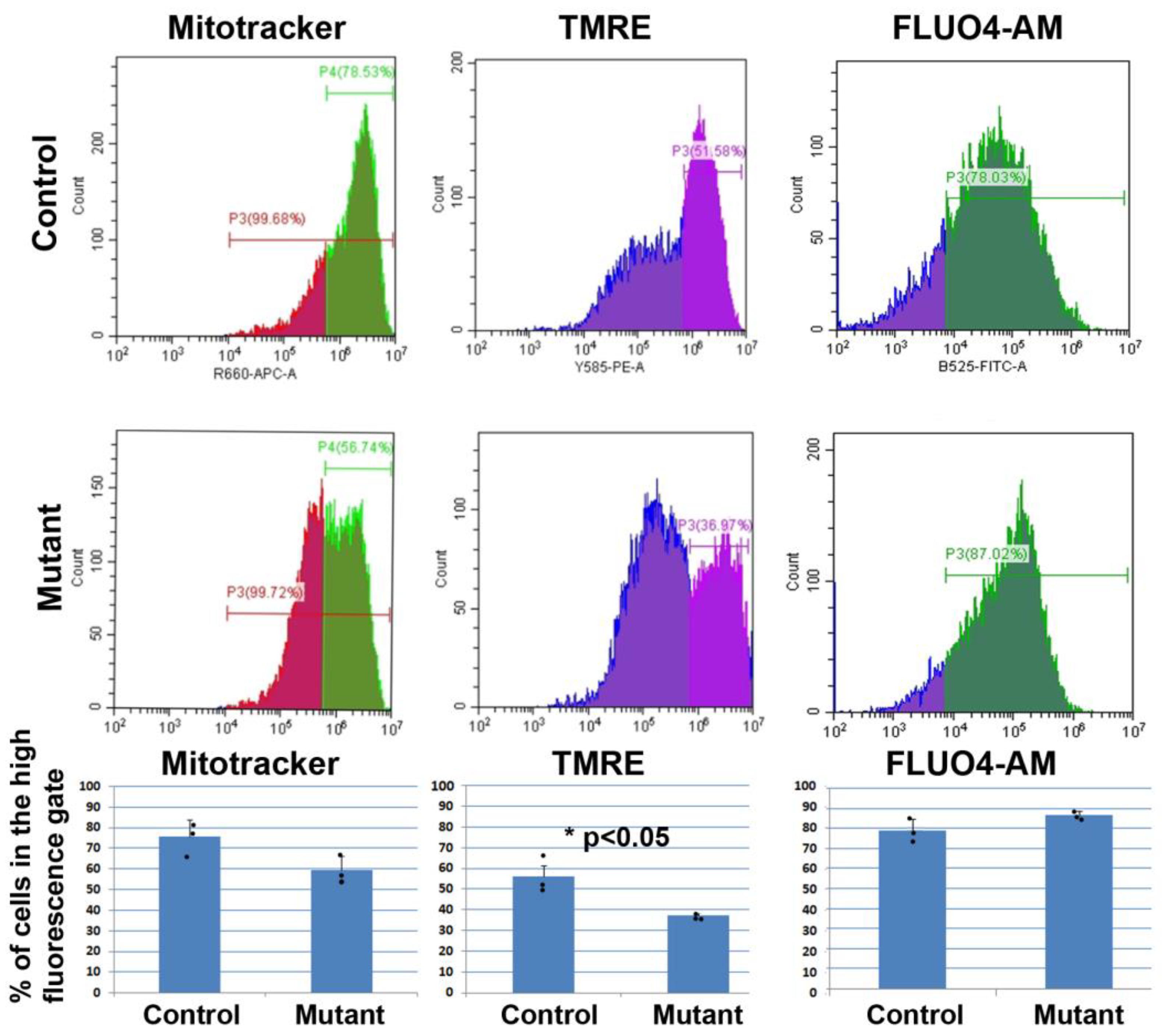
3.1. Silencing of Wt1 in Cultured Neonatal Cardiomyocytes Induces Changes in Mitochondrial Membrane Potential
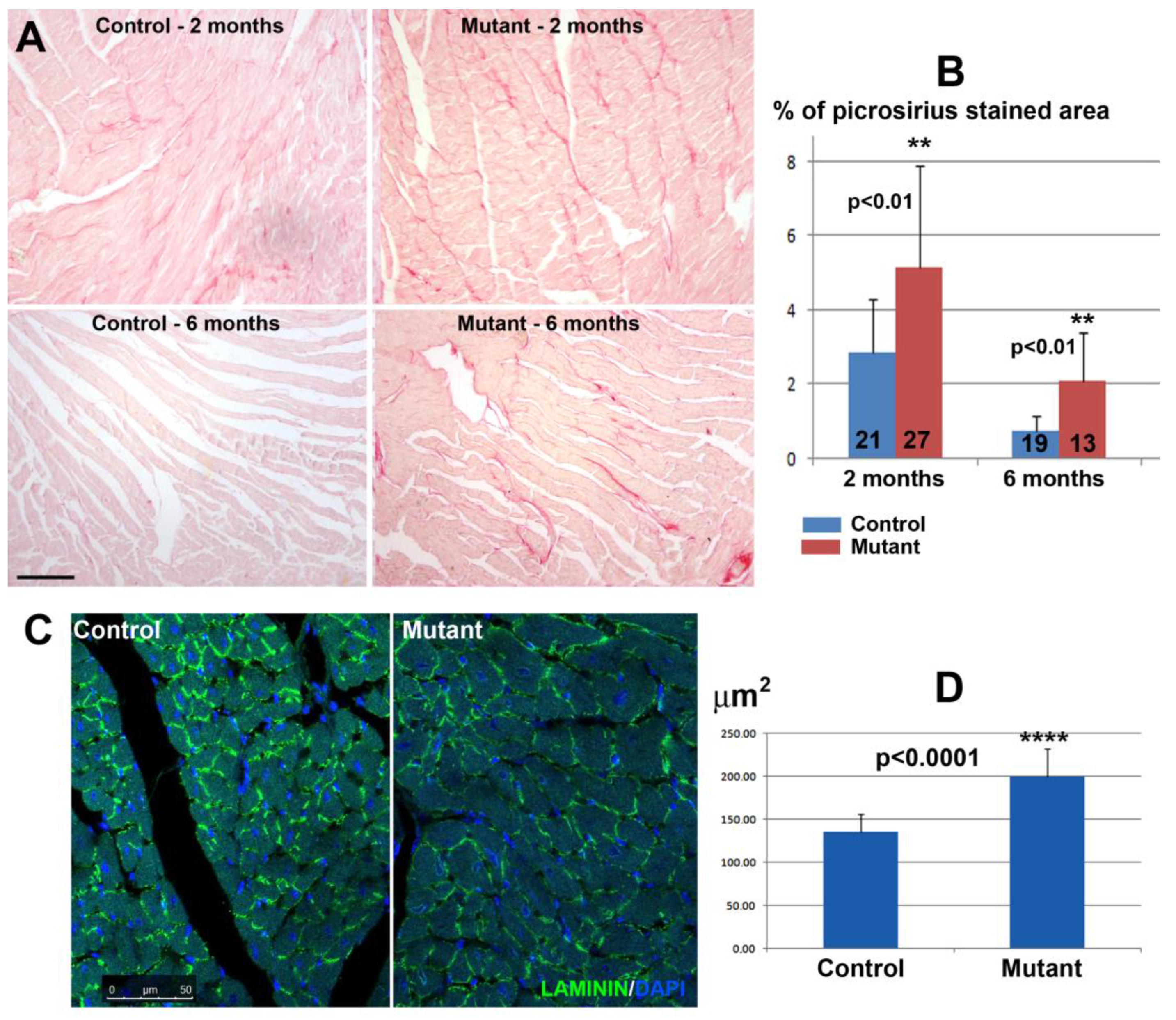
3.2. Conditional Deletion of WT1 in Adult Cardiomyocytes (αMHCmerCremer;WT1loxP/loxP) Induces Fibrosis and Hypertrophy
3.3. Conditional Deletion of WT1 in Adult Cardiomyocytes Induces Mitochondrial Dysfunction
3.4. Conditional Deletion of WT1 in Adult Cardiomyocytes Did Not Substantially Alter Electrocardiogram Parameters
3.5. Proteomic Analysis Shows Alterations in Cardiomyocyte Metabolism after Conditional Deletion of Wt1
3.6. Conditional Deletion of WT1 in Adult Cardiomyocytes Increases the Damage Induced by Doxorubicin Treatment
3.7. Proteomic Analysis of the Hearts from Doxorubicin-Treated Mice Shows Metabolic Alterations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hastie, N.D. Wilms’ tumour 1 (WT1) in development, homeostasis and disease. Development 2017, 144, 2862–2872. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.; Wagner, K.D.; Theres, H.; Englert, C.; Schedl, A.; Scholz, H. Coronary vessel development requires activation of the TrkB neurotrophin receptor by the Wilms’ tumor transcription factor Wt1. Genes Dev. 2005, 19, 2631–2642. [Google Scholar] [CrossRef]
- Martínez-Estrada, O.M.; Lettice, L.A.; Essafi, A.; Guadix, J.A.; Slight, J.; Velecela, V.; Hall, E.; Reichmann, J.; Devenney, P.S.; Hohenstein, P.; et al. Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nat. Genet. 2010, 42, 89–93. [Google Scholar] [CrossRef]
- von Gise, A.; Zhou, B.; Honor, L.B.; Ma, Q.; Petryk, A.; Pu, W.T. WT1 regulates epicardial epithelial to mesenchymal transition through β-catenin and retinoic acid signaling pathways. Dev. Biol. 2011, 356, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Díaz Del Moral, S.; Barrena, S.; Hernández-Torres, F.; Aránega, A.; Villaescusa, J.M.; Gómez Doblas, J.J.; Franco, D.; Jiménez-Navarro, M.; Muñoz-Chápuli, R.; Carmona, R. Deletion of the Wilms’ Tumor Suppressor Gene in the Cardiac Troponin-T Lineage Reveals Novel Functions of WT1 in Heart Development. Front. Cell Dev. Biol. 2021, 9, 683861. [Google Scholar] [CrossRef]
