Three-Dimensional-Derived Echocardiographic Left Ventricular Structure and Function and Indices from the 12-Lead Electrocardiogram across the Menstrual Cycle in Healthy Physically Active Females: An Exploratory Study
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Design
2.3. Participant Evaluation
2.4. Echocardiography
2.5. Electrocardiography
3. Reproducibility Analysis
4. Statistical Analysis
5. Results
6. Discussion
7. Limitations
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morselli, E.; de Souza Santos, R.; Criollo, A.; Nelson, M.; Palmer, B.; Clegg, D. The Effects of Oestrogens and Their Receptors on Cardiometabolic Health. Nat. Rev. Endocrinol. 2017, 13, 352–364. [Google Scholar] [CrossRef]
- Sherman, B.M.; Korenman, S.G. Hormonal Characteristics of the Human Menstrual Cycle throughout Reproductive Life. J. Clin. Investig. 1975, 55, 699–706. [Google Scholar] [CrossRef] [PubMed]
- George, K.P.; Birch, K.M.; Jones, B.; Lea, R. Estrogen Variation and Resting Left Ventricular Structure and Function in Young Healthy Females. Med. Sci. Sports Exerc. 2000, 32, 297. [Google Scholar] [CrossRef] [PubMed]
- Fuenmayor, A.J.; Ramırez, L.; Fuenmayor, A.M. Left Ventricular Function and Autonomic Nervous System Balance during Two Different Stages of the Menstrual Cycleq. Int. J. Cardiol. 2000, 72, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Esformes, J.I.; Norman, F.; Sigley, J.; Birch, K.M. The Influence of Menstrual Cycle Phase upon Postexercise Hypotension. Med. Sci. Sports Exerc. 2006, 38, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Hirose, K.; Daimon, M.; Miyazaki, S.; Chiang, S.-J.; Morimoto-Ichikawa, R.; Maruyama, M.; Kawata, T.; Ohmura, H.; Daida, H. Estrogen Variation during the Menstrual Cycle Does Not Influence Left Ventricular Diastolic Function and Untwisting Rate in Premenopausal Females. J. Cardiol. 2017, 69, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Notomi, Y.; Shiota, T.; Popović, Z.B.; Weaver, J.A.; Oryszak, S.J.; Greenberg, N.L.; White, R.D.; Thomas, J.D.; Setser, R.M.; White, R.D.; et al. Measurement of Ventricular Torsion by Two-Dimensional Ultrasound Speckle Tracking Imaging. J. Am. Coll. Cardiol. 2005, 45, 2034–2041. [Google Scholar] [CrossRef]
- Chen, X.; Xie, H.; Erkamp, R.; Kim, K.; Jia, C.; Rubin, J.M.; O’Donnell, M. 3-D Correlation-Based Speckle Tracking. Ultrason. Imaging 2005, 27, 21–36. [Google Scholar] [CrossRef]
- Zengin, K.; Tokac, M.; Duzenli, M.A.; Soylu, A.; Aygul, N.; Ozdemir, K. Influence of Menstrual Cycle on Cardiac Performance. Maturitas 2007, 58, 70–74. [Google Scholar] [CrossRef]
