Effect of Aerobic Exercise with Blood Flow Restriction on Postexercise Hypotension in Young Adults: The Role of Histamine Receptors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Procedures
2.2. Exercise Protocol
2.3. Measurement of Hemodynamic Responses
2.4. Statistical Analysis
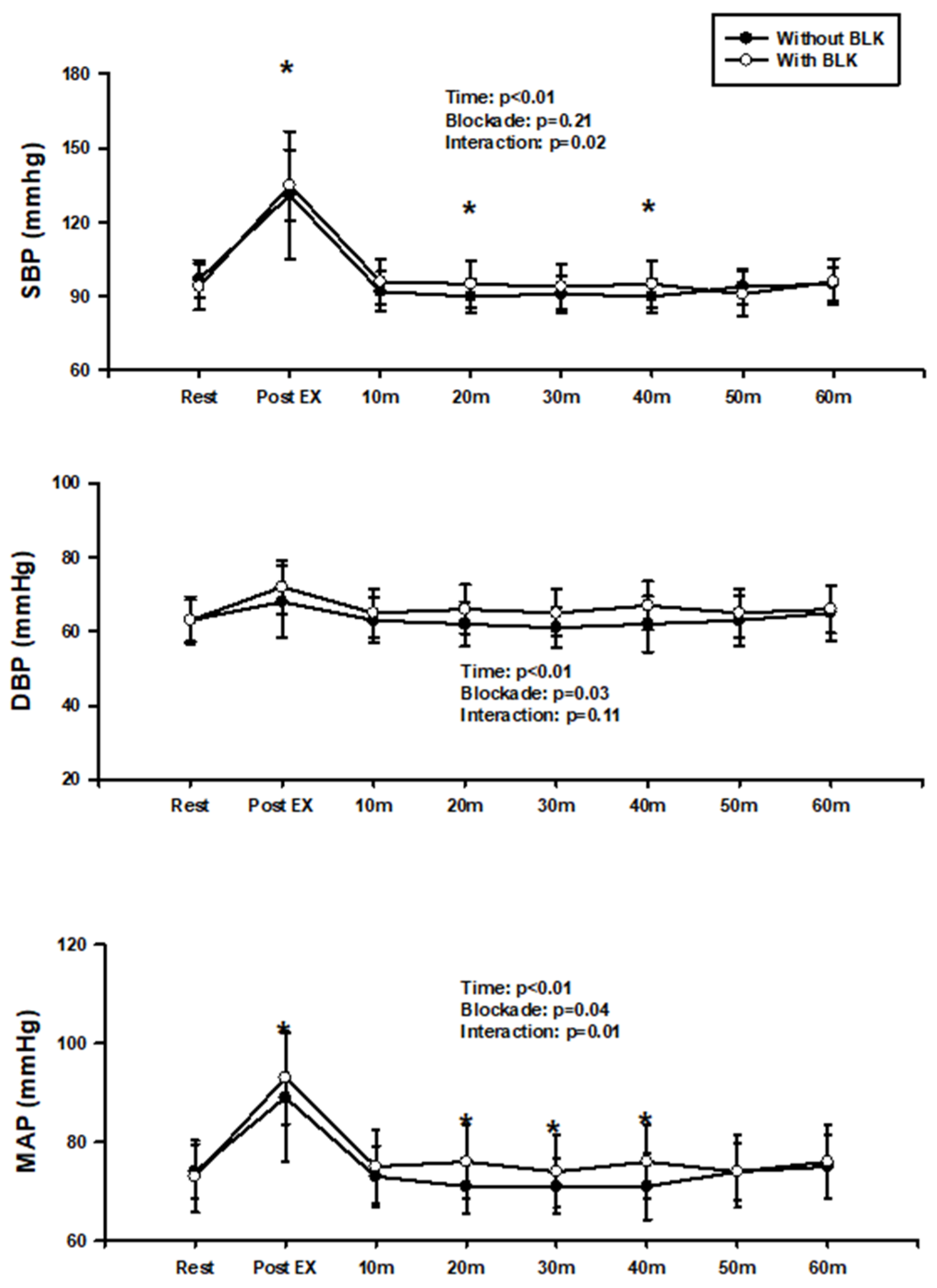
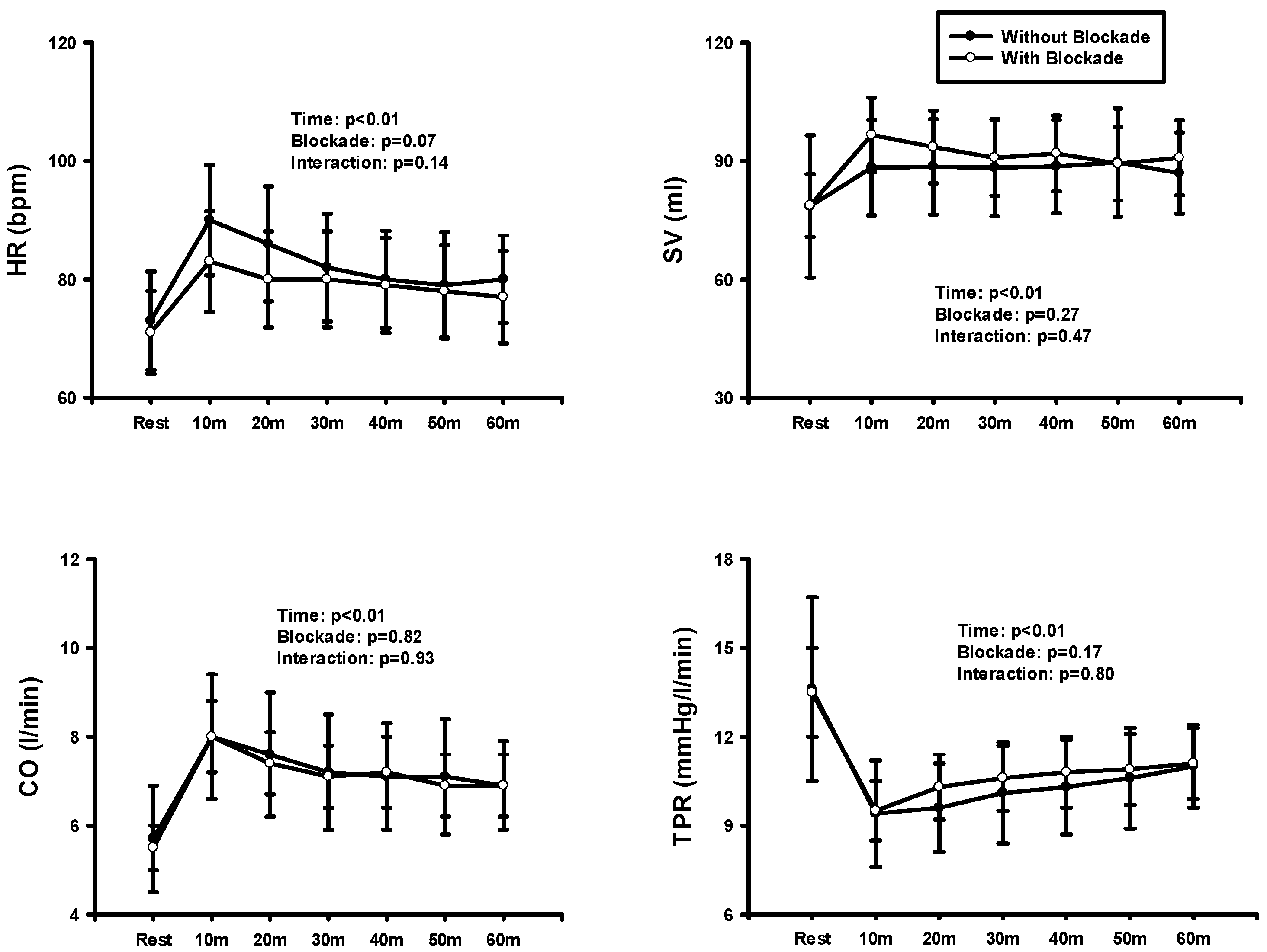
3. Results
4. Discussion
4.1. Limitations of the Study
4.2. Perspective and Significance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Bocalini, D.S.; Bergamin, M.; Evangelista, A.L.; Rica, R.L.; Pontes, F.L.J.; Figueira, A.J.; Serra, A.J.; Rossi, E.M.; Tucci, P.J.F.; Dos Santos, L. Post-exercise hypotension and s variability response after water- and land-ergometry exercise in hypertensive patients. PLoS ONE 2017, 12, e0180216. [Google Scholar] [CrossRef] [PubMed]
- Krzesiak, A.; Lavoie, J.L.; Sebille, S.; Cognard, C.; Bosquet, L.; Delpech, N. Post-exercise hypotension in male spontaneously hypertensive rats: The issue of calculation method. Physiol. Rep. 2023, 11, e15524. [Google Scholar] [CrossRef] [PubMed]
- Mora-Rodriguez, R.; Ortega, J.F.; Morales-Palomo, F.; Ramirez-Jimenez, M.; Moreno-Cabanas, A.; Alvarez-Jimenez, L. Endurance exercise training reduces blood pressure according to the Wilder’s principle. Int. J. Sports Med. 2022, 43, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Kenney, M.J.; Seals, D.R. Postexercise hypotension. Key features, mechanisms, and clinical significance. Hypertension 1993, 22, 653–664. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J.R.; MacDougall, J.D.; Interisano, S.A.; Smith, K.M.; McCartney, N.; Moroz, J.S.; Younglai, E.V.; Tarnopolsky, M.A. Hypotension following mild bouts of resistance exercise and submaximal dynamic exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 79, 148–154. [Google Scholar] [CrossRef]
- Rueckert, P.A.; Slane, P.R.; Lillis, D.L.; Hanson, P. Hemodynamic patterns and duration of post-dynamic exercise hypotension in hypertensive humans. Med. Sci. Sports Exerc. 1996, 28, 24–32. [Google Scholar] [CrossRef]
- Wallace, J.P.; Bogle, P.G.; King, B.A.; Krasnoff, J.B.; Jastremski, C.A. A comparison of 24-h average blood pressures and blood pressure load following exercise. Am. J. Hypertens. 1997, 10, 728–734. [Google Scholar] [CrossRef]
