Assessment of Hypertensive Patients’ Complex Metabolic Status Using Data Mining Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Screening Patients for Hypertension
2.2. Determining Cardiovascular Risk factors in Laboratory Environment
2.3. Laboratory Analysis
2.4. Statistical Analysis
3. Results
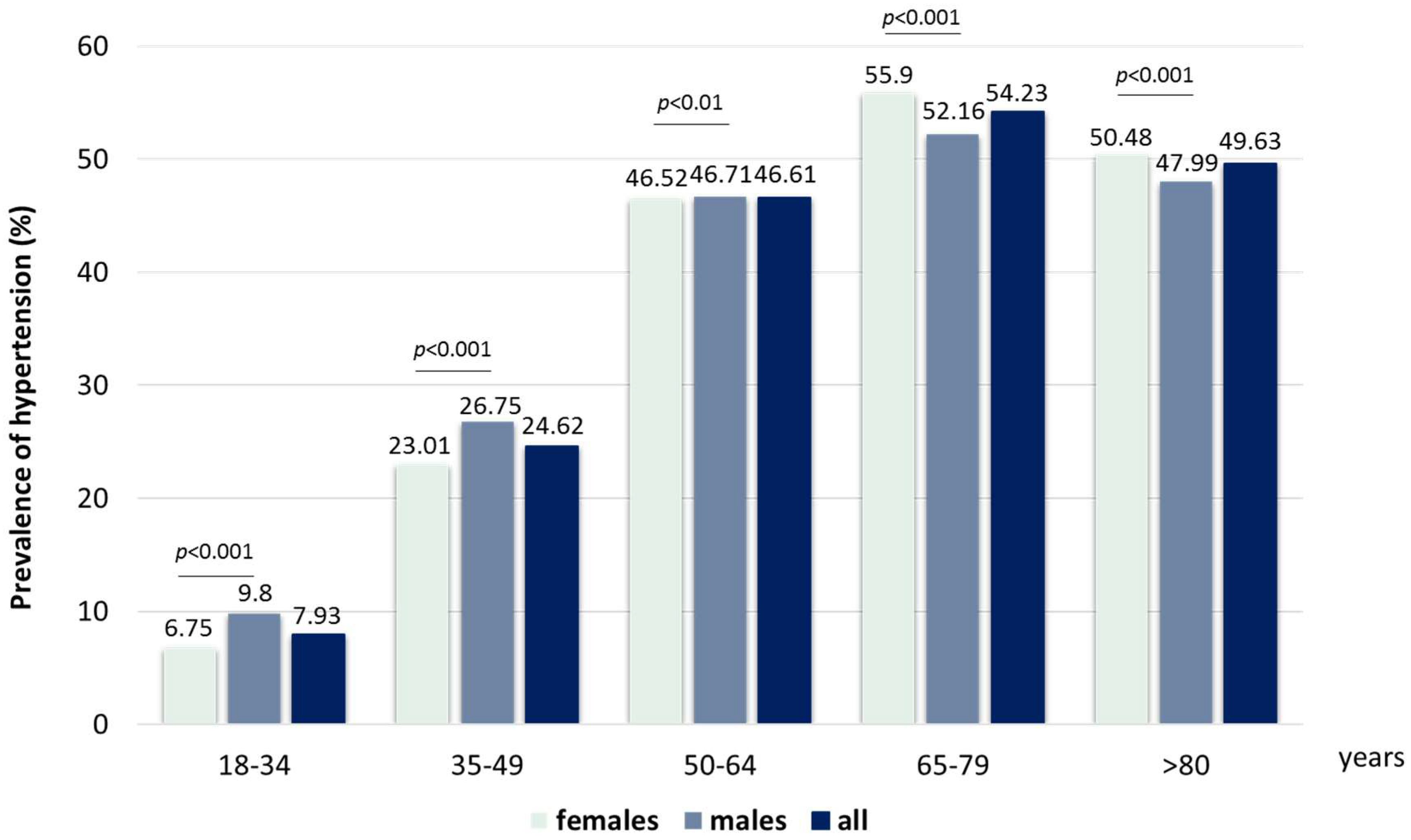
3.1. Identification of Patients with Hypertension
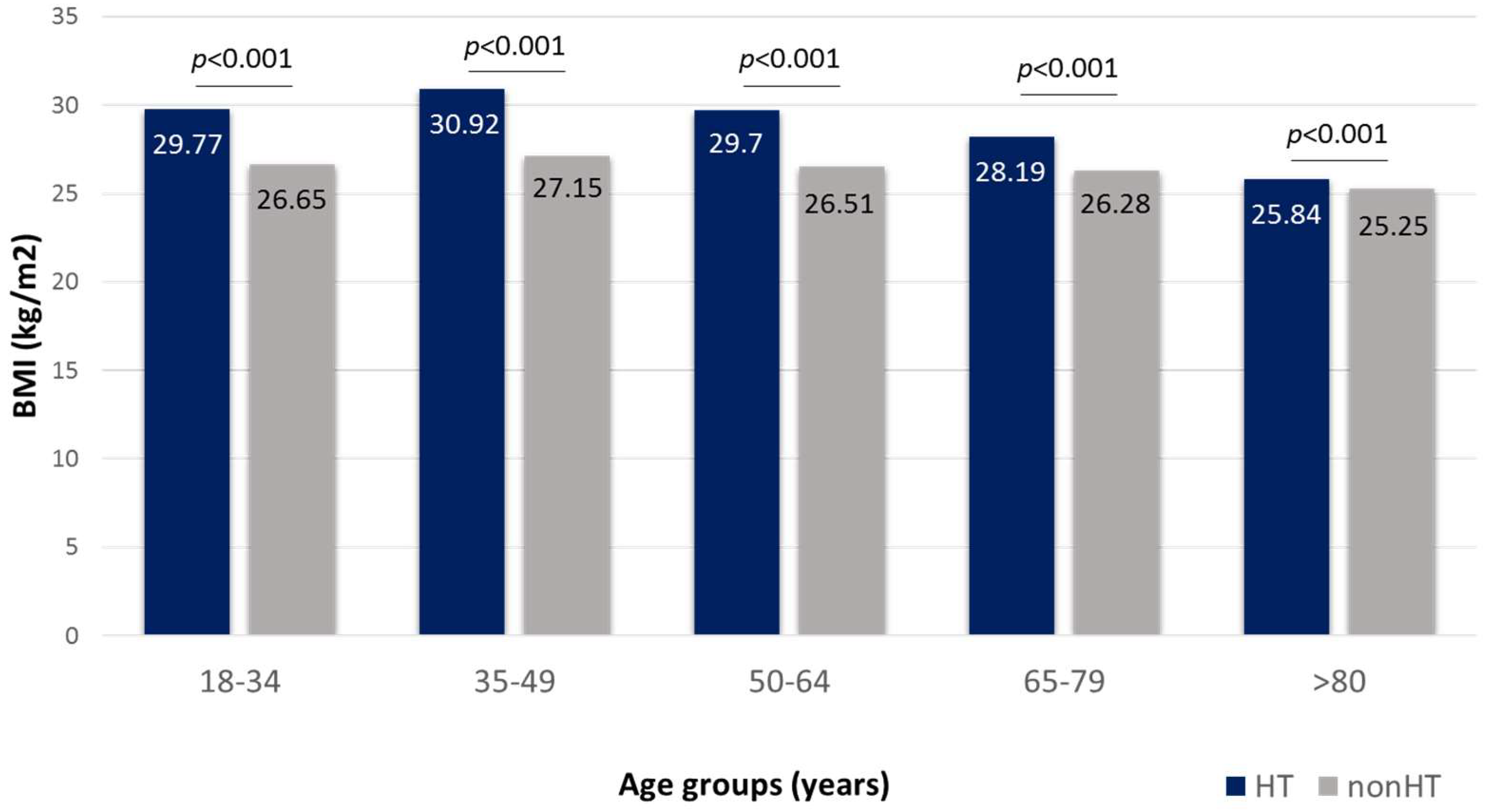
3.2. Body Mass Index
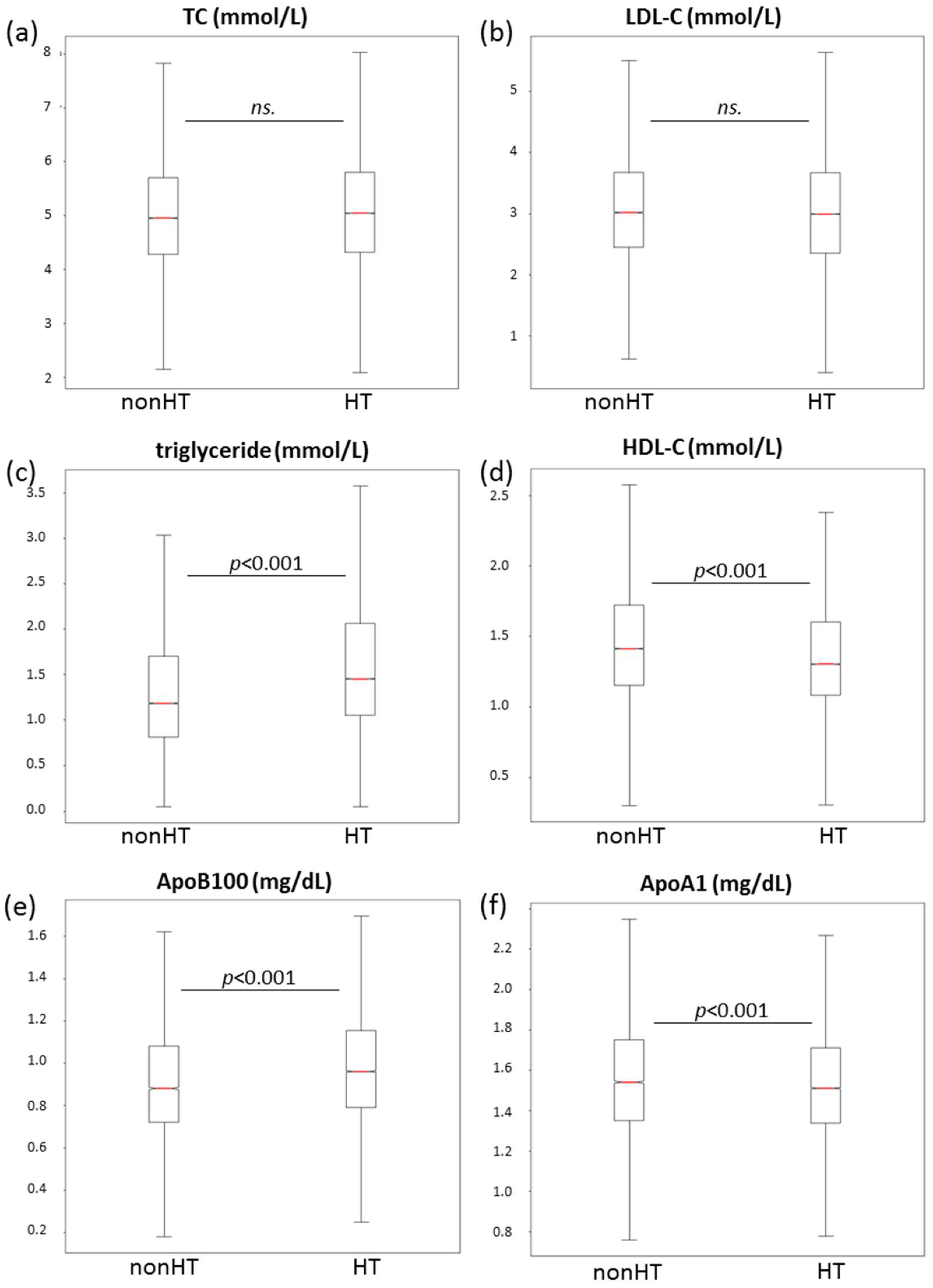
3.3. Lipid Parameters
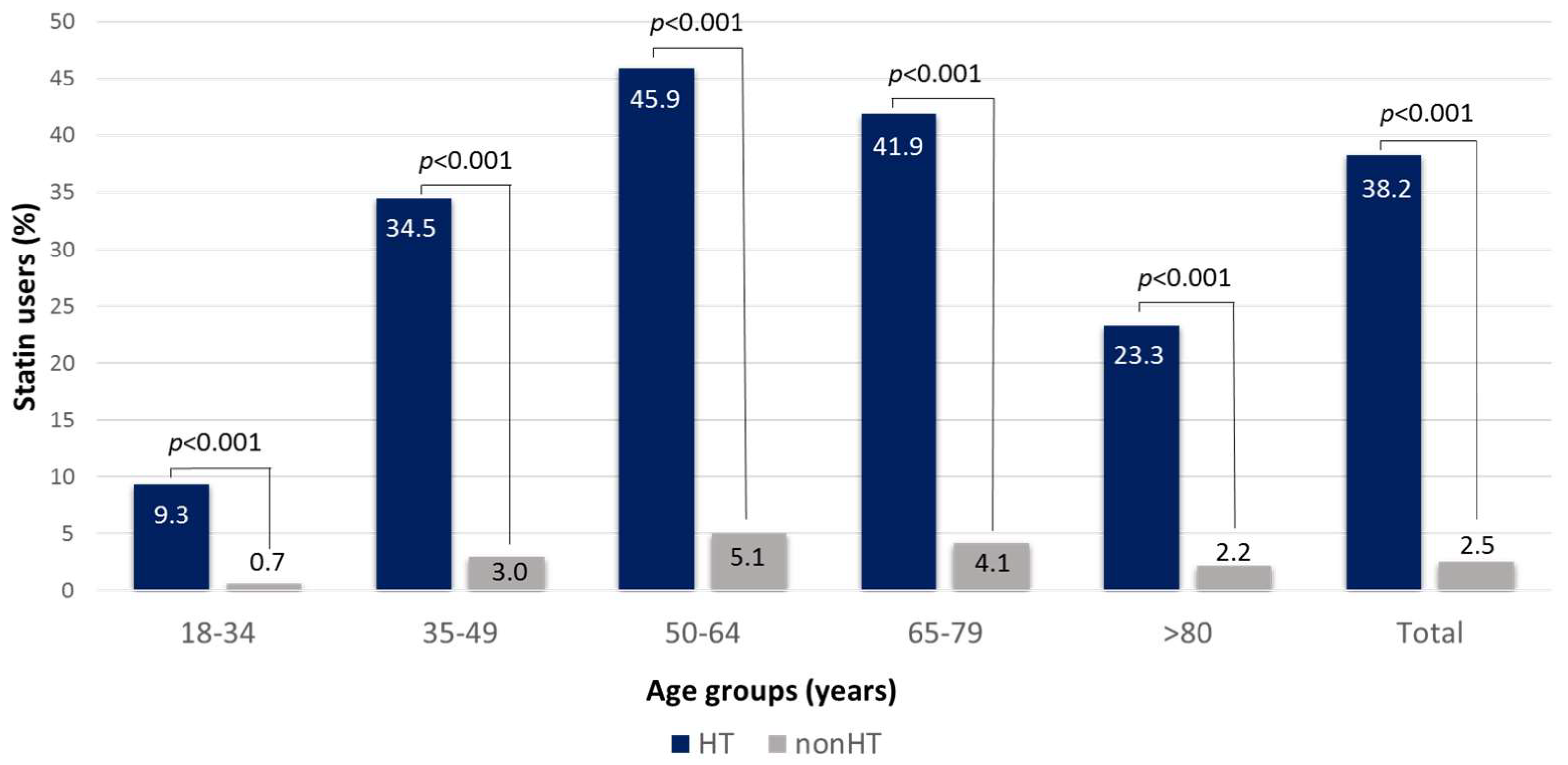
3.4. Identification of Statin Users
3.5. Serum Glucose, Haemoglobin A1c and Uric Acid Levels, Renal and Liver Function
3.6. Concomitant Diseases
3.7. Laboratory Parameters of the Study Population Based on the Grade of Hypertension
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arima, H.; Barzi, F.; Chalmers, J. Mortality patterns in hypertension. J. Hypertens. 2011, 29 (Suppl. S1), S3–S7. [Google Scholar] [CrossRef]
- Lawes, C.M.; Vander Hoorn, S.; Rodgers, A.; International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet 2008, 371, 1513–1518. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet 2017, 389, 37–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies from 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Chow, C.K.; Teo, K.K.; Rangarajan, S.; Islam, S.; Gupta, R.; Avezum, A.; Bahonar, A.; Chifamba, J.; Dagenais, G.; Diaz, R.; et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA 2013, 310, 959–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular disease statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef]