- Addetia, K.; Mazzanti, A.; Maragna, R.; Monti, L.; Yamat, M.; Kukavica, D.; Pagan, E.; Kishiki, K.; Prado, A.; Marino, M.; et al. Value of 3D Echocardiography in the Diagnosis of Arrhythmogenic Right Ventricular Cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 664–677. [Google Scholar] [CrossRef]
- De Bosscher, R.; Claeys, M.; Dausin, C.; Goetschalckx, K.; Claus, P.; Herbots, L.; Ghekiere, O.; Van De Heyning, C.; Paelinck, B.P.; Janssens, K.; et al. Three-Dimensional Echocardiography of the Athlete’s Heart: A Comparison with Cardiac Magnetic Resonance Imaging. Int. J. Cardiovasc. Imaging 2023, 39, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ashraf, M.; Hu, D.; Dai, X.; Xu, Y.; Kenny, B.; Cameron, B.; Nguyen, T.; Xiong, L.; Sahn, D.J. Three-Dimensional Speckle-Tracking Imaging for Left Ventricular Rotation Measurement. J. Ultrasound Med. 2010, 29, 903–909. [Google Scholar] [CrossRef]
- Luis, S.A.; Yamada, A.; Khandheria, B.K.; Speranza, V.; Benjamin, A.; Ischenko, M.; Platts, D.G.; Hamilton-Craig, C.R.; Haseler, L.; Burstow, D.; et al. Use of Three-Dimensional Speckle-Tracking Echocardiography for Quantitative Assessment of Global Left Ventricular Function: A Comparative Study to Three-Dimensional Echocardiography. J. Am. Soc. Echocardiogr. 2014, 27, 285–291. [Google Scholar] [CrossRef]
- Sengupta, P.P.; Korinek, J.; Belohlavek, M.; Narula, J.; Vannan, M.A.; Jahangir, A.; Khandheria, B.K. Left Ventricular Structure and Function. J. Am. Coll. Cardiol. 2006, 48, 1988–2001. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.-L.; Gonçalves, A.; Shah, A.M.; Cheng, S.; Kitzman, D.; Solomon, S.D. Age- and Sex-Related Influences on Left Ventricular Mechanics in Elderly Individuals Free of Prevalent Heart Failure. Circ. Cardiovasc. Imaging 2017, 10, e004510. [Google Scholar] [CrossRef] [PubMed]
- Ammar, K.A.; Paterick, T.E.; Khandheria, B.K.; Jan, M.F.; Kramer, C.; Umland, M.M.; Tercius, A.J.; Baratta, L.; Tajik, A.J. Myocardial Mechanics: Understanding and Applying Three-Dimensional Speckle Tracking Echocardiography in Clinical Practice. Echocardiography 2012, 29, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Marwick, T.H. Measurement of Strain and Strain Rate by Echocardiography. J. Am. Coll. Cardiol. 2006, 47, 1313–1327. [Google Scholar] [CrossRef] [PubMed]
- Dandel, M.; Hetzer, R. Echocardiographic Strain and Strain Rate Imaging—Clinical Applications. Int. J. Cardiol. 2009, 132, 11–24. [Google Scholar] [CrossRef]
- Gowd, B.M.P.; Thompson, P.D. Effect of Female Sex on Cardiac Arrhythmias. Cardiol. Rev. 2012, 20, 297–303. [Google Scholar] [CrossRef]