- Wagner, N.; Ninkov, M.; Vukolic, A.; Cubukcuoglu Deniz, G.; Rassoulzadegan, M.; Michiels, J.F.; Wagner, K.D. Implications of the Wilms’ Tumor Suppressor Wt1 in Cardiomyocyte Differentiation. Int. J. Mol. Sci. 2021, 22, 4346. [Google Scholar] [CrossRef]
- Chau, Y.Y.; Brownstein, D.; Mjoseng, H.; Lee, W.C.; Buza-Vidas, N.; Nerlov, C.; Jacobsen, S.E.; Perry, P.; Berry, R.; Thornburn, A.; et al. Acute multiple organ failure in adult mice deleted for the developmental regulator Wt1. PLoS Genet. 2011, 12, e1002404. [Google Scholar] [CrossRef]
- Ariza, L.; Rojas, A.; Muñoz-Chápuli, R.; Carmona, R. The Wilms’ tumor suppressor gene regulates pancreas homeostasis and repair. PLoS Genet. 2019, 15, e1007971. [Google Scholar] [CrossRef] [PubMed]
- Kendall, T.J.; Duff, C.M.; Boulter, L.; Wilson, D.H.; Freyer, E.; Aitken, S.; Forbes, S.J.; Iredale, J.P.; Hastie, N.D. Embryonic mesothelial-derived hepatic lineage of quiescent and heterogenous scar-orchestrating cells defined but suppressed by WT1. Nat. Commun. 2019, 10, 4688. [Google Scholar] [CrossRef]
- Wagner, K.D.; Wagner, N.; Bondke, A.; Nafz, B.; Flemming, B.; Theres, H.; Scholz, H. The Wilms’ tumor suppressor Wt1 is expressed in the coronary vasculature after myocardial infarction. FASEB J. 2002, 9, 1117–1119. [Google Scholar] [CrossRef]
- Duim, S.N.; Kurakula, K.; Goumans, M.J.; Kruithof, B.P. Cardiac endothelial cells express Wilms’ tumor-1: Wt1 expression in the developing, adult and infarcted heart. J. Mol. Cell Cardiol. 2015, 81, 127–135. [Google Scholar] [CrossRef]
- Duim, S.N.; Smits, A.M.; Kruithof, B.P.; Goumans, M.J. The roadmap of WT1 protein expression in the human fetal heart. J. Mol. Cell Cardiol. 2016, 90, 139–145. [Google Scholar] [CrossRef]
- . Sohal, D.S.; Nghiem, M.; Crackower, M.A.; Witt, S.A.; Kimball, T.R.; Tymitz, K.M.; Penninger, J.M.; Molkentin, J.D. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ. Res. 2001, 89, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Kiermayer, C.; Conrad, M.; Schneider, M.; Schmidt, J.; Brielmeier, M. Optimization of spatiotemporal gene inactivation in mouse heart by oral application of tamoxifen citrate. Genesis 2007, 45, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.; Jian Motamedi, F.; Weerasinghe Arachchige, L.C.; Tison, A.; Bradford, S.T.; Lefebvre, J.; Dolle, P.; Ghyselinck, N.B.; Wagner, K.D.; Schedl, A. Retinoic acid signaling is directly activated in cardiomyocytes and protects mouse hearts from apoptosis after myocardial infarction. Elife 2021, 10, e68280. