Analysis of Superficial Subcutaneous Fat Camper’s and Scarpa’s Fascia in a United States Cohort
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Group Comparisons
3.3. Inter-Rater Reliability
4. Discussion
4.1. Study Results
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joshi, R.; Duong, H. Anatomy, Abdomen and Pelvis, Scarpa Fascia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- MacKay, M.D.; Mudreac, A.; Varacallo, M. Anatomy, Abdomen and Pelvis, Camper Fascia; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Konschake, M.; Zwierzina, M.; Moriggl, B.; Függer, R.; Mayer, F.; Brunner, W.; Schmid, T.; Chen, D.C.; Fortelny, R. The inguinal region revisited: The surgical point of view: An anatomical-surgical mapping and sonographic approach regarding postoperative chronic groin pain following open hernia repair. Hernia 2020, 24, 883–894. [Google Scholar] [CrossRef]
- Del Valle, G.O.; Combs, P.; Qualls, C.; Curet, L.B. Does closure of Camper fascia reduce the incidence of post-cesarean superficial wound disruption? Obstet. Gynecol. 1992, 80, 1013–1016. [Google Scholar] [PubMed]
- Wijaya, W.A.; Liu, Y.; He, Y.; Qing, Y.; Li, Z. Abdominoplasty with Scarpa Fascia Preservation: A Systematic Review and Meta-analysis. Aesthetic Plast. Surg. 2022, 46, 2841–2852. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Ye, L. Efficacy and Safety of Scarpa Fascia Preservation During Abdominoplasty: A Systematic Review and Meta-analysis. Aesthetic Plast. Surg. 2017, 41, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Odobescu, A.; Keith, J.N. Preshaping DIEP Flaps: Simplifying and Optimizing Breast Reconstruction Aesthetics. Plast. Reconstr. Surg. 2021, 147, 1059–1061. [Google Scholar] [CrossRef]
