Abstract
Background: Recent studies have investigated the effects of exercise on the functional capacity of older adults; training with a balance exercise assist robot (BEAR) effectively improves posture. This study compared the clinical safety and efficacy of training using BEAR video games to conventional resistance training in older adults with cardiovascular disease (CVD). Methods: Ninety patients (mean age: 78 years) hospitalized due to worsening CVD were randomized to cardiac rehabilitation (CR) Group R (conventional resistance training) or Group B (training using BEAR). After appropriate therapy, patients underwent laboratory testing and functional evaluation using the timed up-and-go test (TUG), short physical performance battery (SPPB), and functional independence measure (FIM) just before discharge and 4 months after CR. The rates of CVD readmission, cardiac death, and fall-related fractures were monitored. Results: BEAR had no adverse effects during exercise. At 4 months, TUG and SPPB improved significantly in both groups, with no significant difference between them. FIM motor and the Geriatric Nutritional Risk Index were significantly improved in Group B versus Group R. There was no significant difference in cardiac events and fall-related fractures between the two groups. Conclusion: CR with BEAR is safe and comparable to conventional resistance training for improving balance in older adults with CVD.
1. Introduction
Older adults now represent a growing sector of the population since they are living longer, and the world’s population is increasingly ageing [1]. However, scientific evidence for the chronic care of older adults is still lacking, and the assessment of and therapeutic intervention for frailty is a particularly urgent issue. Specifically, in addition to preventing the occurrence of cardiovascular events in patients with cardiovascular disease (CVD), it is necessary to prevent declines in the activities of daily living due to fractures caused by falls, which are particularly frequent in older adults. Falling is known to be influenced not only by muscle strength, but also by balance [2]. Therefore, as part of an industry–academic collaboration, we have developed the balance exercise assist robot (BEAR), a balance-focused exercise therapy, as an intervention for older adults with CVD and verified its effectiveness in preventing patient frailty, as reported elsewhere [3,4,5]. Briefly, BEAR is a stand-up-and-ride transport robot and personal mobility device with a two-wheel inverted balance. When the rider leans forward, the wheels rotate in the same direction, and the robot moves forward until the rider’s body returns to a vertical position [5].
The balance training device we are developing and supporting the demonstration of is characterized by the use of inverted pendulum control robot technology to facilitate the learning of postural control strategies. The motors are controlled to keep the user upright by constantly detecting the user’s posture with sensors.
Recent research has focused on the effects of on in improving the functional capacity of frail older adults [4,6]. Training with BEAR is effective in improving posture. However, little information is available regarding the safety and efficacy of BEAR during cardiac rehabilitation (CR) in older adults with CVD [4]. Therefore, the aim of the present study was to compare the clinical safety and efficacy of postural strategy training using the BEAR system with those of conventional resistance training during CR in older adults with CVD.
2. Materials and Methods
2.1. Study Population
This study was approved by the Nagoya University Certified Clinical Research Review Board and was registered with the Japan Registry of Clinical Trials (registration no. jRCTs042190086). This study was approved by the Ethics Review Board of Nagoya University approved the study (Approval no. 2020) and complied with the Declaration of Helsinki. Written informed consent was obtained from each participant.
