Impact of Intracranial Volume and Brain Volume on the Prognostic Value of Computed Tomography Perfusion Core Volume in Acute Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
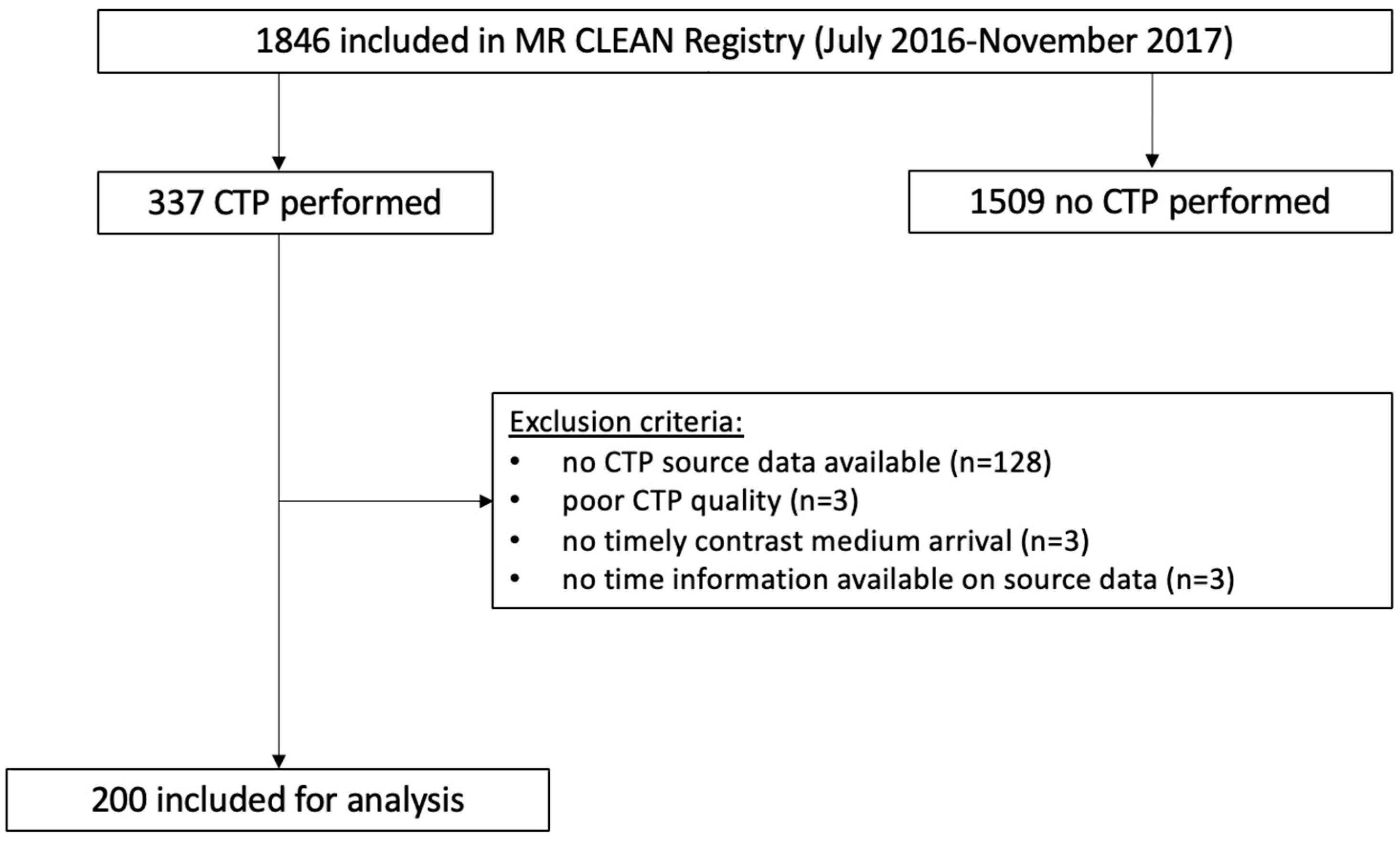
2.1. Patient Selection
2.2. Baseline Image Acquisition, Post-Processing, and Quality Assessment
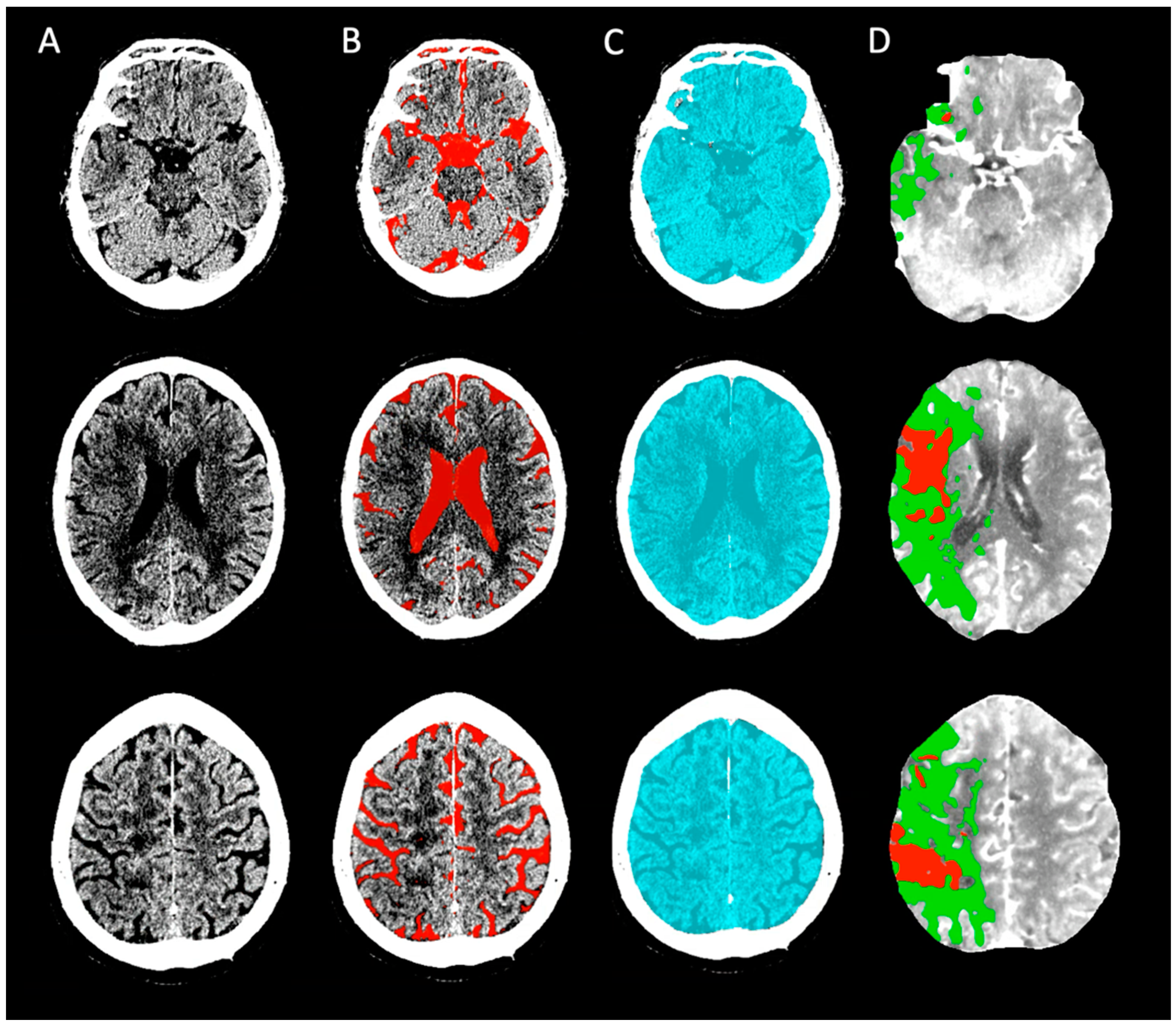
2.3. ICV and Cerebrospinal Fluid (CSF) Assessment
2.4. Statistical Analyses
2.5. Protocol Approval and Patient Consent
2.6. Data Availability
3. Results
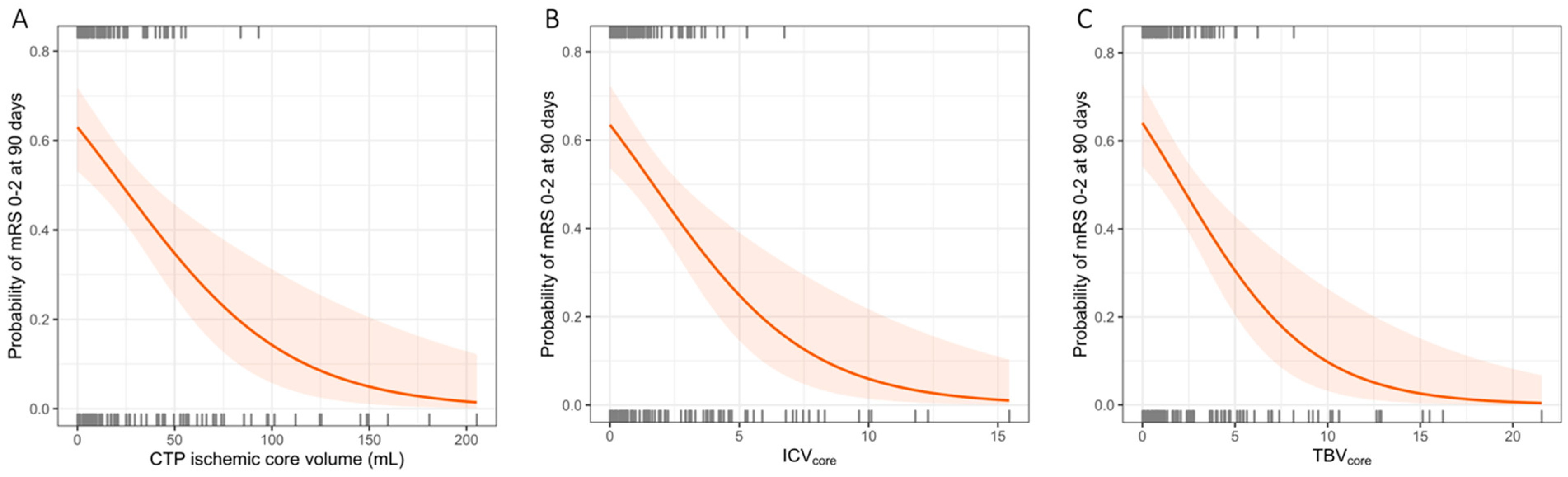
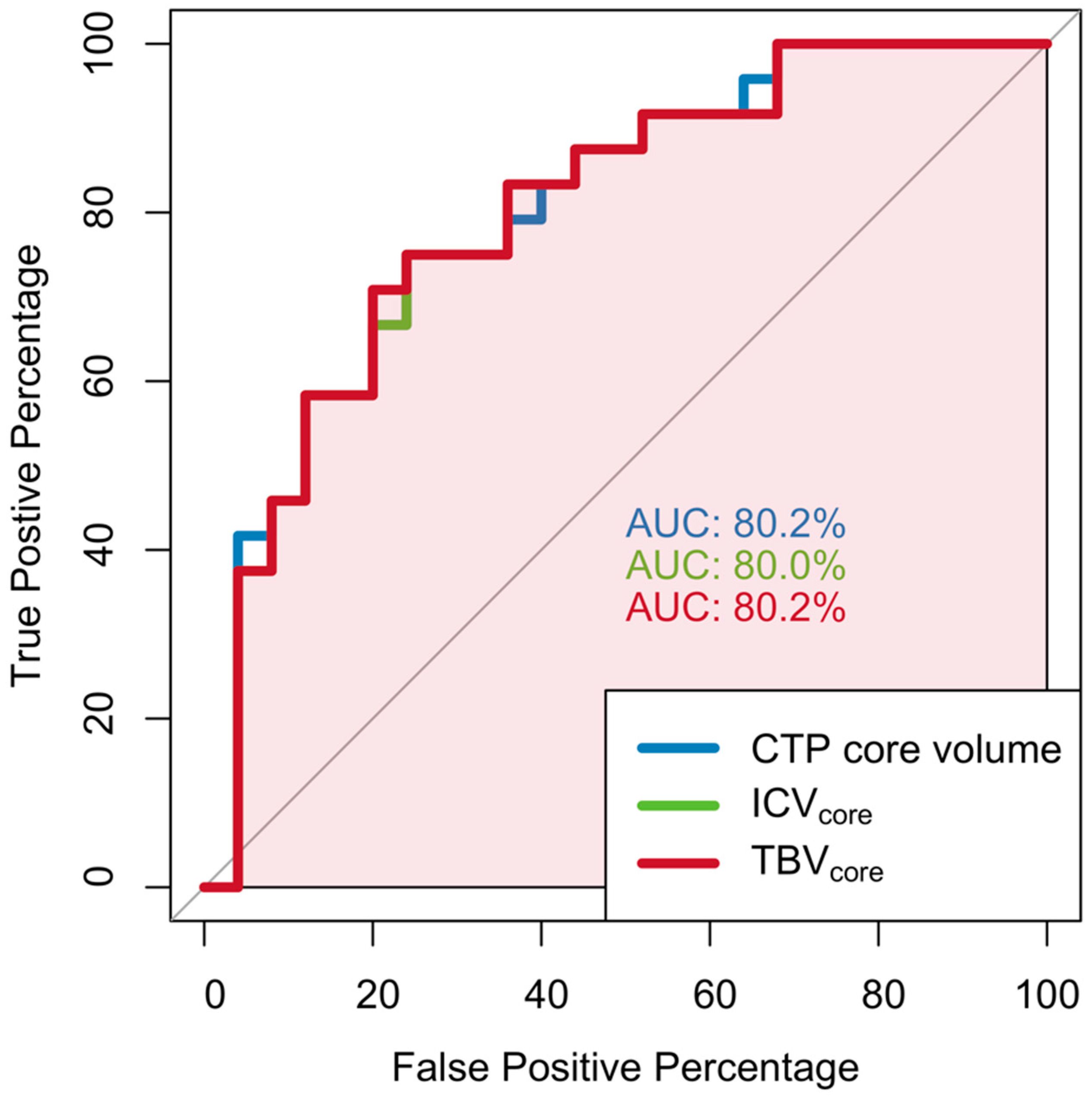
3.1. Associations between CTP-Estimated Core Volume, ICV, TBV, and Functional Outcome
3.2. Associations between CTP-Estimated Core Volume, ICV, TBV, and Functional Independence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Demeestere, J.; Wouters, A.; Christensen, S.; Lemmens, R.; Lansberg, M.G. Review of perfusion imaging in acute ischemic stroke: From time to tissue. Stroke 2020, 51, 1017–1024. [Google Scholar] [CrossRef]
- Koopman, M.S.; Hoving, J.W.; Kappelhof, M.; Berkhemer, O.A.; Beenen, L.F.M.; van Zwam, W.H.; de Jong, H.W.A.M.; Dankbaar, J.W.; Dippel, D.W.J.; Coutinho, J.M.; et al. Association of Ischemic Core Imaging Biomarkers With Post-Thrombectomy Clinical Outcomes in the MR CLEAN Registry. Front. Neurol. 2022, 12, 771367. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; Majoie, C.B.L.M.; Albers, G.W.; Menon, B.K.; Yassi, N.; Sharma, G.; van Zwam, W.H.; van Oostenbrugge, R.J.; Demchuk, A.M.; Guillemin, F.; et al. Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: A meta-analysis of individual patient-level data. Lancet Neurol. 2019, 18, 46–55. [Google Scholar] [CrossRef]
