Lipid Accumulation Product Is Predictive of Cardiovascular Hospitalizations among Patients with Stable Ischemic Heart Disease: Long-Term Follow-Up of the LAERTES Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Biochemical Measurements
2.3. Lipid Accumulation Product at Baseline
2.4. Follow-Up and Clinical Endpoints
2.5. Statistical Analysis
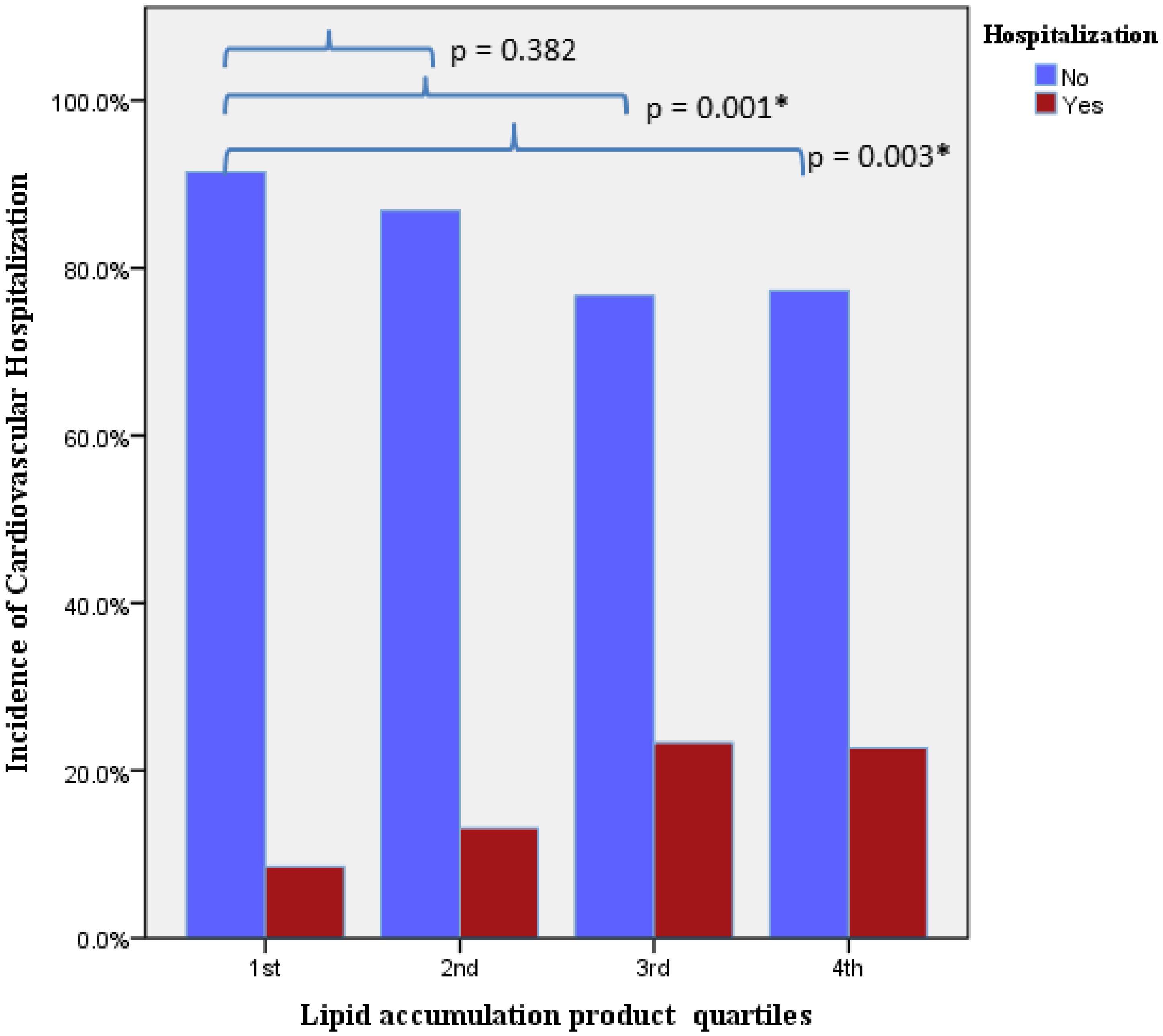
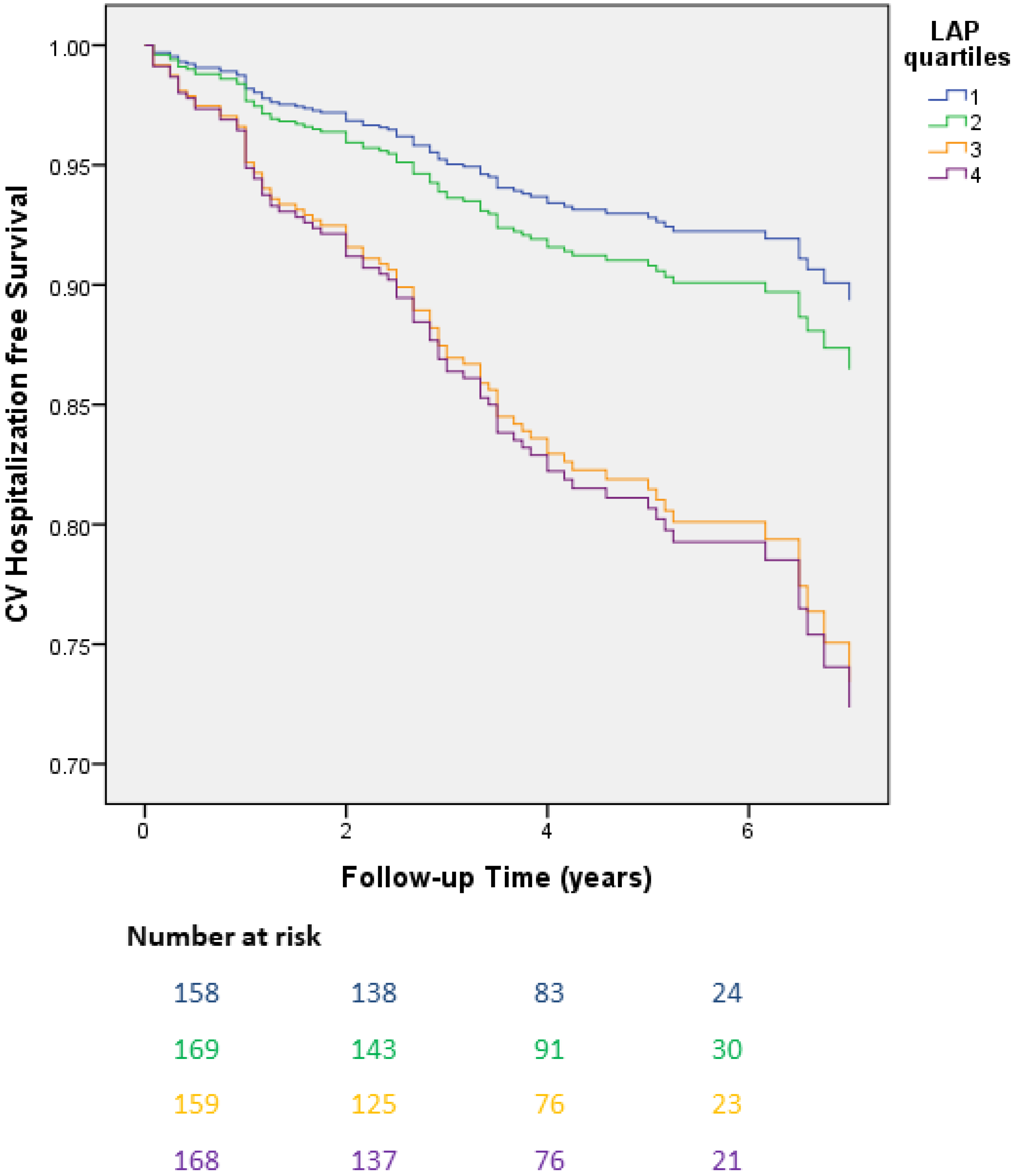
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2021 Update. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Kim, H.J.; Park, S.; Cho, K.-H. Body Mass Index-Related Mortality in Patients with Type 2 Diabetes and Heterogeneity in Obesity Paradox Studies: A Dose-Response Meta-Analysis. PLoS ONE 2017, 12, e0168247. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Kit, B.K.; Orpana, H.; Graubard, B.I. Association of All-Cause Mortality with Overweight and Obesity Using Standard Body Mass Index Categories. JAMA 2013, 309, 71. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Trimarchi, G.; Pizzino, F.; Paradossi, U.; Gueli, I.A.; Palazzini, M.; Gentile, P.; Di Spigno, F.; Ammirati, E.; Garascia, A.; Tedeschi, A.; et al. Charting the Unseen: How Non-Invasive Imaging Could Redefine Cardiovascular Prevention. J. Cardiovasc. Dev. Dis. 2024, 11, 245. [Google Scholar] [CrossRef]
- Duca, F.; Mascherbauer, K.; Donà, C.; Koschutnik, M.; Binder, C.; Nitsche, C.; Halavina, K.; Beitzke, D.; Loewe, C.; Bartko, P.; et al. Association of epicardial adipose tissue on magnetic resonance imaging with cardiovascular outcomes: Quality over quantity? Obesity 2024, 32, 1670–1679. [Google Scholar] [CrossRef]
- Chen, L.Q.; Scheiner, J.; Nashta, N.F.; Weber, J.; Zhou, Q.; Rapelje, K.; Dey, D.; Cao, J.J. Epicardial fat modifies the relationship between coronary calcium score and all-cause mortality: The St. Francis Heart Study. Am. J. Prev. Cardiol. 2024, 19, 100689. [Google Scholar] [CrossRef]