- Kiss, I.; Kékes, E. Hungarian Hypertension Registry. Orv. Hetil. 2014, 155, 764–768. [Google Scholar] [CrossRef] [Green Version]
- Nemcsik, J.; Páll, D.; Nemes-Nagy, Z.; Bacskai, M.; Kovács, T.; Benczúr, B.; Kiss, A.; Ábrahám, G.; Barna, I.; Beaney, T.; et al. May Measurement Month 2019: An analysis of blood pressure screening results from Hungary. Eur. Heart J. Suppl. 2021, 23, B70–B72. [Google Scholar] [CrossRef]
- Paragh, G.; Harangi, M.; Karányi, Z.; Daróczy, B.; Németh, Á.; Fülöp, P. Identifying patients with familial hypercholesterolemia using data mining methods in the Northern Great Plain region of Hungary. Atherosclerosis 2018, 277, 262–266. [Google Scholar] [CrossRef] [Green Version]
- Németh, Á.; Daróczy, B.; Juhász, L.; Fülöp, P.; Harangi, M.; Paragh, G. Assessment of Associations Between Serum Lipoprotein (a) Levels and Atherosclerotic Vascular Diseases in Hungarian Patients With Familial Hypercholesterolemia Using Data Mining and Machine Learning. Front. Genet. 2022, 13, 849197. [Google Scholar] [CrossRef]
- Németh, Á.; Harangi, M.; Daróczy, B.; Juhász, L.; Paragh, G.; Fülöp, P. Identifying Patients with Familial Chylomicronemia Syndrome Using FCS Score-Based Data Mining Methods. J. Clin. Med. 2022, 11, 4311. [Google Scholar] [CrossRef] [PubMed]
- Kovács, B.; Németh, Á.; Daróczy, B.; Karányi, Z.; Maroda, L.; Diószegi, Á.; Nádró, B.; Szabó, T.; Harangi, M.; Páll, D. Determining the prevalence of childhood hypertension and its concomitant metabolic abnormalities using data mining methods in the Northeastern region of Hungary. Front. Cardiovasc. Med. 2022, 9, 1081986. [Google Scholar] [CrossRef] [PubMed]