- Schirmer, M.D.; Donahue, K.L.; Nardin, M.J.; Dalca, A.V.; Giese, A.-K.; Etherton, M.R.; Mocking, S.J.; McIntosh, E.C.; Cole, J.W.; Holmegaard, L.; et al. Brain Volume: An Important Determinant of Functional Outcome After Acute Ischemic Stroke. Mayo Clin. Proc. 2020, 95, 955–965. [Google Scholar] [CrossRef]
- Ganesan, V.; Ng, V.; Chong, W.K.; Kirkham, F.J.; Connelly, A. Lesion volume, lesion location, and outcome after middle cerebral artery territory stroke. Arch. Dis. Child. 1999, 81, 295–300. [Google Scholar] [CrossRef]
- Diprose, W.K.; Diprose, J.P.; Wang, M.T.; Tarr, G.P.; McFetridge, A.; Barber, P.A. Automated Measurement of Cerebral Atrophy and Outcome in Endovascular Thrombectomy. Stroke 2019, 50, 3636–3638. [Google Scholar] [CrossRef] [PubMed]
- Pedraza, M.I.; de Lera, M.; Bos, D.; Calleja, A.I.; Cortijo, E.; Gómez-Vicente, B.; Reyes, J.; Coco-Martín, M.B.; Calonge, T.; Agulla, J.; et al. Brain Atrophy and the Risk of Futile Endovascular Reperfusion in Acute Ischemic Stroke. Stroke 2020, 51, 1514–1521. [Google Scholar] [CrossRef] [PubMed]
- Lauksio, I.; Lindström, I.; Khan, N.; Sillanpää, N.; Hernesniemi, J.; Oksala, N.; Protto, S. Brain atrophy predicts mortality after mechanical thrombectomy of proximal anterior circulation occlusion. J. NeuroInterventional Surg. 2020, 13, 415–420. [Google Scholar] [CrossRef]