- Chawla, V.K.; Choudhary, R.; Binawara, B.K.; Choudhary, S. Haematological and Electrocardiographic Variations during Menstrual Cycle. Pak. J. Physiol. 2010, 6, 18–21. [Google Scholar]
- Khan, S.; Prakash, J.; Rashid, M.; Beg, U.; Kumar, M.; Hussain, G. To Study the Effect of Different Phases of Menstrual Cycle on ECG & Blood Pressure in Healthy Young Adult Females. J. Med. Sci. Clin. Res. 2016, 4, 10406–10414. [Google Scholar] [CrossRef]
- Sharma, S.; Drezner, J.A.; Baggish, A.; Papadakis, M.; Wilson, M.G.; Prutkin, J.M.; La Gerche, A.; Ackerman, M.J.; Borjesson, M.; Salerno, J.C.; et al. International Recommendations for Electrocardiographic Interpretation in Athletes. Eur. Heart J. 2018, 39, 1466–1480. [Google Scholar] [CrossRef] [PubMed]
- Mosteller, R.D. Simplified Calculation of Body-Surface Area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Wagner, G.S. Marriott’s Practical Electrocardiography; Lippincott Williams & Wilkins: Hagerstown, MD, USA, 2001; ISBN 978-0-683-30746-7. [Google Scholar]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Mizukoshi, K.; Takeuchi, M.; Nagata, Y.; Addetia, K.; Lang, R.M.; Akashi, Y.J.; Otsuji, Y. Normal Values of Left Ventricular Mass Index Assessed by Transthoracic Three-Dimensional Echocardiography. J. Am. Soc. Echocardiogr. 2016, 29, 51–61. [Google Scholar] [CrossRef]
- Muraru, D.; Badano, L.P.; Peluso, D.; Dal Bianco, L.; Casablanca, S.; Kocabay, G.; Zoppellaro, G.; Iliceto, S. Comprehensive Analysis of Left Ventricular Geometry and Function by Three-Dimensional Echocardiography in Healthy Adults. J. Am. Soc. Echocardiogr. 2013, 26, 618–628. [Google Scholar] [CrossRef]
- Lee, L.; Cotella, J.I.; Miyoshi, T.; Addetia, K.; Schreckenberg, M.; Hitschrich, N.; Blankenhagen, M.; Amuthan, V.; Citro, R.; Daimon, M.; et al. Normal Values of Left Ventricular Mass by Two-Dimensional and Three-Dimensional Echocardiography: Results from the World Alliance Societies of Echocardiography Normal Values Study. J. Am. Soc. Echocardiogr. 2023, 36, 533–542.e1. [Google Scholar] [CrossRef]
- Häggström, M. Reference Ranges for Estradiol, Progesterone, Luteinizing Hormone and Follicle-Stimulating Hormone during the Menstrual Cycle. Wiki J. Med. 2014, 1, 1–5. [Google Scholar] [CrossRef]
- Subramanya, V.; Zhao, D.; Ouyang, P.; Lima, J.A.; Vaidya, D.; Ndumele, C.E.; Bluemke, D.A.; Shah, S.J.; Guallar, E.; Nwabuo, C.C.; et al. Sex Hormone Levels and Change in Left Ventricular Structure among Men and Post-Menopausal Females: The Multi-Ethnic Study of Atherosclerosis (MESA). Maturitas 2018, 108, 37–44. [Google Scholar] [CrossRef]