- Lonic, D.; Yamaguchi, K.; Chien-Jung Pai, B.; Lo, L.J. Reinforcing the Mucoperiosteal Pocket with the Scarpa Fascia Graft in Secondary Alveolar Bone Grafting: A Retrospective Controlled Outcome Study. Plast. Reconstr. Surg. 2017, 140, 568e–578e. [Google Scholar] [CrossRef] [PubMed]
- Costa-Ferreira, A.; Rebelo, M.; Vásconez, L.O.; Amarante, J. Scarpa fascia preservation during abdominoplasty: A prospective study. Plast. Reconstr. Surg. 2010, 125, 1232–1239. [Google Scholar] [CrossRef]
- Congdon, E.D.; Edson, J.; Yanitelli, S. Gross structure of the subcutaneous layer of the anterior and lateral trunk in the male. Am. J. Anat. 1946, 79, 399–429. [Google Scholar] [CrossRef]
- Frank, K.; Hamade, H.; Casabona, G.; Gotkin, R.H.; O Kaye, K.; Tiryaki, T.; Freytag, D.L.; Bialowas, C.; Koban, K.C.; Cotofana, S. Influences of Age, Gender, and Body Mass Index on the Thickness of the Abdominal Fatty Layers and its Relevance for Abdominal Liposuction and Abdominoplasty. Aesthet. Surg. J. 2019, 39, 1085–1093. [Google Scholar] [CrossRef]
- Harley, O.J.; Pickford, M.A. CT analysis of fat distribution superficial and deep to the Scarpa’s fascial layer in the mid and lower abdomen. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 525–530. [Google Scholar] [CrossRef]
- Zaidi, H.; Aksnes, T.; Åkra, S.; Eggesbø, H.B.; Byrkjeland, R.; Seljeflot, I.; Opstad, T.B. Abdominal Adipose Tissue Associates With Adiponectin and TNFα in Middle-Aged Healthy Men. Front. Endocrinol. 2022, 13, 874977. [Google Scholar] [CrossRef]
- Smith, S.R.; Lovejoy, J.C.; Greenway, F.; Ryan, D.; deJonge, L.; de la Bretonne, J.; Volafova, J.; Bray, G.A. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metab.-Clin. Exp. 2001, 50, 425–435. [Google Scholar] [CrossRef]