- Xiang, M.; Hu, H.; Imai, T.; Nishihara, A.; Sasaki, N.; Ogasawara, T.; Hori, A.; Nakagawa, T.; Yamamoto, S.; Honda, T.; et al. Association between anthropometric indices of obesity and risk of cardiovascular disease in Japanese men. J. Occup. Health 2020, 62, e12098. [Google Scholar] [CrossRef]
- Goh, L.G.H.; Dhaliwal, S.S.; Welborn, T.A.; Lee, A.H.; Della, P.R. Ethnicity and the association between anthropometric indices of obesity and cardiovascular risk in women: A cross-sectional study. BMJ Open 2014, 4, e004702. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Y.; Wang, N.; Zhou, X.; Jiang, Y.; Wang, W.; Zhao, G. Association between anthropometric indicators of obesity and cardiovascular risk factors among adults in Shanghai, China. BMC Public Health 2019, 19, 1035. [Google Scholar] [CrossRef]
- Papathanasiou, K.; Daios, S.; Kalantzis, C.; Malkots, B.; Leventis, I.; Tasoulas, D.; Rallidis, L. Novel insulin resistance indices could be indicative of an adverse cardiometabolic profile among contemporary patients with acute coronary syndrome: Data from CALLINICUS-Hellas Registry. Atherosclerosis 2024, 395 (Suppl. S1), 117946. [Google Scholar] [CrossRef]
- Kahn, H.S. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: A population-based comparison. BMC Cardiovasc. Disord. 2005, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpanah, F.; Barzin, M.; Mirbolouk, M.; Abtahi, H.; Cheraghi, L.; Azizi, F. Lipid accumulation product and incident cardiovascular events in a normal weight population: Tehran Lipid and Glucose Study. Eur. J. Prev. Cardiol. 2016, 23, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Kyrou, I.; Panagiotakos, D.B.; Kouli, G.-M.; Georgousopoulou, E.; Chrysohoou, C.; Tsigos, C.; Tousoulis, D.; Pitsavos, C. Lipid accumulation product in relation to 10-year cardiovascular disease incidence in Caucasian adults: The ATTICA study. Atherosclerosis 2018, 279, 10–16. [Google Scholar] [CrossRef]
