Prenatal Diagnosis of a Ductal-Dependent Branch Pulmonary Artery: Extra Vessels in the 3-Vessel and Trachea View
Abstract
1. Introduction
2. Cases
2.1. Case 1
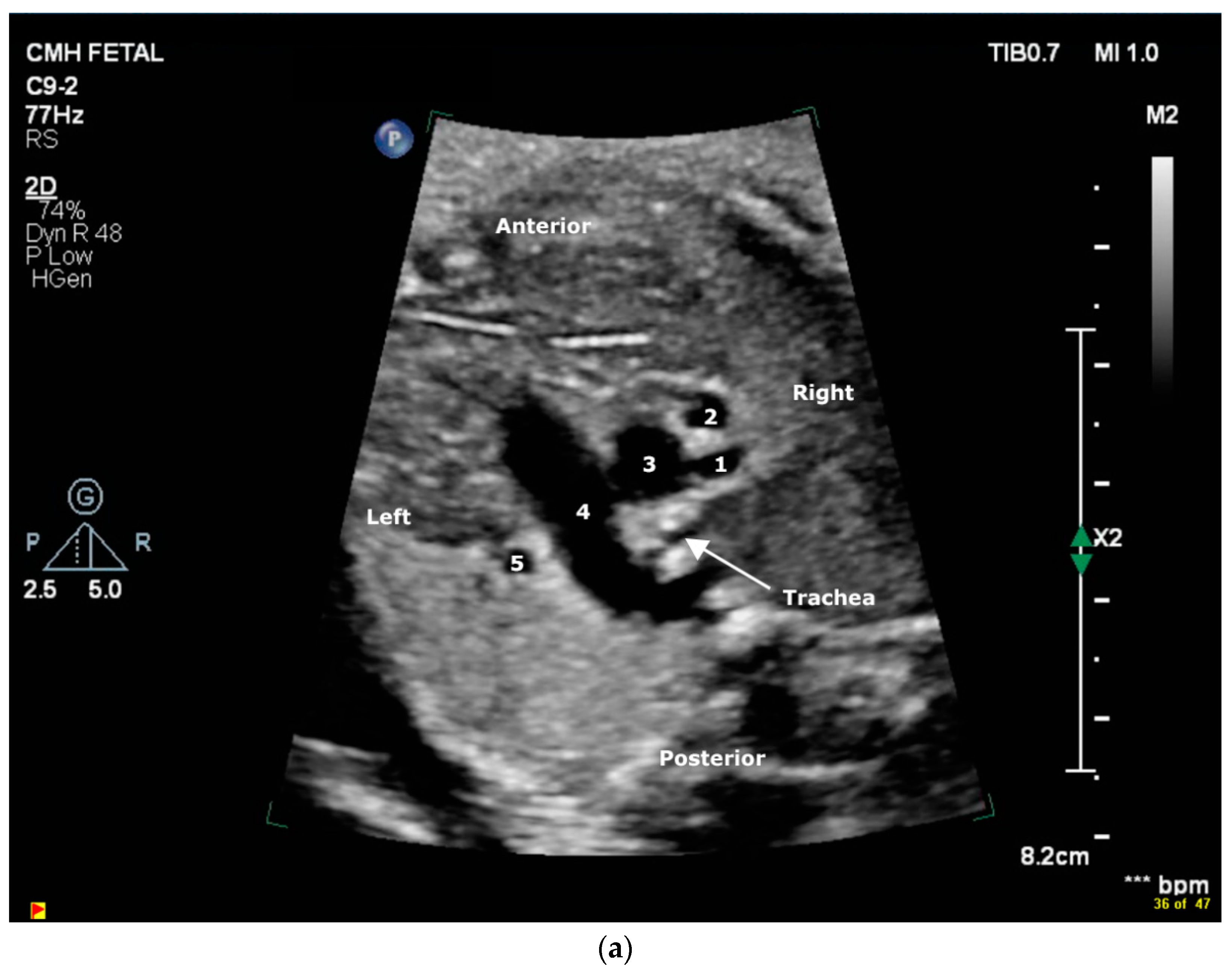
2.2. Case 2
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedberg, M.K.; Silverman, N.H.; Moon-Grady, A.J.; Tong, E.; Nourse, J.; Sorenson, B.; Lee, J.; Hornberger, L.K. Prenatal detection of congenital heart disease. J. Pediatr. 2009, 155, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Tworetzky, W.; McElhinney, D.B.; Reddy, V.M.; Brook, M.M.; Hanley, F.L.; Silverman, N.H. Improved surgical outcome after fetal diagnosis of hypoplastic left heart syndrome. Circulation 2001, 103, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.J.; Lee, Y.H.; Kim, E.S.; Ryu, H.M.; Kim, M.Y.; Choi, H.K.; Cho, K.S.; Kim, A. Three-vessel view of the fetal upper mediastinum: An easy means of detecting abnormalities of the ventricular outflow tracts and great arteries during obstetric screening. Ultrasound Obstet. Gynecol. 1997, 9, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.S.; Wang, E.; Rychik, J.; Soffer, D.; McCann, M.L.; Schwartz, N. Utility of a Single 3-Vessel View in the Evaluation of the Ventricular Outflow Tracts. J. Ultrasound Med. 2015, 34, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- International Society of Ultrasound in Obstetrics and Gynecology; Carvalho, J.S.; Allan, L.D.; Chaoui, R.; Copel, J.A.; DeVore, G.R.; Hecher, K.; Lee, W.; Munoz, H.; Paladini, D.; et al. ISUOG Practice Guidelines (updated): Sonographic screening examination of the fetal heart. Ultrasound Obstet. Gynecol. 2013, 41, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Peirone, A.; Abdullah, M.M.; Dicke, F.; Freedom, R.M.; Smallhorn, J. Echocardiographic evaluation, management and outcomes of bilateral arterial ducts and complex congenital heart disease: 16 years’ experience. Cardiol. Young 2002, 12, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Ten Harkel, A.; Blom, N.A.; Ottenkamp, J. Isolated unilateral absence of a pulmonary artery: A case report and review of the literature. Chest 2002, 122, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Freedom, R.M.; Moes, C.A.F.; Pelech, A.; Smallhorn, J.; Rabinovitch, M.; Olley, P.M.; Williams, W.G.; Trusler, G.A.; Rowe, R.D. Bilateral ductus arteriosus (or remnant): An analysis of 27 patients. Am. J. Cardiol. 1984, 53, 884–891. [Google Scholar] [CrossRef]
- Edwards, J.E. Anomalies of the derivatives of the aortic arch system. Med. Clin. N. Am. 1948, 32, 925–945. [Google Scholar] [CrossRef]
- Priya, S.; Nagpal, P. Atretic Double Aortic Arch: Imaging Appearance of a Rare Anomaly and Differentiation From Its Mimics. Cureus 2020, 12, e9478. [Google Scholar] [CrossRef] [PubMed]
- Acherman, R.J.; Smallhorn, J.F.; Freedom, R.M. Echocardiographic assessment of pulmonary blood supply in patients with pulmonary atresia and ventricular septal defect. J. Am. Coll. Cardiol. 1996, 28, 1308–1313. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aggarwal, N.; Sreedhar, R.; Gadhinglajkar, S.V.; Dharan, B.S.; Babu, S.; Pillai, M.N.; Menon, S. Intraoperative Diagnosis of Major Aortopulmonary Collateral Arteries by Transesophageal Echocardiography. J. Cardiothorac. Vasc. Anesth. 2018, 32, 1794–1799. [Google Scholar] [CrossRef] [PubMed]