- Johns, B.T.; Jamieson, R.K. A Large-Scale Analysis of Variance in Written Language. Cogn. Sci. 2018, 42, 1360–1374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larrañaga, P.; Calvo, B.; Santana, R.; Bielza, C.; Galdiano, J.; Inza, I.; Lozano, J.A.; Armañanzas, R.; Santafé, G.; Pérez, A.; et al. Machine learning in bioinformatics. Brief. Bioinform. 2006, 7, 86–112. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press. 2018, 27, 314–340. [Google Scholar] [CrossRef]
- Worachartcheewan, A.; Schaduangrat, N.; Prachayasittikul, V.; Nantasenamat, C. Data mining for the identification of metabolic syndrome status. EXCLI J. 2018, 17, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Willett, W.C.; Manson, J.E.; Rosner, B.; Stampfer, M.J.; Speizer, F.E.; Colditz, G.A. Body weight, weight change, and risk for hypertension in women. Ann. Intern. Med. 1998, 128, 81–88. [Google Scholar] [CrossRef]
- He, J.; Klag, M.J.; Whelton, P.K.; Chen, J.Y.; Qian, M.C.; He, G.Q. Body mass and blood pressure in a lean population in southwestern China. Am. J. Epidemiol. 1994, 139, 380–389. [Google Scholar] [CrossRef]
- Ramezankhani, A.; Kabir, A.; Pournik, O.; Azizi, F.; Hadaegh, F. Classification-based data mining for identification of risk patterns associated with hypertension in Middle Eastern population: A 12-year longitudinal study. Medicine 2016, 95, e4143. [Google Scholar] [CrossRef]