- Johnson, S.C.; Saykin, A.J.; Baxter, L.C.; Flashman, L.A.; Santulli, R.B.; McAllister, T.W.; Mamourian, A.C. The Relationship between fMRI Activation and Cerebral Atrophy: Comparison of Normal Aging and Alzheimer Disease. NeuroImage 2000, 11, 179–187. [Google Scholar] [CrossRef]
- Minnerup, J.; Wersching, H.; Ringelstein, E.B.; Heindel, W.; Niederstadt, T.; Schilling, M.; Schäbitz, W.-R.; Kemmling, A. Prediction of malignant middle cerebral artery infarction using computed tomography-based intracranial volume reserve measurements. Stroke 2011, 42, 3403–3409. [Google Scholar] [CrossRef]
- Kijonka, M.; Borys, D.; Psiuk-Maksymowicz, K.; Gorczewski, K.; Wojcieszek, P.; Kossowski, B.; Marchewka, A.; Swierniak, A.; Sokol, M.; Bobek-Billewicz, B. Whole Brain and Cranial Size Adjustments in Volumetric Brain Analyses of Sex- and Age-Related Trends. Front. Neurosci. 2020, 14, 278. [Google Scholar] [CrossRef]
- Dieleman, N.; Koek, H.L.; Hendrikse, J. Short-term mechanisms influencing volumetric brain dynamics. NeuroImage Clin. 2017, 16, 507–513. [Google Scholar] [CrossRef]
- Jansen, I.G.H.; Mulder, M.J.H.L.; Goldhoorn, R.-J.B. Endovascular treatment for acute ischaemic stroke in routine clinical practice: Prospective, observational cohort study (MR CLEAN Registry). BMJ 2018, 360, k949. [Google Scholar] [CrossRef]
- Koopman, M.S.; A Berkhemer, O.; Geuskens, R.R.E.G.; Emmer, B.J.; A A van Walderveen, M.; Jenniskens, S.F.M.; van Zwam, W.H.; van Oostenbrugge, R.J.; van der Lugt, A.; Dippel, D.W.J.; et al. Comparison of three commonly used CT perfusion software packages in patients with acute ischemic stroke. J. NeuroInterventional Surg. 2019, 11, 1249–1256. [Google Scholar] [CrossRef]