- Manini, T.M.; Clark, B.C. Blood flow restricted exercise and skeletal muscle health. Exerc. Sport. Sci. Rev. 2009, 37, 78–85. [Google Scholar] [CrossRef]
- Abe, T.; Kearns, C.F.; Manso Filho, H.C.; Sato, Y.; McKeever, K.H. Muscle, tendon, and somatotropin responses to the restriction of muscle blood flow induced by KAATSU-walk training. Equine Vet. J. 2006, 38, 345–348. [Google Scholar] [CrossRef]
- Cook, S.B.; Clark, B.C.; Ploutz-Snyder, L.L. Effects of exercise load and blood-flow restriction on skeletal muscle function. Med. Sci. Sports Exerc. 2007, 39, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Takarada, Y.; Nakamura, Y.; Aruga, S.; Onda, T.; Miyazaki, S.; Ishii, N. Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. J. Appl. Physiol. 2000, 88, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Takarada, Y.; Takazawa, H.; Sato, Y.; Takebayashi, S.; Tanaka, Y.; Ishii, N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J. Appl. Physiol. 2000, 88, 2097–2106. [Google Scholar] [CrossRef]
- Yamanaka, T.; Farley, R.S.; Caputo, J.L. Occlusion training increases muscular strength in division IA football players. J. Strength. Cond. Res. 2012, 26, 2523–2529. [Google Scholar] [CrossRef] [PubMed]
- Domingos, E.; Polito, M.D. Blood pressure response between resistance exercise with and without blood flow restriction: A systematic review and meta-analysis. Life Sci. 2018, 209, 122–131. [Google Scholar] [CrossRef]
- Maior, A.S.; Simao, R.; Martins, M.S.; de Salles, B.F.; Willardson, J.M. Influence of Blood Flow Restriction During Low-Intensity Resistance Exercise on the Postexercise Hypotensive Response. J. Strength. Cond. Res. 2015, 29, 2894–2899. [Google Scholar] [CrossRef]
- Picon, M.M.; Chulvi, I.M.; Cortell, J.T.; Tortosa, J.; Alkhadar, Y.; Sanchis, J.; Laurentino, G. Acute Cardiovascular Responses after a Single Bout of Blood Flow Restriction Training. Int. J. Exerc. Sci. 2018, 11, 20–31. [Google Scholar]
- Araujo, J.P.; Silva, E.D.; Silva, J.C.; Souza, T.S.; Lima, E.O.; Guerra, I.; Sousa, M.S. The acute effect of resistance exercise with blood flow restriction with hemodynamic variables on hypertensive subjects. J. Hum. Kinet. 2014, 43, 79–85. [Google Scholar] [CrossRef]