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.-D.; El Maï, M.; Ladomery, M.; Belali, T.; Leccia, N.; Michiels, J.-F.; Wagner, N. Altered VEGF Splicing Isoform Balance in Tumor Endothelium Involves Activation of Splicing Factors Srpk1 and Srsf1 by the Wilms’ Tumor Suppressor Wt1. Cells 2019, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Hattori, F.; Chen, H.; Yamashita, H.; Tohyama, S.; Satoh, Y.S.; Yuasa, S.; Li, W.; Yamakawa, H.; Tanaka, T.; Onitsuka, T.; et al. Nongenetic method for purifying stem cell-derived cardiomyocytes. Nat. Methods 2010, 7, 61–66. [Google Scholar] [CrossRef]
- Creed, S.; McKenzie, M. Measurement of Mitochondrial Membrane Potential with the Fluorescent Dye Tetramethylrhodamine Methyl Ester (TMRM). Methods Mol. Biol. 2019, 1928, 69–76. [Google Scholar] [CrossRef]
- Li, D.L.; Wang, Z.V.; Ding, G.; Tan, W.; Luo, X.; Criollo, A.; Xie, M.; Jiang, N.; May, H.; Kyrychenko, V.; et al. Doxorubicin Blocks Cardiomyocyte Autophagic Flux by Inhibiting Lysosome Acidification. Circulation 2016, 133, 1668–1687. [Google Scholar] [CrossRef]
- Merten, K.E.; Feng, W.; Zhang, L.; Pierce, W.; Cai, J.; Klein, J.B.; Kang, Y.J. Modulation of cytochrome C oxidase-va is possibly involved in metallothionein protection from doxorubicin cardiotoxicity. J. Pharmacol. Exp. Ther. 2005, 315, 1314–1319. [Google Scholar] [CrossRef]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius red staining: A useful tool to appraise collagen networks in normal and pathological tissues. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Parra, V.; Eisner, V.; Chiong, M.; Criollo, A.; Moraga, F.; Garcia, A.; Härtel, S.; Jaimovich, E.; Zorzano, A.; Hidalgo, C.; et al. Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovasc. Res. 2008, 77, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Santulli, G.; Xie, W.; Reiken, S.R.; Marks, A.R. Mitochondrial calcium overload is a key determinant in heart failure. Proc. Natl. Acad. Sci. USA 2015, 112, 11389–11394. [Google Scholar] [CrossRef]
- Ritchie, M.F.; Yue, C.; Zhou, Y.; Houghton, P.J.; Soboloff, J. Wilms tumor 682 suppressor 1 (WT1) and early growth response 1 (EGR1) are regulators of STIM1 683 expression. J. Biol. Chem. 2010, 285, 10591–10596. [Google Scholar] [CrossRef] [PubMed]