- Liebeskind, D.S.; Bracard, S.; Guillemin, F.; Jahan, R.; Jovin, T.G.; Majoie, C.B.; Mitchell, P.J.; van der Lugt, A.; Menon, B.K.; Román, L.S.; et al. eTICI reperfusion: Defining success in endovascular stroke therapy. J. NeuroInterventional Surg. 2019, 11, 433–438. [Google Scholar] [CrossRef]
- Brudfors, M.; Balbastre, Y.; Flandin, G.; Nachev, P.; Ashburner, J. Flexible Bayesian Modelling for Nonlinear Image Registration. In Lecture Notes in Computer Science, Proceedings of the Medical Image Computing and Computer Assisted Intervention–MICCAI 2020, Lima, Peru, 4–8 October 2020; Martel, A.L., Abolmaesumi, P., Stoyanov, D., Mateus, D., Zuluaga, M.A., Zhou, S.K., Racoceanu, D., Joskowicz, L., Eds.; Springer: Cham, Switzerland, 2020; Volume 12263. [Google Scholar] [CrossRef]
- van Swieten, J.C.; Koudstaal, P.J.; Visser, M.C.; Schouten, H.J.; van Gijn, J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988, 19, 604–607. [Google Scholar] [CrossRef]
- Grund, B.; Sabin, C. Analysis of biomarker data: Logs, odds ratios, and receiver operating characteristic curves. Curr. Opin. HIV AIDS 2010, 5, 473–479. [Google Scholar] [CrossRef]
- Kauw, F.; E Bernsen, M.L.; Dankbaar, J.W.; de Jong, H.W.; Kappelle, L.J.; Velthuis, B.K.; van der Worp, H.B.; van der Lugt, A.; Roos, Y.B.; Yo, L.S.; et al. Cerebrospinal fluid volume improves prediction of malignant edema after endovascular treatment of stroke. Int. J. Stroke 2022, 18, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Luijten, S.P.; Compagne, K.C.; van