- Burke, J.H.; Ehlert, F.A.; Kruse, J.T.; Parker, M.A.; Goldberger, J.J.; Kadish, A.H. Gender-Specific Differences in the QT Interval and the Effect of Autonomic Tone and Menstrual Cycle in Healthy Adults. Am. J. Cardiol. 1997, 79, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Endres, S.; Mayuga, K.A.; de Cristofaro, A.; Taneja, T.; Goldberger, J.J.; Kadish, A.H. Menstrual Cycle and ST Height. Ann. Noninvasive Electrocardiol. 2004, 9, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.M.C.; Leonardo, F.; Rosano, G.M.C.; De Luca, F.; Sarrel, P.M.; Beale, C.M.; Collins, P. Cyclical Variation in Paroxysmal Supraventricular Tachycardia in Females. Lancet 1996, 347, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Colenso-Semple, L.M.; D’Souza, A.C.; Elliott-Sale, K.J.; Phillips, S.M. Current Evidence Shows No Influence of Females’s Menstrual Cycle Phase on Acute Strength Performance or Adaptations to Resistance Exercise Training. Front. Sports Act. Living 2023, 5, 1054542. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.H.; Dumanski, S.M.; Ahmed, S.B. Female Sex-Specific Considerations to Improve Rigor and Reproducibility in Cardiovascular Research. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H279–H287. [Google Scholar] [CrossRef]
- Elliott-Sale, K.J.; Minahan, C.L.; de Jonge, X.A.K.J.; Ackerman, K.E.; Sipilä, S.; Constantini, N.W.; Lebrun, C.M.; Hackney, A.C. Methodological Considerations for Studies in Sport and Exercise Science with Females as Participants: A Working Guide for Standards of Practice for Research on Females. Sports Med. 2021, 51, 843–861. [Google Scholar] [CrossRef]



| Parameter | Follicular Phase (Mean ± SD) | Ovulation Phase (Mean ± SD) | Luteal Phase (Mean ± SD) | p-Value |
|---|---|---|---|---|
| Age (y) | 21 ± 2 | - | - | - |
| Height (m) | 1.67 ± 0.07 | - | - | - |
| Weight (kg) | 69 ± 11 | 69 ± 12 | 70 ± 12 | 0.160 |
| BMI (kg/m2) | 25 ± 4 | 25 ± 4 | 25 ± 4 | 0.450 |
| BSA (m2) | 1.8 ± 0.2 | 1.8 ± 0.2 | 1.8 ± 0.2 | 0.216 |
| Systolic blood pressure (mmHg) | 124 ± 11 | 119 ± 7 | 122 ± 11 | 0.401 |
| Diastolic blood pressure (mmHg) | 73 ± 7 | 71 ± 8 | 67 ± 8 | 0.015 *^ |
| Parameter | Follicular Phase (Mean ± SD) | Ovulation Phase (Mean ± SD) | Luteal Phase (Mean ± SD) | p-Value |
|---|---|---|---|---|
| LVEDV (mL) | 105 ± 24 | 105 ± 26 | 108 ± 27 | 0.444 |
| LVEDVi (mL/m2) | 60 ± 14 | 58 ± 13 | 59 ± 13 | 0.681 |
| LVESV (mL) | 48 ± 10 | 49 ± 10 | 51 ± 11 | 0.194 |
| LVEF (%) | 54 ± 3 | 53 ± 3 | 52 ± 4 | 0.403 |
| SV (mL) | 57 ± 15 | 56 ± 16 | 57 ± 17 | 0.885 |
| CO (mL) | 3.7 ± 0.7 | 3.9 ± 0.9 | 3.9 ± 1.0 | 0.305 |
| LVM (g) | 142 ± 25 | 140 ± 23 | 147 ± 22 | 0.032 # |
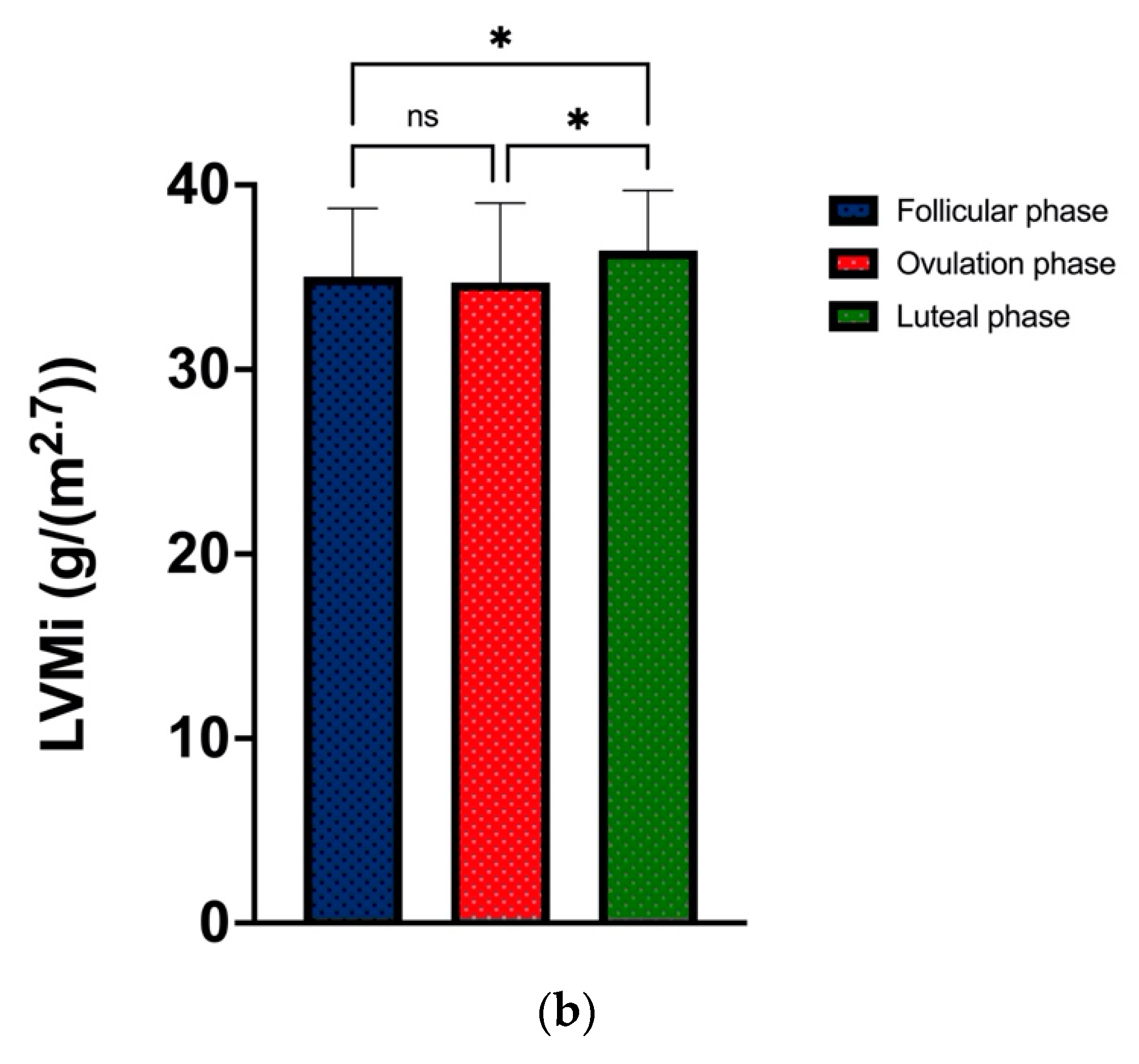
| LVMi (g/m2.7) | 35.0 ± 3.7 | 34.7 ± 4.3 | 36.4 ± 3.3 | 0.026 *^ |