- Jeong, H.J.; Moon, P.D.; Kim, S.J.; Seo, J.U.; Kang, T.H.; Kim, J.J.; Kang, I.C.; Um, J.Y.; Kim, H.M.; Hong, S.H. Activation of hypoxia-inducible factor-1 regulates human histidine decarboxylase expression. Cell Mol. Life Sci. 2009, 66, 1309–1319. [Google Scholar] [CrossRef]
- Savany, A.; Cronenberger, L. Properties of histidine decarboxylase from rat gastric mucosa. Eur. J. Biochem. 1982, 123, 593–599. [Google Scholar] [CrossRef]
- Schayer, R.W. Histamine and Hyperaemia of Muscular Exercise. Nature 1964, 201, 195. [Google Scholar] [CrossRef]
- Halliwill, J.R.; Buck, T.M.; Lacewell, A.N.; Romero, S.A. Postexercise hypotension and sustained postexercise vasodilatation: What happens after we exercise? Exp. Physiol. 2013, 98, 7–18. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.L.; Beasley, J.M.; Halliwill, J.R. H2-receptor-mediated vasodilation contributes to postexercise hypotension. J. Appl. Physiol. 2006, 100, 67–75. [Google Scholar] [CrossRef]
- Romero, S.A.; Minson, C.T.; Halliwill, J.R. The cardiovascular system after exercise. J. Appl. Physiol. 2017, 122, 925–932. [Google Scholar] [CrossRef]
- Ely, M.R.; Ratchford, S.M.; La Salle, D.T.; Trinity, J.D.; Wray, D.W.; Halliwill, J.R. Effect of histamine-receptor antagonism on leg blood flow during exercise. J. Appl. Physiol. 2020, 128, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.M.; Stebbins, C.L.; Lee, O.T.; Nho, H.; Lee, J.H.; Chun, J.M.; Kim, K.A.; Kim, J.K. Augmentation of the exercise pressor reflex in prehypertension: Roles of the muscle metaboreflex and mechanoreflex. Appl. Physiol. Nutr. Metab. 2013, 38, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, M.J.; Conway, L.; Warmington, S.A. Blood flow restriction walking and physical function in older adults: A randomized control trial. J. Sci. Med. Sport 2017, 20, 1041–1046. [Google Scholar] [CrossRef]
- Ely, M.R.; Mangum, J.E.; Needham, K.W.; Minson, C.T.; Halliwill, J.R. Effect of histamine-receptor antagonism on the circulating inflammatory cell and cytokine response to exercise: A pilot study. Physiol. Rep. 2024, 12, e15936. [Google Scholar] [CrossRef]