- Maier, L.S.; Bers, D.M. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc. Res. 2007, 73, 631–640. [Google Scholar] [CrossRef]
- Kamatham, S.; Waters, C.M.; Schwingshackl, A.; Mancarella, S. TREK-1 protects the heart against ischemia-reperfusion-induced injury and from adverse remodeling after myocardial infarction. Pflugers. Arch. 2019, 471, 1263–1272. [Google Scholar] [CrossRef]
- Inoue, Y.Y.; Ambale-Venkatesh, B.; Mewton, N.; Volpe, G.J.; Ohyama, Y.; Sharma, R.K.; Wu, C.O.; Liu, C.Y.; Bluemke, D.A.; Soliman, E.Z.; et al. Electrocardiographic Impact of Myocardial Diffuse Fibrosis and Scar: MESA (Multi-Ethnic Study of Atherosclerosis). Radiology 2017, 282, 690–698. [Google Scholar] [CrossRef]
- Lexow, J.; Poggioli, T.; Sarathchandra, P.; Santini, M.P.; Rosenthal, N. Cardiac fibrosis in mice expressing an inducible myocardial-specific Cre driver. Dis. Model. Mech. 2013, 6, 1470–1476. [Google Scholar] [CrossRef]
- Koitabashi, N.; Bedja, D.; Zaiman, A.L.; Pinto, Y.M.; Zhang, M.; Gabrielson, K.L.; Takimoto, E.; Kass, D.A. Avoidance of transient cardiomyopathy in cardiomyocyte-targeted tamoxifen-induced MerCreMer gene deletion models. Circ. Res. 2009, 105, 12–15. [Google Scholar] [CrossRef]
- Rouhi, L.; Fan, S.; Cheedipudi, S.M.; Olcum, M.; Jeong, H.H.; Zhao, Z.; Gurha, P.; Marian, A.J. Effects of tamoxifen inducible MerCreMer on gene expression in cardiac myocytes in mice. J. Cardiovasc. Aging 2022, 2, 8. [Google Scholar] [CrossRef]
- Oakey, L.A.; Fletcher, R.S.; Elhassan, Y.S.; Cartwright, D.M.; Doig, C.L.; Garten, A.; Thakker, A.; Maddocks, O.D.K.; Zhang, T.; Tennant, D.A.; et al. Metabolic tracing reveals novel adaptations to skeletal muscle cell energy production pathways in response to NAD+ depletion. Wellcome Open Res. 2019, 3, 147. [Google Scholar] [CrossRef] [PubMed]
- País, S.M.; Téllez-Iñón, M.T.; Capiati, D.A. Serine/threonine protein phosphatases type 2A and their roles in stress signaling. Plant Signal Behav. 2009, 4, 1013–1015. [Google Scholar] [CrossRef]