| Concentricity (g/mL2/3) | 6.4 ± 0.6 | 6.4 ± 0.6 | 6.6 ± 0.5 | 0.352 |
| GLS (%) | −18.4 ± 2.1 | −18.0 ± 2.6 | −17.6 ± 4.8 | 0.796 |
| GCS (%) | −15.8 ± 2.8 | −16.8 ± 2.9 | −17.2 ± 2.6 | 0.107 |
| GRS (%) | 47.7 ± 8.7 | 48.6 ± 7.8 | 52.0 ± 9.2 | 0.049 # |
| Torsion (°) | 9.5 ± 4.1 | 8.9 ± 3.9 | 7.9 ± 3.5 | 0.409 |
| FP Mean ± SD (Range) | OP Mean ± SD (Range) | LP Mean ± SD (Range) | p-Value | |
|---|---|---|---|---|
| Heart rate (beats. min−1) | 66 ± 16 | 73 ± 16 | 70 ± 15 | 0.062 |
| P duration (ms) | 100 ± 12 | 98 ± 9 | 98 ± 15 | 0.648 |
| PR interval (ms) | 152 ± 25 | 150 ± 22 | 149 ± 24 | 0.739 |
| QRS duration (ms) | 87 ± 9 | 88 ± 9 | 89 ± 10 | 0.236 |
| QT corrected (Bazett) (ms) | 415 ± 14 | 417 ± 15 | 411 ± 23 | 0.494 |
| QRS axis (o) | 63 ± 19 | 63 ± 25 | 66 ± 21 | 0.632 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morrison, B.N.; Campbell, A.J.; Coté, A.T.; Mohammad, A.; Sambrook, L.; Robinson, G.; George, K.; Oxborough, D. Three-Dimensional-Derived Echocardiographic Left Ventricular Structure and Function and Indices from the 12-Lead Electrocardiogram across the Menstrual Cycle in Healthy Physically Active Females: An Exploratory Study. J. Cardiovasc. Dev. Dis. 2023, 10, 331. https://doi.org/10.3390/jcdd10080331
Morrison BN, Campbell AJ, Coté AT, Mohammad A, Sambrook L, Robinson G, George K, Oxborough D. Three-Dimensional-Derived Echocardiographic Left Ventricular Structure and Function and Indices from the 12-Lead Electrocardiogram across the Menstrual Cycle in Healthy Physically Active Females: An Exploratory Study. Journal of Cardiovascular Development and Disease. 2023; 10(8):331. https://doi.org/10.3390/jcdd10080331
Chicago/Turabian StyleMorrison, Barbara N., Allison J. Campbell, Anita T. Coté, Aleah Mohammad, Laura Sambrook, Georgia Robinson, Keith George, and David Oxborough. 2023. "Three-Dimensional-Derived Echocardiographic Left Ventricular Structure and Function and Indices from the 12-Lead Electrocardiogram across the Menstrual Cycle in Healthy Physically Active Females: An Exploratory Study" Journal of Cardiovascular Development and Disease 10, no. 8: 331. https://doi.org/10.3390/jcdd10080331
APA StyleMorrison, B. N., Campbell, A. J., Coté, A. T., Mohammad, A., Sambrook, L., Robinson, G., George, K., & Oxborough, D. (2023). Three-Dimensional-Derived Echocardiographic Left Ventricular Structure and Function and Indices from the 12-Lead Electrocardiogram across the Menstrual Cycle in Healthy Physically Active Females: An Exploratory Study. Journal of Cardiovascular Development and Disease, 10(8), 331. https://doi.org/10.3390/jcdd10080331