- Garg, D.C.; Eshelman, F.N.; Weidler, D.J. Pharmacokinetics of ranitidine following oral administration with ascending doses and with multiple-fixed doses. J. Clin. Pharmacol. 1985, 25, 437–443. [Google Scholar] [CrossRef]
- Russell, T.; Stoltz, M.; Weir, S. Pharmacokinetics, pharmacodynamics, and tolerance of single- and multiple-dose fexofenadine hydrochloride in healthy male volunteers. Clin. Pharmacol. Ther. 1998, 64, 612–621. [Google Scholar] [CrossRef]
- Charloux, A.; Lonsdorfer-Wolf, E.; Richard, R.; Lampert, E.; Oswald-Mammosser, M.; Mettauer, B.; Geny, B.; Lonsdorfer, J. A new impedance cardiograph device for the non-invasive evaluation of cardiac output at rest and during exercise: Comparison with the “direct” Fick method. Eur. J. Appl. Physiol. 2000, 82, 313–320. [Google Scholar] [CrossRef]
- Neto, G.R.; Sousa, M.S.C.; Costa, P.B.; Salles, B.F.; Novaes, G.S.; Novaes, J.S. Hypotensive effects of resistance exercises with blood flow restriction. J. Strength. Cond. Res. 2015, 29, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, R.; Miao, J.H.; Rehman, A. Physiology, Cardiovascular. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024; pp. 1–19. [Google Scholar]
- Halliwill, J.R.; Taylor, J.A.; Eckberg, D.L. Impaired sympathetic vascular regulation in humans after acute dynamic exercise. J. Physiol. 1996, 495 Pt 1, 279–288. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.L.; Halliwill, J.R. H1 and H2 receptors mediate postexercise hyperemia in sedentary and endurance exercise-trained men and women. J. Appl. Physiol. 2006, 101, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Halliwill, J.R.; Minson, C.T.; Joyner, M.J. Effect of systemic nitric oxide synthase inhibition on postexercise hypotension in humans. J. Appl. Physiol. 2000, 89, 1830–1836. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, J.M.; Pricher, M.P.; Wilkins, B.W.; Holowatz, L.A.; Halliwill, J.R. Postexercise hypotension is not explained by a prostaglandin-dependent peripheral vasodilation. J. Appl. Physiol. 2005, 98, 447–453. [Google Scholar] [CrossRef]
- Hecksteden, A.; Grutters, T.; Meyer, T. Association between postexercise hypotension and long-term training-induced blood pressure reduction: A pilot study. Clin. J. Sport. Med. 2013, 23, 58–63. [Google Scholar] [CrossRef]