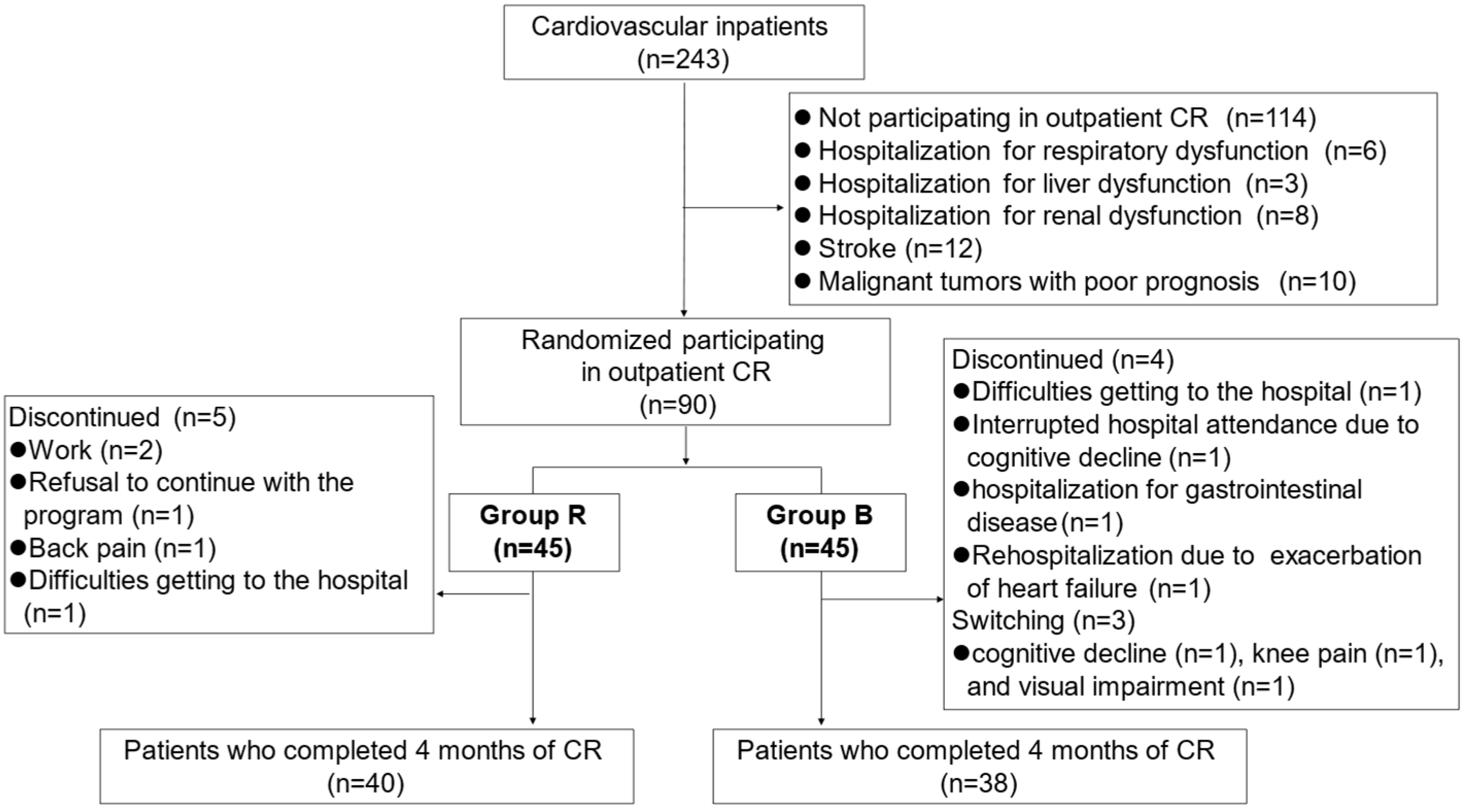
This prospective interventional study involved 243 patients who were at least 65 years old and who had been hospitalized for worsening CVD at the Department of Cardiology, National Center for Geriatrics and Gerontology (Obu, Japan), between December 2019 and December 2021. Figure 1 shows a flowchart of participant enrollment.
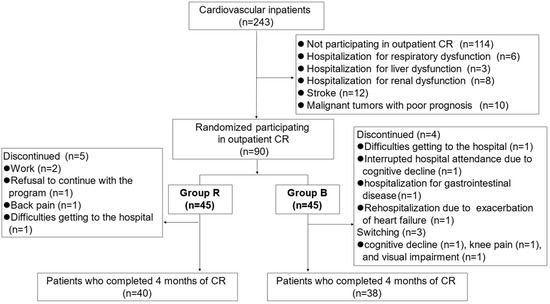
Figure 1.