- Jakobsson, P.J.; Mancini, J.A.; Riendeau, D.; Ford-Hutchinson, A.W. Identification and characterization of a novel microsomal enzyme with glutathione-dependent transferase and peroxidase activities. J. Biol. Chem. 1997, 272, 22934–22939. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, B. Doxorubicin induces cardiotoxicity through upregulation of death receptors mediated apoptosis in cardiomyocytes. Sci. Rep. 2017, 7, 44735. [Google Scholar] [CrossRef] [PubMed]
- Terentyev, D.; Viatchenko-Karpinski, S.; Györke, I.; Volpe, P.; Williams, S.C.; Györke, S. Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: Mechanism for hereditary arrhythmia. Proc. Natl. Acad. Sci. USA 2003, 100, 11759–11764. [Google Scholar] [CrossRef]
- van Wijk, B.; Gunst, Q.D.; Moorman, A.F.; van den Hoff, M.J. Cardiac regeneration from activated epicardium. PLoS ONE 2012, 7, e44692. [Google Scholar] [CrossRef]
- Wagner, N.; Wagner, K.-D. Every Beat You Take—The Wilms′ Tumor Suppressor WT1 and the Heart. Int. J. Mol. Sci. 2021, 22, 7675. [Google Scholar] [CrossRef]



| 8 | RR Interval (ms) | Heart Rate (BPM) | PR Interval (ms) | P Duration (ms) | QRS Interval (ms) | QT Interval (ms) | QTc (ms) | JT Interval (ms) | Tpeak Tend Interval (ms) | P Amplitude (µV) | Q Amplitude (µV) | R Amplitude (µV) | S Amplitude (µV) | ST Height (µV) | T Amplitude (µV) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 249 ± 47.2 | 252 ± 52.0 | 39.1 ± 5.6 | 15.9 ± 2.4 | 14.6 ± 1.6 | 33.4 ± 3.5 | 67.9 ± 9.5 | 18.7 ± 3.7 | 12.5 ± 3.0 | 34.4 ± 23.6 | 10.9 ± 6.5 | 375 ± 96.5 | −15.6 ± 71.6 | 41.9 ± 54.0 | 114.3 ± 49.0 |
| N = 31 | N = 31 | N = 29 | N = 29 | N = 31 | N = 31 | N = 31 | N = 31 | N = 31 | N = 30 | N = 31 | N = 31 | N = 31 | N = 31 | N = 31 | |
| Mutant | 247.8 ± 46.4 | 250.5 ± 34.2 | 38.1 ± 5.6 | 16.6 ± 2.6 | 13.6 ± 1.3 | 32.2 ± 3.7 | 65.2 ± 8.3 | 18.4 ± 3.4 | 11.9 ± 2.8 | 34.3 ± 31.9 | 9.65 ± 5.3 | 352.5 ± 89.0 | 9.16 ± 43.6 | 57.1 ± 32.0 | 104.6 ± 36.1 |
| N = 25 | N = 25 | N = 25 | N = 25 | N = 25 | N = 25 | N = 25 | N = 25 | N = 25 | N = 25 | N = 25 | N = 25 | N = 25 | N = 25 | N = 25 | |
| Student’s t | 0.923 | 0.896 | 0.522 | 0.304 | 0.014 * | 0.227 | 0.270 | 0.718 | 0.408 | 0.982 | 0.448 | 0.371 | 0.136 | 0.221 | 0.409 |
| ORA-PATHWAY-KEGG | |||
|---|---|---|---|
| Gene set description | Ratio mutant/control | p-value | FDR |
| Valine, leucine, and isoleucine degradation | 7.92 | <0.001 | 0.016 |
| Glutathione metabolism | 6.93 | <0.001 | 0.022 |