- Liu, S.; Goodman, J.; Nolan, R.; Lacombe, S.; Thomas, S.G. Blood pressure responses to acute and chronic exercise are related in prehypertension. Med. Sci. Sports Exerc. 2012, 44, 1644–1652. [Google Scholar] [CrossRef]

| Variables | (n = 10) |
|---|---|
| Age (yrs) | 23.3 ± 2.8 |
| Height (cm) | 175.2 ± 6.5 |
| Weight (kg) | 72.2 ± 8.6 |
| Body mass index (kg/m2) | 23.6 ± 2.9 |
| SBP (mmHg) | 96 ± 6.5 |
| DBP (mmHg) | 63 ± 6.0 |
| MAP (mmHg) | 74 ± 5.5 |
| Values | (n = 10) | |
|---|---|---|
| Control | Blockade | |
| HR (bpm) | 73 ± 8.3 | 71 ± 7.0 |
| SV (mL) | 79.8 ± 18.0 | 78.7 ± 7.9 |
| CO (L/min) | 5.8 ± 1.2 | 5.5 ± 0.5 |
| SBP (mmHg) | 98 ± 8 | 95 ± 9 |
| DBP (mmHg) | 64 ± 6 | 63 ± 6 |
| MAP (mmHg) | 75 ± 6 | 73 ± 7 |
| TPR (mmHg/L/min) | 13.6 ± 3.1 | 13.5 ± 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, D.; Song, D.S.; Boyer, W.; Gillum, T.; Sullivan, S.; Liwanag, N.; Yoon, I.; Kim, J.-K. Effect of Aerobic Exercise with Blood Flow Restriction on Postexercise Hypotension in Young Adults: The Role of Histamine Receptors. J. Cardiovasc. Dev. Dis. 2024, 11, 326. https://doi.org/10.3390/jcdd11100326
Seo D, Song DS, Boyer W, Gillum T, Sullivan S, Liwanag N, Yoon I, Kim J-K. Effect of Aerobic Exercise with Blood Flow Restriction on Postexercise Hypotension in Young Adults: The Role of Histamine Receptors. Journal of Cardiovascular Development and Disease. 2024; 11(10):326. https://doi.org/10.3390/jcdd11100326
Chicago/Turabian StyleSeo, Dongnyeuck, Dae Sik Song, William Boyer, Trevor Gillum, Sean Sullivan, Nailiyah Liwanag, Iltark Yoon, and Jong-Kyung Kim. 2024. "Effect of Aerobic Exercise with Blood Flow Restriction on Postexercise Hypotension in Young Adults: The Role of Histamine Receptors" Journal of Cardiovascular Development and Disease 11, no. 10: 326. https://doi.org/10.3390/jcdd11100326
APA StyleSeo, D., Song, D. S., Boyer, W., Gillum, T., Sullivan, S., Liwanag, N., Yoon, I., & Kim, J.-K. (2024). Effect of Aerobic Exercise with Blood Flow Restriction on Postexercise Hypotension in Young Adults: The Role of Histamine Receptors. Journal of Cardiovascular Development and Disease, 11(10), 326. https://doi.org/10.3390/jcdd11100326