- Jafari, A.; Najafipour, H.; Shadkam, M.; Aminizadeh, S. Evaluation of the novel three lipid indices for predicting five- and ten-year incidence of cardiovascular disease: Findings from Kerman coronary artery disease risk factors study (KERCADRS). Lipids Health Dis. 2023, 22, 169. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, R.; Li, S.; Cai, R.; Ni, F.; Zheng, H.; Hu, R.; Sun, T. Association between Four Anthropometric Indexes and Metabolic Syndrome in US Adults. Front. Endocrinol. 2022, 13, 889785. [Google Scholar] [CrossRef]
- Khanmohammadi, S.; Tavolinejad, H.; Aminorroaya, A.; Rezaie, Y.; Ashraf, H.; Vasheghani-Farahani, A. Association of lipid accumulation product with type 2 diabetes mellitus, hypertension, and mortality: A systematic review and meta-analysis. J. Diabetes Metab. Disord. 2022, 21, 1943–1973. [Google Scholar] [CrossRef]
- Kityo, A.; Lee, S.-A. Association of cardiometabolic factors and insulin resistance surrogates with mortality in participants from the Korean Genome and Epidemiology Study. Lipids Health Dis. 2023, 22, 210. [Google Scholar] [CrossRef]
- Rallidis, L.S.; Tellis, C.C.; Lekakis, J.; Rizos, I.; Varounis, C.; Charalampopoulos, A.; Zolindaki, M.; Dagres, N.; Anastasiou-Nana, M.; Tselepis, A.D. Lipoprotein-Associated Phospholipase A2 Bound on High-Density Lipoprotein Is Associated with Lower Risk for Cardiac Death in Stable Coronary Artery Disease Patients. J. Am. Coll. Cardiol. 2012, 60, 2053–2060. [Google Scholar] [CrossRef]
- Besseling, J.; Kindt, I.; Hof, M.; Kastelein, J.J.; Hutten, B.A.; Hovingh, G.K. Severe heterozygous familial hypercholesterolemia and risk for cardiovascular disease: A study of a cohort of 14,000 mutation carriers. Atherosclerosis 2014, 233, 219–223. [Google Scholar] [CrossRef]
- Zakerkish, M.; Hoseinian, A.; Alipour, M.; Payami, S.P. The Association between Cardio-metabolic and hepatic indices and anthropometric measures with metabolically obesity phenotypes: A cross-sectional study from the Hoveyzeh Cohort Study. BMC Endocr. Disord. 2023, 23, 122. [Google Scholar] [CrossRef] [PubMed]
- Tongdee, P.; Nimkuntod, P. Novel Mathematic Indexes to Identify Subclinical Atherosclerosis in Different Obesity Phenotypes of Perimenopausal/Menopausal Women. J. Med. Assoc. 2016, 99 (Suppl. S7), S62–S68. [Google Scholar]