| Cardiac muscle contraction | 6.83 | <0.001 | 0.015 |
| PPAR signaling pathway | 6.26 | <0.001 | 0.016 |
| Parkinson disease | 6.16 | <0.00001 | 0.001 |
| Oxidative phosphorylation | 5.96 | <0.0001 | 0.003 |
| Carbon metabolism | 5.92 | <0.001 | 0.006 |
| Ribosome | 5.10 | <0.001 | 0.013 |
| Non-alcoholic fatty liver disease (NAFLD) | 4.70 | <0.001 | 0.015 |
| Metabolic pathways | 1.84 | <0.001 | 0.022 |
| ORA Phenotype | |||
| Gene set description | Ratio mutant/control | p-value | FDR |
| Increased fatty acid level | 8.49 | <0.0001 | 0.028 |
| Abnormal fatty acid level | 6.61 | <0.001 | 0.003 |
| Abnormal circulating free fatty acids level | 6.57 | <0.0001 | 0.035 |
| Abnormal free fatty acids level | 6.26 | <0.0001 | 0.041 |
| Abnormal heartbeat | 5.63 | <0.00001 | 0.007 |
| Abnormal metabolism | 3.72 | <0.001 | 0.003 |
| GSEA analysis pathway according to WikiPathways | |||
| Gene set description | NES mutant/control | p-value | FDR |
| Electron transport chain | −2.68 | <0.0001 | <0.0001 |
| Oxidative phosphorylation | −3.26 | <0.0001 | <0.0001 |
| RR Interval (ms) | Heart Rate (BPM) | PR Interval (ms) | P Duration (ms) | QRS Interval (ms) | QT Interval (ms) | QTc (ms) | JT Interval (ms) | Tpeak Tend Interval (ms) | P Amplitude (µV) | Q Amplitude (µV) | R Amplitude (µV) | S Amplitude (µV) | ST Height (µV) | T Amplitude (µV) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 253.3 ± 19.6 | 238.6 ± 17.9 | 40.5 ± 1.8 | 15.0 ± 1.0 | 13.7 ± 1.3 | 29.0 ± 4.4 | 57.7 ± 9.5 | 14.2 ± 2.6 | 8.7 ± 2.1 | 43.0 ± 12.5 | 12.2 ± 2.8 | 314.9 ± 88.2 | 5.29 ± 82.9 | 48.6 ± 33.3 | 72.6 ± 25.3 | |
| Three months | N = 9 | N = 9 | N = 8 | N = 8 | N = 9 | N = 9 | N = 9 | N = 9 | N = 9 | N = 9 | N = 9 | N = 9 | N = 9 | N = 9 | N = 9 | |
| Mutant | 269.2 ± 35.2 | 232.5 ± 229.0 | 36.1 ± 5.7 | 14.7 ± 2.2 | 13.5 ± 1.2 | 33.2 ± 4.7 | 64.8 ± 6.5 | 19.8 ± 4.3 | 13.6 ± 3.9 | 20.7 ± 36.4 | 12.3 ± 4.0 | 300.6 ± 71.6 | 5.4 ± 53.8 | 74.1 ± 34.5 | 122.7 ± 44.0 | |
| N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | ||
| Student’s t | 0.280 | 0.562 | 0.059 | 0.688 | 0.711 | 0.102 | 0.133 | 0.008 ** | 0.007 ** | 0.110 | 0.968 | 0.747 | 0.786 | 0.174 | 0.015 * | |
| Control | 232.9 ± 45.7 | 267.4 ± 52.9 | 37.8 ± 4.1 | 14.5 ± 2.8 | 13.3 ± 1.1 | 29.7 ± 5.6 | 63.7 ± 15.0 | 13.8 ± 4.5 | 8.67 ± 3.4 | 25.6 ± 3.0 | 12.2 ± 4.3 | 331.0 ± 103.1 | 20.2 ± 88.4 | 55.1 ± 35.0 | 58.2 ± 50.0 | |