- Ames, R.P. Hyperlipidemia in hypertension: Causes and prevention. Am. Heart J. 1991, 122, 1219–1224. [Google Scholar] [CrossRef]
- Stahel, P.; Xiao, C.; Hegele, R.A.; Lewis, G.F. The Atherogenic Dyslipidemia Complex and Novel Approaches to Cardiovascular Disease Prevention in Diabetes. Can. J. Cardiol. 2018, 34, 595–604. [Google Scholar] [CrossRef]
- Neaton, J.D.; Wentworth, D. Serum cholesterol, blood pressure, cigarette smoking, and death from coronary heart disease. Overall findings and differences by age for 316,099 white men. Multiple Risk Factor Intervention Trial Research Group. Arch. Intern. Med. 1992, 152, 56–64. [Google Scholar] [CrossRef]
- Gaddey, H.L.; Holder, K.K. Unintentional Weight Loss in Older Adults. Am. Fam. Physician 2021, 104, 34–40. [Google Scholar] [PubMed]
- Grayson, P.C.; Kim, S.Y.; LaValley, M.; Choi, H.K. Hyperuricemia and incident hypertension: A systematic review and meta-analysis. Arthritis. Care. Res. 2011, 63, 102–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, P.J.; Stason, W.B.; Demartini, F.E.; Sommers, S.C.; Laragh, J.H. Hyperuricemia in primary and renal hypertension. N. Engl. J. Med. 1966, 275, 457–464. [Google Scholar] [CrossRef] [PubMed]
- De Becker, B.; Borghi, C.; Burnier, M.; van de Borne, P. Uric acid and hypertension: A focused review and practical recommendations. J. Hypertens. 2019, 37, 878–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feig, D.I. The role of uric acid in the pathogenesis of hypertension in the young. J. Clin. Hypertens. 2012, 14, 346–352. [Google Scholar] [CrossRef]