- Cartolano, F.D.C.; Pappiani, C.; de Freitas, M.C.P.; Neto, A.M.F.; Carioca, A.A.F.; Damasceno, N.R.T. Is Lipid Accumulation Product Associated with an Atherogenic Lipoprotein Profile in Brazilian Subjects? Arq. Bras. Cardiol. 2018, 110, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Gan, S.; Zhou, Q.; Yu, F.; Zhou, H.; Lu, H.; Jin, J.; Liu, Q.; Deng, Z. Positive correlation between lipid accumulation product index and arterial stiffness in Chinese patients with type 2 diabetes. Front. Endocrinol. 2023, 14, 1277162. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Meng, X.; Huang, H.; Jing, J.; Pan, Y.; Mei, L.; Jin, A.; Wang, Y.; Wei, T.; Cai, X. Higher visceral adiposity index and lipid accumulation product in relation to increased risk of atherosclerotic burden in community-dwelling older adults. Exp. Gerontol. 2023, 174, 112115. [Google Scholar] [CrossRef]
- Khan, S.U.; Khan, M.U.; Valavoor, S.; Khan, M.S.; Okunrintemi, V.; Mamas, M.A.; Leucker, T.M.; Blaha, M.J.; Michos, E.D. Association of lowering apolipoprotein B with cardiovascular outcomes across various lipid-lowering therapies: Systematic review and meta-analysis of trials. Eur. J. Prev. Cardiol. 2020, 27, 1255–1268. [Google Scholar] [CrossRef]
- Marston, N.A.; Giugliano, R.P.; Melloni, G.E.M.; Park, J.-G.; Morrill, V.; Blazing, M.A.; Ference, B.; Stein, E.; Stroes, E.S.; Braunwald, E.; et al. Association of Apolipoprotein B–Containing Lipoproteins and Risk of Myocardial Infarction in Individuals with and without Atherosclerosis. JAMA Cardiol. 2022, 7, 250. [Google Scholar] [CrossRef]
- Ioachimescu, A.G.; Brennan, D.M.; Hoar, B.M.; Hoogwerf, B.J. The Lipid Accumulation Product and All-cause Mortality in Patients at High Cardiovascular Risk: A PreCIS Database Study. Obesity 2010, 18, 1836–1844. [Google Scholar] [CrossRef]