- Hakim, O.; Bello, O.; Ladwa, M.; Peacock, J.L.; Umpleby, A.M.; Charles-Edwards, G.; Amiel, S.A.; Goff, L.M. The Link between Obesity and Inflammatory Markers in the Development of Type 2 Diabetes in Men of Black African and White European Ethnicity. Nutrients 2020, 12, 3796. [Google Scholar] [CrossRef]
- Khan, F.A.A.; Fatima, M. Abdominoplasty without Drains or Progressive Tension Suturing. Aesthetic Plast. Surg. 2021, 45, 1660–1666. [Google Scholar] [CrossRef] [PubMed]
- Pokrywczynska, M.; Jundzill, A.; Rasmus, M.; Adamowicz, J.; Balcerczyk, D.; Buhl, M.; Warda, K.; Buchholz, L.; Gagat, M.; Grzanka, D.; et al. Understanding the role of mesenchymal stem cells in urinary bladder regeneration-a preclinical study on a porcine model. Stem Cell. Res. Ther. 2018, 9, 328. [Google Scholar] [CrossRef]
- Anfinan, N.; Sait, K.H. Appropriate Management of Subcutaneous Tissue of Midline Abdominal Incisions. Cureus 2020, 12, e6549. [Google Scholar] [CrossRef]
- Murouchi, T. Transient obturator nerve block with fascia iliaca compartment block following local infiltration for open inguinal herniorrhaphy. JA Clin. Rep. 2018, 4, 63. [Google Scholar] [CrossRef]
- Lisiecki, J.; Kozlow, J.H.; Agarwal, S.; Ranganathan, K.; Terjimanian, M.N.; Rinkinen, J.; Brownley, R.C.; Enchakalody, B.; Wang, S.C.; Levi, B. Abdominal wall dynamics after component separation hernia repair. J. Surg. Res. 2015, 193, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Lancerotto, L.; Stecco, C.; Macchi, V.; Porzionato, A.; Stecco, A.; De Caro, R. Layers of the abdominal wall: Anatomical investigation of subcutaneous tissue and