- Pugh, D.; Gallacher, P.J.; Dhaun, N. Management of Hypertension in Chronic Kidney Disease. Drugs 2019, 79, 365–379. [Google Scholar] [CrossRef] [Green Version]
- Leiba, A.; Fishman, B.; Twig, G.; Gilad, D.; Derazne, E.; Shamiss, A.; Shohat, T.; Ron, O.; Grossman, E. Association of Adolescent Hypertension With Future End-stage Renal Disease. JAMA Intern. Med. 2019, 179, 517–523. [Google Scholar] [CrossRef]
- Rosenberg, J.; Sola, O.; Visconti, A. Approach to Elevated Liver Enzymes. Prim. Care 2023, 50, 363–376. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, L.; Chen, S. Differences in Lipid Profiles and Atherogenic Indices Between Hypertensive and Normotensive Populations: A Cross-Sectional Study of 11 Chinese Cities. Front. Cardiovasc. Med. 2022, 9, 887067. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H.; Matsushita, K.; Aboyans, V.; Hess, C.N.; Hicks, C.W.; Kwan, T.W.; McDermott, M.M.; Misra, S.; Ujueta, F.; on American Heart Association Council on Epidemiology and Prevention; et al. Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions: A Scientific Statement From the American Heart Association. Circulation 2021, 144, e171–e191. [Google Scholar] [CrossRef] [PubMed]

| 18–34 Years | 35–49 Years | 50–64 Years | 65–79 Years | >80 Years | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HT | nonHT | HT | nonHT | HT | nonHT | HT | nonHT | HT | nonHT | |
| n | 22,438 | 260,548 | 54,197 | 165,904 | 109,883 | 125,873 | 90,891 | 76,700 | 18,210 | 18,484 |
| TC (mmol/L) | 4.80 ± 1.01 | 4.79 ± 1.05 | 5.26 ± 1.14 | 5.28 ± 1.18 | 5.22 ± 1.19 | 5.35 ± 1.31 | 4.98 ± 1.20 | 4.99 ± 1.33 | 4.74 ± 1.20 | 4.64 ± 2.31 |