Flowchart of participant enrollment.
Participants performed a cardiopulmonary exercise test (CPX); underwent laboratory measurements, including GNRI, which is a screening tool used to assess nutritional status in older people and is primarily based on serum albumin and BMI [7], echocardiography, and a physical function evaluation; and completed questionnaires, including the Functional Independence Measure (FIM), Geriatric Depression Scale (GDS), and Japanese version of the Montreal Cognitive Assessment (MoCA-J), once their condition had stabilized after appropriate therapy.
Patients with structural heart disease, including coronary artery disease (i.e., having experienced angina pectoris or myocardial infarction, with a history of revascularization procedures); symptomatic heart failure (non-ischemic cardiomyopathy, ischemia, tachycardia, bradycardia, valvular disease, or hypertension); or other CVD (aortic disease, peripheral artery disease, and other vascular diseases), were eligible for inclusion in the study. Non-ischemic cardiomyopathies were defined as ventricular myocardial abnormalities in the absence of coronary artery disease or valvular, pericardial, or congenital heart disease [8]. Tachycardia and bradycardia included atrial, supraventricular, and ventricular arrhythmias, sick sinus syndrome, and atrioventricular block in the absence of structural heart disease. Valvular heart disease was diagnosed on the basis of hemodynamic or echocardiographic findings or a history of valvular or congenital cardiac surgery, according to the American College of Cardiology/American Heart Association guidelines [9]. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or a history of treatment for hypertension. Worsening heart failure was defined as a clinical syndrome comprising symptoms and/or signs due to structural and/or functional cardiac abnormalities accompanied by elevated natriuretic peptide concentrations and/or objective evidence of pulmonary or systemic congestion [10].