- Welch, K.; Hanley, F.; Johnston, T.; Cailes, C.; Shah, M.J. Isolated unilateral absence of the right proximal pulmonary artery: Surgical repair and follow-up. Ann. Thorac. Surg. 2005, 79, 1399–1402. [Google Scholar] [CrossRef] [PubMed]
- Sreeram, N.; Asante-Korang, A.; Ladusans, E. Distal ductal origin of the right pulmonary artery: Prospective diagnosis and primary repair in infancy. Int. J. Cardiol. 1992, 35, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, K.R.; Karamlou, T.; Yoo, S.-J.; Williams, W.G.; Freedom, R.M.; McCrindle, B.M. Outcomes in 45 children with ductal origin of the distal pulmonary artery. Ann. Thorac. Surg. 2006, 81, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Fontana, G.P.; Spach, M.S.; Effmann, E.L.; Sabiston, D.C., Jr. Origin of the right pulmonary artery from the ascending aorta. Ann. Surg. 1987, 206, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Kruzliak, P.; Syamasundar, R.P.; Novak, M.; Pechanova, O.; Kovacova, G. Unilateral absence of pulmonary artery: Pathophysiology, symptoms, diagnosis and current treatment. Arch. Cardiovasc. Dis. 2013, 106, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Koga, H.; Hidaka, T.; Miyako, K.; Suga, N.; Takahashi, N. Age-related clinical characteristics of isolated congenital unilateral absence of a pulmonary artery. Pediatr. Cardiol. 2010, 31, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Mery, C.M.; Molina, K.M.; Krishnamurthy, R.; Fraser, C.D., Jr.; Justino, H. Pulmonary artery resuscitation for isolated ductal origin of a pulmonary artery. J. Thorac. Cardiovasc. Surg. 2014, 148, 2235–2244.e1. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.; Quinn, R.; Moran, A.; Donnelly, J. Ductal origin of the pulmonary artery in isolation: A case series. Pediatr. Cardiol. 2010, 31, 997–1001. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goyal, A.; Kiaffas, M.; Swanson, T.; Kathol, M.J.; Shah, S.; Madan, N. Prenatal Diagnosis of a Ductal-Dependent Branch Pulmonary Artery: Extra Vessels in the 3-Vessel and Trachea View. J. Cardiovasc. Dev. Dis. 2024, 11, 55. https://doi.org/10.3390/jcdd11020055
Goyal A, Kiaffas M, Swanson T, Kathol MJ, Shah S, Madan N. Prenatal Diagnosis of a Ductal-Dependent Branch Pulmonary Artery: Extra Vessels in the 3-Vessel and Trachea View. Journal of Cardiovascular Development and Disease. 2024; 11(2):55. https://doi.org/10.3390/jcdd11020055
Chicago/Turabian StyleGoyal, Anmol, Maria Kiaffas, Tara Swanson, Melanie J. Kathol, Sanket Shah, and Nitin Madan. 2024. "Prenatal Diagnosis of a Ductal-Dependent Branch Pulmonary Artery: Extra Vessels in the 3-Vessel and Trachea View" Journal of Cardiovascular Development and Disease 11, no. 2: 55. https://doi.org/10.3390/jcdd11020055
APA StyleGoyal, A., Kiaffas, M., Swanson, T., Kathol, M. J., Shah, S., & Madan, N. (2024). Prenatal Diagnosis of a Ductal-Dependent Branch Pulmonary Artery: Extra Vessels in the 3-Vessel and Trachea View. Journal of Cardiovascular Development and Disease, 11(2), 55. https://doi.org/10.3390/jcdd11020055