Es, A.C.; Roos, Y.B.; Majoie, C.B.; van Oostenbrugge, R.J.; van Zwam, W.H.; Dippel, D.W.; Wolters, F.J.; van der Lugt, A.; et al. Brain atrophy and endovascular treatment effect in acute ischemic stroke: A secondary analysis of the MR CLEAN trial. Int. J. Stroke 2021, 17, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Kaginele, P.; Beer-Furlan, A.; Joshi, K.C.; Kadam, G.; Achanaril, A.; Levy, E.; Waqas, M.; Siddiqui, A.; Rai, H.; Snyder, K.; et al. Brain Atrophy and Leukoaraiosis Correlate with Futile Stroke Thrombectomy. J. Stroke Cerebrovasc. Dis. 2021, 30, 105871. [Google Scholar] [CrossRef] [PubMed]
- Bu, N.; Khlif, M.S.; Lemmens, R.; Wouters, A.; Fiebach, J.B.; Chamorro, A.; Ringelstein, E.B.; Norrving, B.; Laage, R.; Grond, M.; et al. Imaging Markers of Brain Frailty and Outcome in Patients With Acute Ischemic Stroke. Stroke 2021, 52, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- McDonough, R.V.; Ospel, J.M.; Campbell, B.C.; Hill, M.D.; Saver, J.L.; Dippel, D.W.; Demchuk, A.M.; Majoie, C.B.; Brown, S.B.; Mitchell, P.J.; et al. Functional Outcomes of Patients ≥85 Years With Acute Ischemic Stroke Following EVT: A HERMES Substudy. Stroke 2022, 53, 2220–2226. [Google Scholar] [CrossRef]
| MR CLEAN Registry CTP Subgroup (n = 200) | Overall MR CLEAN Registry (n = 1755) | |
|---|---|---|
| Clinical | ||
| Age (yr)—median (IQR) | 71 (56–80) | 72 (62–81) |
| Female—n (%) | 83 (42) | 889 (51) |
| NIHSS at baseline—median (IQR) [known in] | 16 (12–20) [n = 197] | 16 (11–19) |