Patients with severe respiratory dysfunction (i.e., receiving long-term oxygen therapy for respiratory disease), liver dysfunction (Child–Pugh score Class C), stroke, renal dysfunction (reduced glomerular filtration rate and albuminuria category G5), malignant tumors with a prognosis of <1 year, and visual or hearing impairments that could interfere with playing the video games were excluded from the study. Patients in whom CR was contraindicated as per the Guidelines for Rehabilitation in Patients with Cardiovascular Disease of the Japanese Circulation Society [11] and patients who did not understand how to operate the BEAR system were also excluded from the study. After applying both inclusion and exclusion criteria, we finally included 90 consecutive CVD patients (Figure 1).
2.2. Balance Exercise Assist Robot
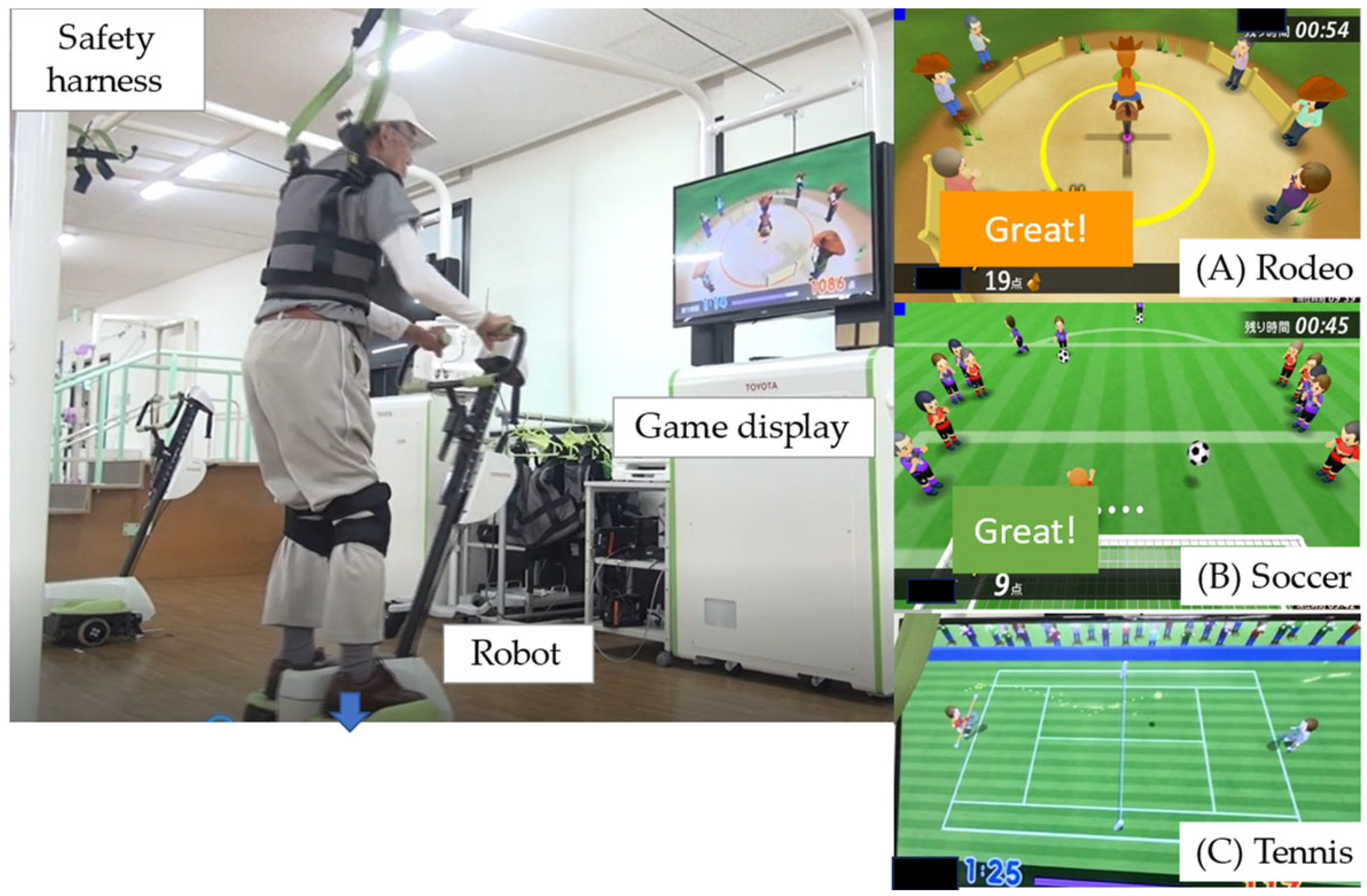
The BEAR (Toyota Motor Corporation, Aichi, Japan) used in the present study had two wheels, an in-wheel motor controlled by an inverted pendulum system, and a foot plate on either side (Figure 2; Supplementary Video S1), as shown in our previous study [4]. The BEAR moves backwards and forwards and left and right according to shifts in the operator’s center of gravity. Balance exercise that promotes movement in these four directions is achieved by playing three video games, each of which focuses on different skills: (1) a skiing game, which requires left–right movement; (2) a rodeo game, which requires the operator to keep the robot stationary in the face of irregular disturbances; and (3) a tennis game, which involves forward–backward movement (Figure 2). The level of difficulty was adjusted to suit each individual, and the users performed the repetitive movements automatically [12]. To ensure safety during the exercises, we prepared a 2.4 m × 2.0 m space in the exercise room for the exclusive use of participants. The participants wore a safety harness to limit the risk of falls, but no lifting force was applied to reduce weight bearing.