| LDL-C (mmol/L) | 2.94 ± 0.85 | 2.94 ± 0.97 | 3.22 ± 0.94 | 3.31 ± 0.97 | 3.11 ± 0.9 | 3.31 ± 1.07 | 2.89 ± 1.01 | 3.02 ± 1. 9 | 2.72 ± 0.97 | 2.72 ± 1.07 |
| TG (mmol/L) | 1.25 (0.87–1.87) | 1.01 (0.72–1.5) | 1.56 (1.09–2.30) | 1.28 (0.9–1.90) | 1.56 (1.13–2.20) | 1.40 (1.00–1.98) | 1.40 (1.04–1.90) | 1.30 (0.97–1.80) | 1.20 (0.91–1.60) | 1.11 (0.87–1.50) |
| HDL-C (mmol/l) | 1.38 ± 0.41 | 1.50 ± 0.58 | 1.34 ± 0.41 | 1.45 ± 0.45 | 1.36 ± 0.41 | 1.43 ± 0.47 | 1.37 ± 0.41 | 1.38 ± 0.45 | 1.38 ± 0.42 | 1.46 ± 0.75 |
| ApoB100 (g/L) | 0.88 ± 0.27 | 0.86 ± 0.86 | 1.00 ± 0.28 | 0.98 ± 0.28 | 1.02 ± 0.28 | 1.01 ± 0.29 | 0.97 ± 0.28 | 0.96 ± 0.28 | 0.93 ± 0.26 | 0.92 ± 0.27 |
| ApoA1 (g/L) | 1.52 ± 0.33 | 1.57 ± 0.87 | 1.52 ± 0.29 | 1.56 ± 0.38 | 1.54 ± 0.29 | 1.57 ± 0.33 | 1.54 ± 0.30 | 1.53 ± 0.32 | 1.52 ± 0.33 | 1.54 ± 0.31 |
| glucose (mmol/L) | 5.10 (4.7–5.6) | 4.98 (4.6–5.4) | 5.50 (5.0–6.3) | 5.29 (5.3–7.0) | 5.89 (5.3–7.0) | 5.60 (5.0–6.4) | 6.10 (5.4–7.3) | 5.78 (5.1–6.9) | 6.20 (5.5–7.3) | 5.93 (5.2–7.1) |
| HbA1c (%) | 5.4 (5.1–6.0) | 5.3 (5.0–5.6) | 6.0 (5.5–7.2) | 5.8 (5.4–6.8) | 6.4 (5.8–7.5) | 6.1 (5,6–7.1) | 6.4 (5.8–7.5) | 6.4 (5.7–7.5) | 6.3 (5.8–7.3) | 6.0 (5.5–7.0) |
| uric acid (µmol/L) | 297 ± 84.2 | 265 ± 75.5 | 308 ± 89.0 | 278 ± 87.1 | 322 ± 92.9 | 297 ± 96.7 | 333 ± 103.1 | 314 ± 112.2 | 344 ± 120.7 | 335 ± 142.5 |
| urea (mmol/L) | 4.30 (3.55–5.19) | 4.13 (3.40–5.0) | 4.89 (4.06–5.83) | 4.65 (3.80–5.60) | 5.70 (4.70–7.0) | 5.33 (4.38–6.60) | 6.90 (5.53–9.02) | 6.50 (5.20–8.50) | 8.20 (6.30–11.1) | 7.90 (6.0–10.9) |