- Wehr, E.; Pilz, S.; Boehm, B.O.; März, W.; Obermayer-Pietsch, B. The Lipid Accumulation Product Is Associated with Increased Mortality in Normal Weight Postmenopausal Women. Obesity 2011, 19, 1873–1880. [Google Scholar] [CrossRef]
- Zhao, Q.; Cheng, Y.-J.; Xu, Y.-K.; Zhao, Z.-W.; Liu, C.; Sun, T.-N.; Zhou, Y.-J. Comparison of various insulin resistance surrogates on prognostic prediction and stratification following percutaneous coronary intervention in patients with and without type 2 diabetes mellitus. Cardiovasc. Diabetol. 2021, 20, 190. [Google Scholar] [CrossRef]
- Dong, T.; Lin, W.; Zhou, Q.; Yang, Y.; Liu, X.; Chen, J.; Liu, H.; Zhang, C. Association of adiposity indicators with cardiometabolic multimorbidity risk in hypertensive patients: A large cross-sectional study. Front. Endocrinol. 2024, 15, 1302296. [Google Scholar] [CrossRef] [PubMed]
- Reiner, Z.; Catapano, A.L.; De Backer, G.; Graham, I.; Taskinen, M.R.; Wiklund, O.; Agewall, S.; Alegria, E.; Chapman, M.J.; Durrington, P.N.; et al. ESC/EAS Guidelines for the management of dyslipidaemias: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur. Heart J. 2011, 32, 1769–1818. [Google Scholar] [CrossRef] [PubMed]
| CV Hospitalization | ||||
|---|---|---|---|---|
| Parameter | (N = 770) | Yes (N = 127) | No (N = 643) | p-Value |
| Age (years) | 62 (55–69) | 62 (55–69) | 63 (55–69) | 0.54 |
| Female gender (%) | 13 | 17 | 12 | 0.07 |
| Smoking history (%) | 79 | 78 | 80 | 0.71 |
| Hypertension (%) | 66 | 75 | 64 | 0.006 |
| Diabetes mellitus (%) | 33 | 40 | 32 | 0.06 |
| Hypercholesterolemia (%) | 55 | 58 | 54 | 0.63 |
| FHx premature CAD (%) | 38 | 42 | 37 | 0.07 |
| Prior ACS (%) | 80 | 81 | 79 | 0.11 |
| Prior revascularization (PCI or CABG) [%]. | 77 | 78 | 75 | 0.52 |
| Waist circumference (cm) | 102 ± 12 | 104 ± 11 | 102 ± 12 | 0.03 |
| BMI (kg/m2) | 28 (26–32) | 29 (26–32) | 28 (26–31) | 0.025 |
| Obesity; BMI > 30 Kg/m2 (%) | 33 | 42 | 30 | 0.006 |
| LVEF (%) | 51 (45–60) | 50 (43–60) | 55 (45–60) | 0.63 |
| Total cholesterol (mg/dL) | 161 (138–191) | 160 (140–188) | 158 (136–189) | 0.36 |
| LDL-C (mg/dL) | 95 (78–119) | 94 (80–120) | 95 (76–117) | 0.33 |