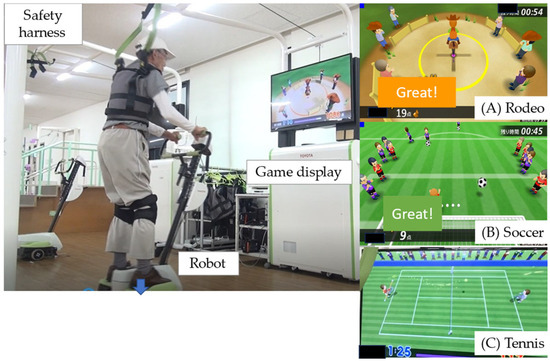
Figure 2.
The balance exercise assist robot (BEAR) system. The BEAR moves backwards and forwards, as well as left and right, according to shifts in the operator’s center of gravity. These movements can be incorporated into balance exercises by playing three video games: (A) rodeo; (B) soccer; and (C) tennis.
2.3. CR Program
A 4-month prospective blinded randomized single-center trial was conducted. Randomization was stratified, with allocation adjusted for gender parity. To control for information bias, a prospective randomized open-label blinded end-point (PROBE) study design was used, with evaluations performed by physical therapists not involved in the study.
Examinations were performed when the patients were in a stable condition, after appropriate therapy for their disease. The intervention was performed on an outpatient basis for 1 h once a week for 4 months, for a total of 16 sessions. After 4 months, all patients were reassessed. The efficacy and safety of BEAR were tested in a randomized controlled trial (RCT) using the two-group randomization method described below. Patients were assigned at the start of CR and randomized 1:1 to either conventional and established resistance training (Group R) or BEAR (Group B).
2.3.1. Resistance Training Group (Group R)
Group R received resistance training as usual CR. Patients performed exercise (30 min aerobic exercise and 30 min resistance training), and were evaluated before and 4 months after the start of training. Resistance training was performed to improve muscular endurance and muscular strength by loading peripheral skeletal muscles and was adapted to individual muscular strength using body weight and tubes.
2.3.2. BEAR Group (Group B)
Patients performed 30 min aerobic exercise using an ergometer plus 30 min balance exercises with the BEAR system. Each session of balance training with the BEAR system consisted of twelve episodes: four rounds of each of the three games, with each round lasting 1.5 min.
2.4. Measurements
Peak oxygen uptake (VO2peak), gait speed (10 m gait test) the Short Physical Performance Battery (SPPB) score, timed up-and-go (TUG) time, and knee extensor strength were assessed just prior to discharge, when patients were medically stable, and at the end of the 4-month intervention period.
2.4.1. Frailty
Frailty was defined according to the Japanese version of the Cardiovascular Health Study criteria (J-CHS) [13]. The J-CHS assesses five components, namely weight loss, physical activity, fatigue, muscle weakness, and gait speed, and it classifies people as being frail, pre-frail, or robust. Frailty is defined as the presence of signs or symptoms associated with at least three of the five components; pre-frailty is defined as the presence of signs or symptoms consistent with one or two components; and robust is defined as having no characteristics attributable to any of the components.
2.4.2. Peak Oxygen Uptake
Each patient underwent a CPX (Minato Medical Science, Osaka, Japan) on a cycle ergometer at a progressively increasing work rate to maximum tolerance. The CPX was conducted in accordance with the recommendations of the American Thoracic Society and American College of Chest Physicians [14]. Gas exchange data were obtained breath by breath, and VO2peak was determined as the highest oxygen uptake value recorded.
2.4.3. Gait Speed
To determine gait speed, we used the 10 m gait test. Participants were instructed to walk twice along a 16 m walkway at a comfortable pace [15]. The time taken to cover 10 m along the walkway was measured and used to calculate gait speed.
2.4.4. Short Physical Performance Battery
The SPPB evaluates lower limb function [16] through three components: balance tests (closed leg standing, semitandem standing, and tandem standing), walking time, and standing from a seated position. The reliability, validity, and feasibility of the SPPB in older adults have been reported elsewhere [17]. The maximum SPPB score is 12 points; the higher the score, the better the physical function.
2.4.5. Timed Up-and-Go
The TUG time includes the time required for a participant to stand up from an armchair, walk 3 m, turn, return to the chair, and sit down again [18].