superficial fascia. Surg. Radiol. Anat. 2011, 33, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.V.; Murthy, V.L.; Abbasi, S.A.; Blankstein, R.; Kwong, R.Y.; Goldfine, A.B.; Jerosch-Herold, M.; Lima, J.A.C.; Ding, J.; Allison, M.A. Visceral adiposity and the risk of metabolic syndrome across body mass index: The MESA Study. JACC Cardiovasc. Imaging 2014, 7, 1221–1235. [Google Scholar] [CrossRef]
- Tatsumi, Y.; Nakao, Y.M.; Masuda, I.; Higashiyama, A.; Takegami, M.; Nishimura, K.; Watanabe, M.; Ohkubo, T.; Okamura, T.; Miyamoto, Y. Risk for metabolic diseases in normal weight individuals with visceral fat accumulation: A cross-sectional study in Japan. BMJ Open 2017, 7, e013831. [Google Scholar] [CrossRef]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef]
- Golan, R.; Shelef, I.; Rudich, A.; Gepner, Y.; Shemesh, E.; Chassidim, Y.; Harman-Boehm, I.; Henkin, Y.; Schwarzfuchs, D.; Avraham, S.B.; et al. Abdominal superficial subcutaneous fat: A putative distinct protective fat subdepot in type 2 diabetes. Diabetes Care 2012, 35, 640–647. [Google Scholar] [CrossRef]
- Zelicha, H.; Kloting, N.; Kaplan, A.; Meir, A.Y.; Rinott, E.; Tsaban, G.; Chassidim, Y.; Bluher, M.; Ceglarek, U.; Isermann, B.; et al. The effect of high-polyphenol Mediterranean diet on visceral adiposity: The DIRECT PLUS randomized controlled trial. BMC Med. 2022, 20, 327. [Google Scholar] [CrossRef] [PubMed]
- Brand, T.; van den Munckhof, I.C.L.; van der Graaf, M.; Schraa, K.; Dekker, H.M.; Joosten, L.A.B.; Netea, M.G.; Riksen, N.P.; de Graaf, J.; Rutten, J.H.W. Superficial vs Deep Subcutaneous Adipose Tissue: Sex-Specific Associations With Hepatic Steatosis and Metabolic Traits. J. Clin. Endocrinol. Metab. 2021, 106, e3881–e3889. [Google Scholar] [CrossRef]