| HDL-C (mg/dL) | 41 (35–49) | 40 (34–46) | 42 (36–49) | 0.012 |
| Triglycerides (mg/dL) | 122 (89–173) | 134 (92–191) | 120 (89–170) | 0.03 |
| HbA1c (%) | 6.5 (5.8–8.1) | 6.5 (5.9–8.5) | 6.5 (5.7–7.7) | 0.29 |
| Lp(a) (mg/dL) | 13 (6–30) | 11 (7–28) | 13 (6–30) | 0.85 |
| LAP (cm × mmol/L) | 50.6 (32.7–80.3) | 60 (38.8–97.6) | 47 (31.8–80) | 0.002 |
| ASA (%) | 86 | 88 | 86 | 0.68 |
| Clopidogrel (%) | 18 | 16 | 19 | 0.75 |
| ACEi (%) | 58 | 59 | 58 | 0.87 |
| Beta-blocker (%) | 83 | 83 | 83 | 0.88 |
| Statin (%) | 89 | 89 | 89 | 0.97 |
| Ezetimibe (%) | 8 | 6 | 10 | 0.14 |
| Multivessel CAD (%) | 60.1 | 66.6 | 58.6 | 0.19 |
| CV Hospitalization | ||||
|---|---|---|---|---|
| Total (N = 770) | Yes (N = 127) | No (N = 643) | p-Value | |
| Primary Endpoint Components | 16.5 | |||
| ACS (%) | 12.4 | 75 | 0 | <0.001 |
| Non-fatal stroke (%) | 1.5 | 9 | 0 | <0.001 |
| Ventricular arrhythmias (%) | 2.6 | 16 | 0 | <0.001 |
| Medical treatment | ||||
| Antiplatelets (%) | 95 | 96 | 94 | 0.73 |
| LLT (%) | 91 | 90 | 91 | 0.96 |
| Beta-blockers (%) | 81.5 | 92 | 79 | 0.13 |
| ACEi (%) | 75.4 | 92 | 71 | 0.04 |
| Smoking persistence (%) | 21.6 | 27 | 20 | 0.46 |
| LDL-C < 70 mg/dL (%) | 17 | 13 | 17 | 0.12 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Parameter | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| LVEF (per %) | 0.98 | 0.97–1.00 | 0.07 | |||
| Smoking persistence | 1.45 | 0.60–3.49 | 0.40 | |||
| Hypertension | 1.68 | 1.16–2.41 | 0.005 | 1.57 | 1.03–2.39 | 0.03 |
| Diabetes mellitus | 1.35 | 0.98–1.88 | 0.06 | |||
| Hypercholesterolemia | 1.00 | 0.99–1.00 | 0.58 | |||
| Lipoprotein(a) (per mg/dL) | 1.00 | 0.99–1.00 | 0.86 | |||
| Triglycerides (per mg/dL) | 1.00 | 1.00–1.00 | 0.12 | |||
| HDL-C (per mg/dL) | 0.96 | 0.92–1.10 | 0.09 | |||
| Baseline BMI (per kg/m2) | 1.06 | 1.02–1.09 | 0.001 | 1.01 | 0.95–1.07 | 0.65 |
| BMI at follow-up (per kg/m2) | 1.01 | 0.97–1.06 | 0.45 | |||
| Waist (per cm) | 1.01 | 1.00–1.03 | 0.01 | 0.99 | 0.96–1.01 | 0.48 |