| Transfer from primary stroke center—n (%) | 19 (10) | 962 (55) |
| IVT administered—n (%) | 144 (72) | 1282 (73) |
| Onset-to-imaging time (min) *—median (IQR) [known in] | 79 (56–137) [N = 194] | 76(53–128) [N = 1279] |
| Onset-to-groin time (min)—median (IQR) [known in] | 153 (120–223) [N = 196] | 185(144–243) [N = 1740] |
| Imaging | ||
| Occlusion location on baseline CTA—n (%) [known in] Intracranial ICA ICA-T M1 M2 Other | [N = 198] 6 (3) 35 (18) 121 (61) 35 (18) 1 (1) | [N = 1657] 76 (4) 342 (20) 974 (56) 295 (17) 6 (0.3) |
| ASPECTS—median (IQR) [known in] | 9 (8–10) [N = 199] | 9 (8–10) [N = 1713] |
| Collateral status—n (%) [known in] 0 1 2 3 | [N = 197] 8 (4) 79 (40) 82 (42) 28 (14) | [N = 1693] 89 (5) 635 (36) 643 (37) 290 (17) |
| Baseline ischemic core volume on CTP (mL)—median (IQR) | 13 (5–41) | NA |
| Baseline penumbra volume on CTP (mL)—median (IQR) | 96 (58–123) | NA |
| Intracranial volume (ICV) (mL)—median (IQR) Males Females | 1377 (1283–1456) 1435 (1378–1502) 1280 (1224–1330) | NA |
| Total brain volume (TBV) (mL)—median (IQR) Males Females | 1109 (1020–1196) 1170 (1106–1233) 1020 (976–1082) | NA |
| CSF volume (mL)—median (IQR) Males Females | 258 (229–296) 266 (237–303) 246 (216–286) | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoving, J.W.; Konduri, P.R.; Tolhuisen, M.L.; Koopman, M.S.; van Voorst, H.; Van Poppel, L.M.; Daems, J.D.; van Es, A.C.G.M.; van Walderveen, M.A.A.; Lingsma, H.F.; et al. Impact of Intracranial Volume and Brain Volume on the Prognostic Value of Computed Tomography Perfusion Core Volume in Acute Ischemic Stroke. J. Cardiovasc. Dev. Dis. 2024, 11, 80. https://doi.org/10.3390/jcdd11030080