- Serfaty, D.; Rein, M.; Schwarzfuchs, D.; Shelef, I.; Gepner, Y.; Bril, N.; Cohen, N.; Shemesh, E.; Sarusi, B.; Kovsan, J.; et al. Abdominal fat sub-depots and energy expenditure: Magnetic resonance imaging study. Clin. Nutr. 2017, 36, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.H.; Li, W.C.; Sheu, B.F.; Liao, S.-C.; Chen, J.-Y.; Chang, K.-C.; Tsai, Y.-W. Correlation between body composition and risk factors for cardiovascular disease and metabolic syndrome. Biofactors 2012, 38, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Shea, J.L.; King, M.T.; Yi, Y.; Gulliver, W.; Sun, G. Body fat percentage is associated with cardiometabolic dysregulation in BMI-defined normal weight subjects. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 741–747. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Silva, C.; Galofré, J.C.; Escalada, J.; Santos, S.; Millán, D.; Vila, N.; Ibañez, P.; Gil, M.J.; Valentí, V.; et al. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int. J. Obes. 2012, 36, 286–294. [Google Scholar] [CrossRef]
- Liberato, S.C.; Maple-Brown, L.; Bressan, J.; Hills, A.P. The relationships between body composition and cardiovascular risk factors in young Australian men. Nutr. J. 2013, 12, 108. [Google Scholar] [CrossRef]
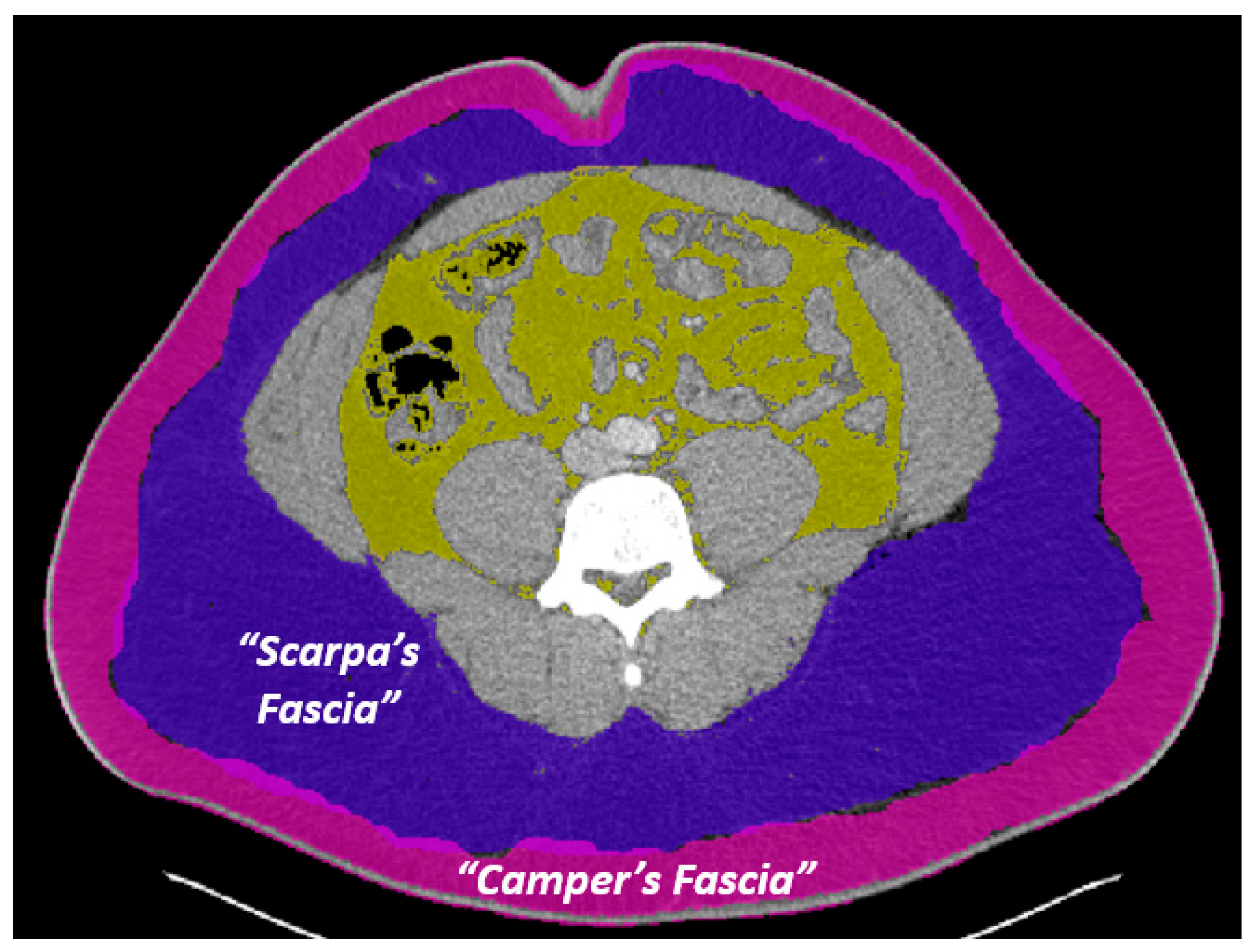
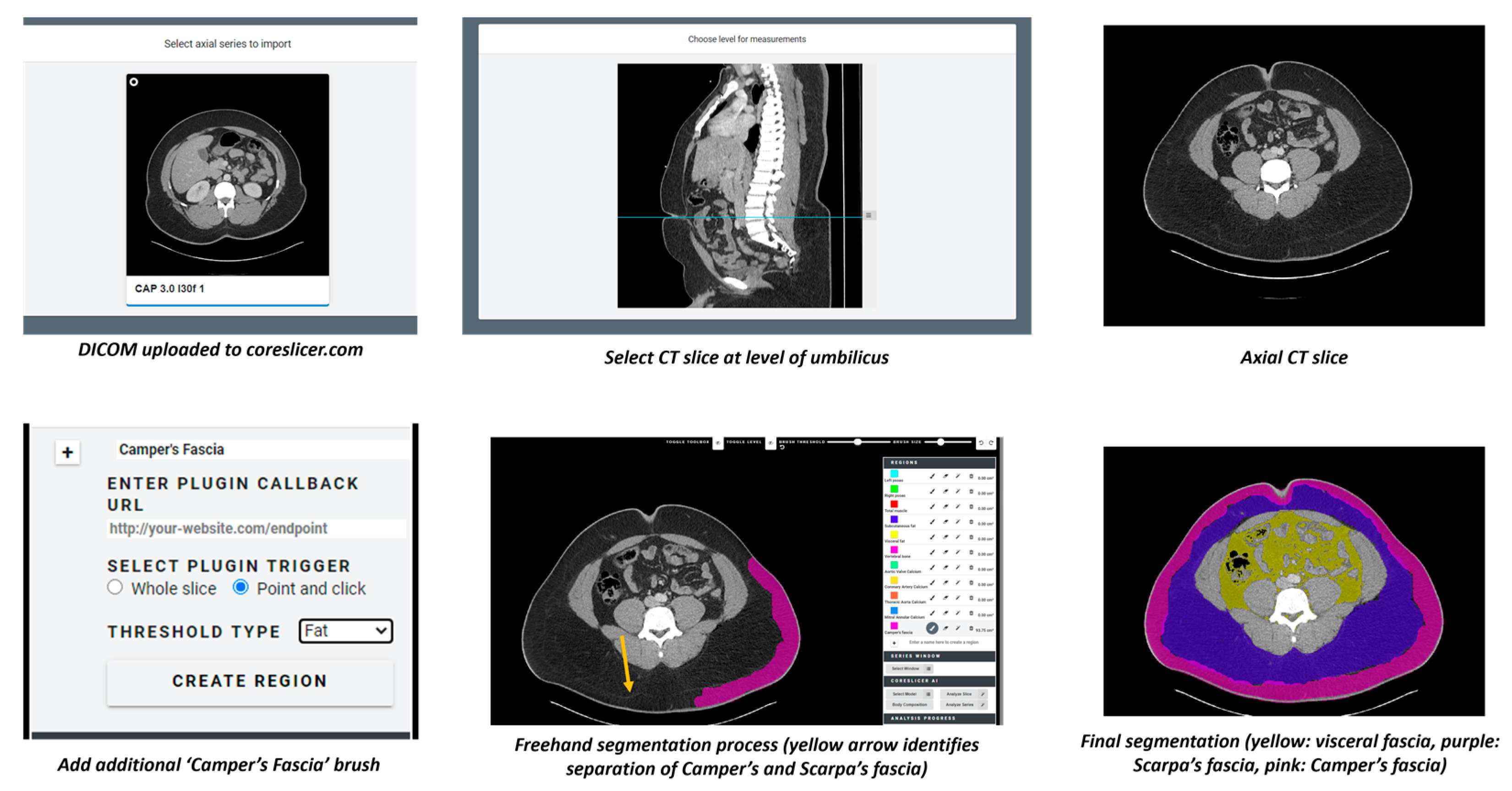

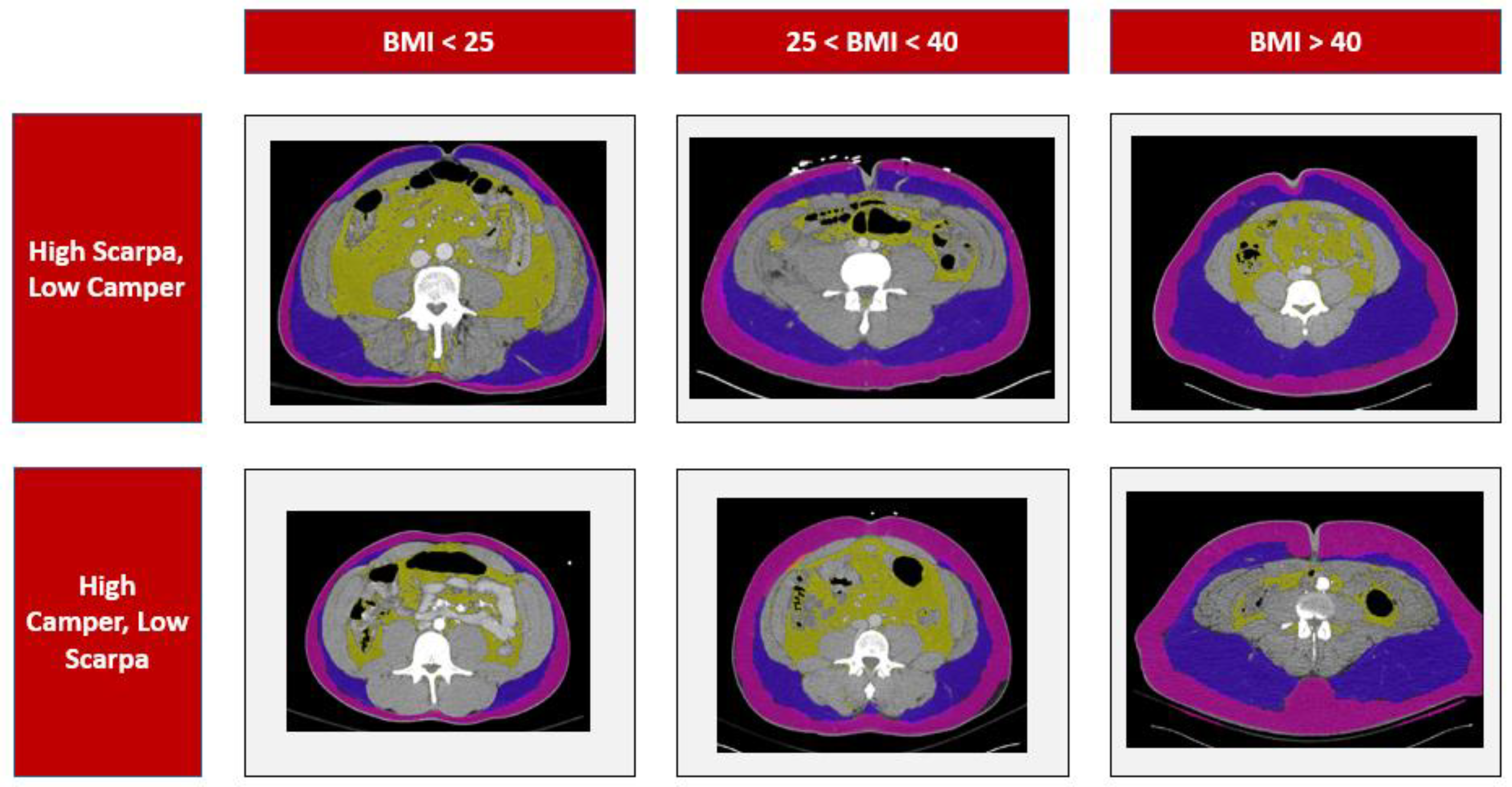



| Demographics | Female | Male | Total |
|---|---|---|---|
| n | 53 | 405 | 458 |
| Race, n (%) | |||
| Asian | 0 (0%) | 4 (1%) | 4 (0.9%) |
| Black | 43 (81.1%) | 346 (85.4%) | 389 (84.9%) |
| Other | 0 (0%) | 5 (1.2%) | 5 (1.1%) |