| creatinine (µmol/L) | 68.3 (57.8–81.2) | 66.0 (56.3–78.0) | 71.0 (61.0–83.7) | 69.0 (59.5–80.5) | 75.0 (64.0–89.7) | 72.0 (61.0–84.7) | 83.0 (68.9–103.9) | 79.3 (66.0–98.0) | 89.3 (72.0–116.0) | 88.0 (70.6–114.5) |
| AST (U/L) | 20.7 (17.1–26.3) | 19.0 (16.0–24.0) | 21.9 (18.0–29.0) | 20.7 (17.0–26.5) | 22.2 (18.2–30.0) | 21.9 (18.0–29.0) | 22.0 (18.0–30.3) | 21.9 (17.0–30.7) | 23.0 (18.0–33.0) | 22.3 (17.0–34.0) |
| ALT (U/L) | 21.0 (15.0–34.0 | 18.0 (13.0–26.9) | 24.5 (17.5–36.2) | 21.0 (15.0–32.0) | 23.0 (17.3–33.0) | 21.0 (15.5–31.0) | 19.7 (15–27.8) | 19 (13.7–27) | 17.0 (12.8–25.8) | 16.0 (12.0–25.0) |
| GGT (U/L) | 21.8 (14.3–37.6) | 18.0 (13.0–28.0) | 33.2 (20.3–61.0) | 26.3 (17–49.7) | 35.8 (22.8–66.0) | 33.0 (20.4–63.0) | 31.4 (20.3–57.3) | 30.0 (19.0–59.0) | 28.0 (17.7–53.3) | 25.0 (16.0–47.5) |
| Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|
| n | 99 601 | 43 283 | 41 990 |
| TC (mmol/L) | 5.08 ± 1.19 | 5.15 ± 1.15 | 5.15 ± 1.1 |
| LDL-C (mmol/L) | 3.06 ± 0.99 | 3.07 ± 0.99 | 3.04 ± 0.96 |
| TG (mmol/L) | 1.41 (1.01–2.01) | 1.48 (1.08–2.07) | 1.54 (1.12–2.17) |
| HDL-C (mmol/l) | 1.36 ± 0.41 | 1.37 ± 0.41 | 1.36 ± 0.39 |
| ApoB100 (g/L) | 0.98 ± 0.28 | 0.99 ± 0.28 | 1.0 ± 0.28 |
| ApoA1 (g/L) | 1.52 ± 0.31 | 1.54 ± 0.34 | 1.53 ± 0.29 |
| glucose (mmol/L) | 5.7 (5.1–6.6) | 5.9 (5.3–7.0) | 6.0 (5.4–7.2) |
| HbA1c (%) | 6.1 (5.6–7.3) | 6.3 (5.7–7.4) | 6.3 (5.7–7.5) |
| uric acid (µmol/L) | 313 ± 95.1 | 323 ± 94.4 | 333 ± 91.4 |