Hoving JW, Konduri PR, Tolhuisen ML, Koopman MS, van Voorst H, Van Poppel LM, Daems JD, van Es ACGM, van Walderveen MAA, Lingsma HF, et al. Impact of Intracranial Volume and Brain Volume on the Prognostic Value of Computed Tomography Perfusion Core Volume in Acute Ischemic Stroke. Journal of Cardiovascular Development and Disease. 2024; 11(3):80. https://doi.org/10.3390/jcdd11030080
Chicago/Turabian StyleHoving, Jan W., Praneeta R. Konduri, Manon L. Tolhuisen, Miou S. Koopman, Henk van Voorst, Laura M. Van Poppel, Jasper D. Daems, Adriaan C. G. M. van Es, Marianne A. A. van Walderveen, Hester F. Lingsma, and et al. 2024. "Impact of Intracranial Volume and Brain Volume on the Prognostic Value of Computed Tomography Perfusion Core Volume in Acute Ischemic Stroke" Journal of Cardiovascular Development and Disease 11, no. 3: 80. https://doi.org/10.3390/jcdd11030080
APA StyleHoving, J. W., Konduri, P. R., Tolhuisen, M. L., Koopman, M. S., van Voorst, H., Van Poppel, L. M., Daems, J. D., van Es, A. C. G. M., van Walderveen, M. A. A., Lingsma, H. F., Dippel, D. W. J., Van Zwam, W. H., Marquering, H. A., Majoie, C. B. L. M., & Emmer, B. J., on behalf of the MR CLEAN Registry Investigators. (2024). Impact of Intracranial Volume and Brain Volume on the Prognostic Value of Computed Tomography Perfusion Core Volume in Acute Ischemic Stroke. Journal of Cardiovascular Development and Disease, 11(3), 80. https://doi.org/10.3390/jcdd11030080