| Unknown | 2 (3.8%) | 10 (2.5%) | 12 (2.6%) |
| White | 8 (15.1%) | 40 (9.9%) | 48 (10.5%) |
| Age, mean ± SD (min–max) | 31.2 ± 11.7 (16–78) | 31.5 ± 12 (15–82) | 31.5 ± 12 (15–82) |
| BMI, mean ± SD (min–max) | 28.4 ± 7.18 (18.3–47.7) | 25.8 ± 5.66 (15.3–56.7) | 26.1 ± 0.28 (15.3–56.7) |
| Total Fat, mean ± SD (min–max) | 423 ± 237 (46–1080) | 214 ± 210(4–1080) | 239 ± 224 (4–1080) |
| Superficial Fat, mean ± SD (min–max) | 357 ± 199 (39–944) | 163 ± 162 (2–763) | 185 ± 178 (2–944) |
| Camper’s Fascia, mean ± SD (min–max) | 192 ± 97 (27–438) | 84 ± 75 (1–449) | 96.6 ± 85.2 (1–449) |
| Camper’s Total Ratio, mean ± SD (min–max) | 0.487 ± 0.103 (0.262–0.698) | 0.455 ± 0.123 (0.122–0.8) | 0.459 ± 0.121(0.122–0.8) |
| Camper’s Superficial Ratio, mean ± SD (min–max) | 0.567 ± 0.0955 (0.338–0.784) | 0.593 ± 0.127 (0.241–1) | 0.59 ± 0.124 (0.241–1) |
| Scarpa’s Fascia, mean ± SD (min–max) | 165 ± 113 (12–506) | 78.5 ± 93.1 (0–479) | 89.3 ± 4.6 (0–506) |
| Scarpa’s Total Ratio, mean ± SD (min–max) | 0.369 ± 0.0853 (0.185–0.621) | 0.31 ± 0.101 (0–0.604) | 0.317 ± 0.101 (0–0.621) |
| Scarpa’s Superficial Ratio, mean ± SD (min–max) | 0.433 ± 0.0955 (0.216–0.662) | 0.407 ± 0.127 (0–0.759) | 0.411 ± 0.124 (0–0.759) |
| Visceral Fat, mean ± SD (min–max) | 66 ± 56.3 (4–205) | 51.6 ± 60.2 (1–316) | 53.3 ± 59.9 (1–316) |
| Visceral Total Ratio, mean ± SD (min–max) | 0.144 ± 0.0879 (0.03–0.376) | 0.234 ± 0.116 (0.036–0.723) | 0.224 ± 0.117 (0.03–0.723) |
| Camper’s: Total Fat Ratio | Female | Male |
|---|---|---|
| Mean | 0.486 | 0.453 |
| SD | 0.1022 | 0.1226 |
| p-value | 0.0656 | |
| 95% CI of Difference | −0.002107 to 0.06704 | |
| Camper’s: Superficial Fat Ratio | Female | Male |
| Mean | 0.5666 | 0.5919 |