| Obese (BMI > 30 kg/m2) | 1.59 | 1.14–2.2 | 0.05 | 1.27 | 0.85–1.88 | 0.23 |
| LAP quartile (4th vs. 1st) | 2.36 | 1.37–4.04 | 0.02 | 2.20 | 1.12–4.34 | 0.02 |
| Age (per year) | 0.99 | 0.98–1.01 | 0.90 | |||
| Female gender | 0.71 | 0.47–1.07 | 0.10 | |||
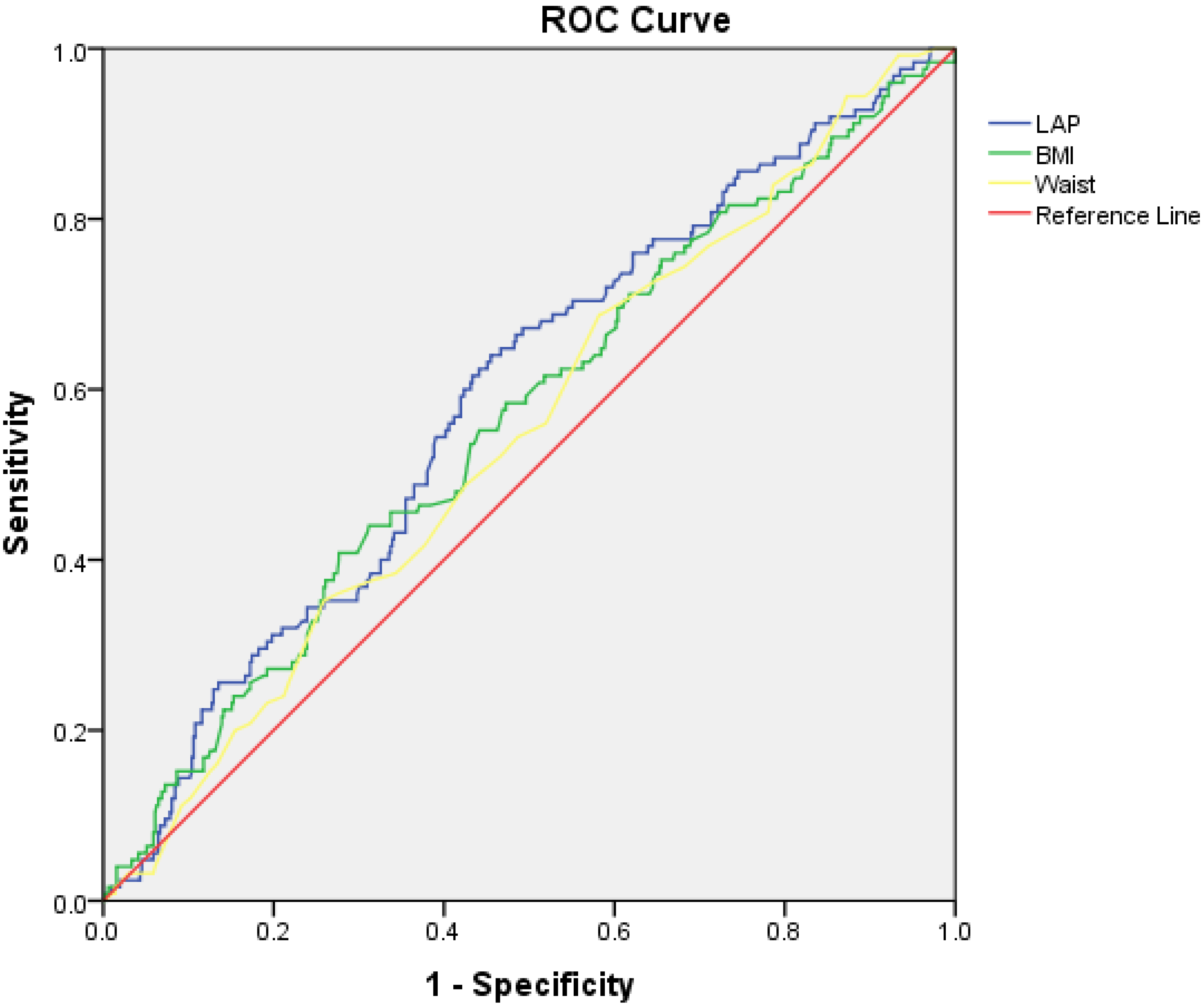
| Variable | Area under the Curve | 95% Confidence Intervals | p-Value |
|---|---|---|---|
| LAP | 0.59 | 0.53–0.64 | 0.002 |
| BMI | 0.56 | 0.50–0.62 | 0.026 |
| Waist circumference | 0.54 | 0.49–0.60 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papathanasiou, K.A.; Roussos, C.E.; Armylagos, S.; Rallidis, S.L.; Rallidis, L.S. Lipid Accumulation Product Is Predictive of Cardiovascular Hospitalizations among Patients with Stable Ischemic Heart Disease: Long-Term Follow-Up of the LAERTES Study. J. Cardiovasc. Dev. Dis. 2024, 11, 316. https://doi.org/10.3390/jcdd11100316
Papathanasiou KA, Roussos CE, Armylagos S, Rallidis SL, Rallidis LS. Lipid Accumulation Product Is Predictive of Cardiovascular Hospitalizations among Patients with Stable Ischemic Heart Disease: Long-Term Follow-Up of the LAERTES Study. Journal of Cardiovascular Development and Disease. 2024; 11(10):316. https://doi.org/10.3390/jcdd11100316
Chicago/Turabian StylePapathanasiou, Konstantinos A., Christos Eleftherios Roussos, Stylianos Armylagos, Stylianos L. Rallidis, and Loukianos S. Rallidis. 2024. "Lipid Accumulation Product Is Predictive of Cardiovascular Hospitalizations among Patients with Stable Ischemic Heart Disease: Long-Term Follow-Up of the LAERTES Study" Journal of Cardiovascular Development and Disease 11, no. 10: 316. https://doi.org/10.3390/jcdd11100316
APA StylePapathanasiou, K. A., Roussos, C. E., Armylagos, S., Rallidis, S. L., & Rallidis, L. S. (2024). Lipid Accumulation Product Is Predictive of Cardiovascular Hospitalizations among Patients with Stable Ischemic Heart Disease: Long-Term Follow-Up of the LAERTES Study. Journal of Cardiovascular Development and Disease, 11(10), 316. https://doi.org/10.3390/jcdd11100316