| Six months | N = 9 | N = 9 | N = 8 | N = 8 | N = 9 | N = 8 | N = 8 | N = 8 | N = 8 | N = 8 | N = 9 | N = 9 | N = 9 | N = 9 | N = 9 | |
| Mutant | 256.9 ± 4.5 | 240.4 ± 43.7 | 38.8 ± 4.6 | 14.6 ± 0.95 | 14.0 ± 1.7 | 30.9 ± 3.9 | 61.7 ± 10.8 | 16.9 ± 3.0 | 11.0 ± 2.5 | 48.9 ± 34.0 | 10.0 ± 4.4 | 307.9 ± 68.2 | −17.4 ± 63.2 | 55.5 ± 42.8 | 114.5 ± 35.8 | |
| N = 6 | N = 6 | N = 5 | N = 5 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | ||
| Student’s t | 0.883 | 0.179 | 0.790 | 0.787 | 0.852 | 0.854 | 0.8589 | 0.852 | 0.851 | 0.622 | 0.226 | 0.175 | 0.942 | 0.322 | 0.538 |
| ORA-PATHWAY-WIKI | |||
|---|---|---|---|
| Gene set description | Ratio mutant/control | p-value | FDR |
| Fatty acid oxidation | 54.18 | <0.001 | 0.035 |
| Fatty acid beta oxidation | 23.90 | <0.001 | 0.021 |
| Glycolysis and gluconeogenesis | 21.25 | <0.0001 | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz del Moral, S.; Benaouicha, M.; Villa del Campo, C.; Torres, M.; Wagner, N.; Wagner, K.-D.; Muñoz-Chápuli, R.; Carmona, R. Cardiomyocyte-Specific Wt1 Is Involved in Cardiac Metabolism and Response to Damage. J. Cardiovasc. Dev. Dis. 2023, 10, 211. https://doi.org/10.3390/jcdd10050211
Díaz del Moral S, Benaouicha M, Villa del Campo C, Torres M, Wagner N, Wagner K-D, Muñoz-Chápuli R, Carmona R. Cardiomyocyte-Specific Wt1 Is Involved in Cardiac Metabolism and Response to Damage. Journal of Cardiovascular Development and Disease. 2023; 10(5):211. https://doi.org/10.3390/jcdd10050211
Chicago/Turabian StyleDíaz del Moral, Sandra, Maha Benaouicha, Cristina Villa del Campo, Miguel Torres, Nicole Wagner, Kay-Dietrich Wagner, Ramón Muñoz-Chápuli, and Rita Carmona. 2023. "Cardiomyocyte-Specific Wt1 Is Involved in Cardiac Metabolism and Response to Damage" Journal of Cardiovascular Development and Disease 10, no. 5: 211. https://doi.org/10.3390/jcdd10050211
APA StyleDíaz del Moral, S., Benaouicha, M., Villa del Campo, C., Torres, M., Wagner, N., Wagner, K.-D., Muñoz-Chápuli, R., & Carmona, R. (2023). Cardiomyocyte-Specific Wt1 Is Involved in Cardiac Metabolism and Response to Damage. Journal of Cardiovascular Development and Disease, 10(5), 211. https://doi.org/10.3390/jcdd10050211