| SD | 0.09515 | 0.1262 |
| p-value | 0.1602 | |
| 95% CI | −0.06061 to 0.01004 | |
| Scarpa’s: Total Fat Ratio | Female | Male |
| Mean | 0.3692 | 0.3101 |
| SD | 0.08511 | 0.1006 |
| p-value | <0.0001 | |
| 95% CI of Difference | 0.03067 to 0.08747 | |
| Scarpa’s: Superficial Fat Ratio | Female | Male |
| Mean | 0.4334 | 0.4081 |
| SD | 0.09515 | 0.1262 |
| p-value | 0.1602 | |
| 95% CI of Difference | −0.01004 to 0.06060 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.Z.; Ganapathy, A.; Nayak, Y.; Mejias, C.; Bishop, G.L.; Mellnick, V.M.; Ballard, D.H. Analysis of Superficial Subcutaneous Fat Camper’s and Scarpa’s Fascia in a United States Cohort. J. Cardiovasc. Dev. Dis. 2023, 10, 347. https://doi.org/10.3390/jcdd10080347
Chen DZ, Ganapathy A, Nayak Y, Mejias C, Bishop GL, Mellnick VM, Ballard DH. Analysis of Superficial Subcutaneous Fat Camper’s and Scarpa’s Fascia in a United States Cohort. Journal of Cardiovascular Development and Disease. 2023; 10(8):347. https://doi.org/10.3390/jcdd10080347
Chicago/Turabian StyleChen, David Z., Aravinda Ganapathy, Yash Nayak, Christopher Mejias, Grace L. Bishop, Vincent M. Mellnick, and David H. Ballard. 2023. "Analysis of Superficial Subcutaneous Fat Camper’s and Scarpa’s Fascia in a United States Cohort" Journal of Cardiovascular Development and Disease 10, no. 8: 347. https://doi.org/10.3390/jcdd10080347
APA StyleChen, D. Z., Ganapathy, A., Nayak, Y., Mejias, C., Bishop, G. L., Mellnick, V. M., & Ballard, D. H. (2023). Analysis of Superficial Subcutaneous Fat Camper’s and Scarpa’s Fascia in a United States Cohort. Journal of Cardiovascular Development and Disease, 10(8), 347. https://doi.org/10.3390/jcdd10080347