| urea (mmol/L) | 5.43 (4.36–6.95) | 5.8 (4.68–7.5) | 6.03 (4.9–7.9) |
| creatinine (µmol/L) | 74.4 (62.5–89.0) | 77.0 (65.0–92.9) | 79.0 (66.4–97.3) |
| AST (U/L) | 22.0 (18.0–29.7) | 22.5 (18.5–30.0) | 22.5 (18.5–29.5) |
| ALT (U/L) | 22.0 (16.0–32.0) | 22.1 (16.4–32.5) | 22.5 (16.7–32.3) |
| GGT (U/L) | 31.0 (19.3–57.5) | 33.3 (21.0–60.3) | 34.0 (21.5–61.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, B.; Németh, Á.; Daróczy, B.; Karányi, Z.; Maroda, L.; Diószegi, Á.; Harangi, M.; Páll, D. Assessment of Hypertensive Patients’ Complex Metabolic Status Using Data Mining Methods. J. Cardiovasc. Dev. Dis. 2023, 10, 345. https://doi.org/10.3390/jcdd10080345
Kovács B, Németh Á, Daróczy B, Karányi Z, Maroda L, Diószegi Á, Harangi M, Páll D. Assessment of Hypertensive Patients’ Complex Metabolic Status Using Data Mining Methods. Journal of Cardiovascular Development and Disease. 2023; 10(8):345. https://doi.org/10.3390/jcdd10080345
Chicago/Turabian StyleKovács, Beáta, Ákos Németh, Bálint Daróczy, Zsolt Karányi, László Maroda, Ágnes Diószegi, Mariann Harangi, and Dénes Páll. 2023. "Assessment of Hypertensive Patients’ Complex Metabolic Status Using Data Mining Methods" Journal of Cardiovascular Development and Disease 10, no. 8: 345. https://doi.org/10.3390/jcdd10080345
APA StyleKovács, B., Németh, Á., Daróczy, B., Karányi, Z., Maroda, L., Diószegi, Á., Harangi, M., & Páll, D. (2023). Assessment of Hypertensive Patients’ Complex Metabolic Status Using Data Mining Methods. Journal of Cardiovascular Development and Disease, 10(8), 345. https://doi.org/10.3390/jcdd10080345