2.4.6. Muscle Strength of Knee Extension
The strength of knee extension was measured using a handheld dynamometer (µ-tas F-1; Anima, Tokyo, Japan). Knee extension was tested while the participant was seated, with the knee and hip flexed at 90° [19]. Each strength test was performed twice, and the best result was recorded.
2.4.7. Functional Independence Measure
The FIM was developed to evaluate the rehabilitation of patients with disabilities, and it comprises two domains: motor and cognitive [20]. The motor domain (FIM motor) consists of 13 items: eating; grooming; bathing; dressing the upper body; dressing the lower body; toileting; bladder management; bowel management; transfer to bed, chair, or wheelchair; transfer to toilet; transfer to tub or shower; walking/wheelchair; and stairs. The cognitive domain (FIM cognitive) consists of five items: comprehension, expression, social interaction, problem solving, and memory. Items in both domains are scored from 1 to 7 (1, total assistance; 2, maximum assistance; 3, moderate assistance; 4, minimal contact assistance; 5, supervision; 6, modified independence; and 7, complete independence). The minimum total FIM score is 18 points, and the maximum is 126 points; minimum FIM motor and FIM cognitive scores are 13 and 5 points, respectively, and maximum FIM motor and FIM cognitive scores are 91 and 35 points, respectively. FIM scores were obtained by two physical therapists at discharge and 4 months.
2.4.8. Bioelectrical Impedance Analysis
Body composition was assessed using multiple-frequency bioelectrical impedance analysis (S10; InBody, Seoul, Republic of Korea) using six different frequencies (1, 5, 50, 250, 500, and 1000 kHz). Patients remained supine on the bed with their arms and legs abducted, and reusable contact electrodes were placed on the first and third fingers of both hands and the medial and lateral sides of both ankles. The phase angle was calculated from the relationship between the resistance and reactance vectors based on bioelectrical impedance measurements. We used a resistance and reactance of 50 kHz to calculate the phase angle, and the phase angle in the whole body was used in the analyses. Evaluation of the phase angle has been shown to have high test–retest reliability and accuracy [21]. We also calculated the skeletal muscle index (SMI) by dividing the limb skeletal muscle mass (kg) by height squared (m2). The phase angle and SMI were measured before discharge and at 4 months.
2.5. Follow-Up
To evaluate whether the rate of cardiac events differed between patients in Groups R and B, we prospectively followed all patients for the occurrence of primary events, defined as cardiac death (from worsening congestive heart failure or sudden death) or unscheduled readmission for heart failure. Non-cardiac deaths were excluded from the analysis. The secondary endpoint was fall-related fractures.
2.6. Statistical Analysis
Continuous variables are reported as the mean ± SD, and categorical data are reported as percentages of all patients. Parameters obtained just before discharge after patients had been medically stabilized were compared with values obtained at the end of the 4-month intervention period using paired t-tests and Mann–Whitney U tests. The normality of the data distribution was confirmed using the Shapiro–Wilk test. The significance of differences between the two groups was assessed using the Chi-squared test for categorical variables and the Mann–Whitney rank-sum test for continuous variables. Cumulative cardiac event-free survival estimates were calculated using the Kaplan–Meier method, and the significance of differences was evaluated using a stratified log-rank test. All statistical analyses were performed using SPSS 17.0 (SPSS, Chicago, IL, USA). p < 0.05 was considered statistically significant.
3. Results
3.1. Baseline Characteristics
By June 2021, 90 patients were enrolled in the RCT; their clinical characteristics are presented in Table 1. The mean age was 78 years, 50% of patients were male, and the mean body mass index (BMI) was 22.7 kg/m2. There were no significant differences in age, sex, BMI, atrial fibrillation, coronary risk factors, underlying disease, medication use, echocardiographic data, CPX parameters, or frailty between Groups R and B (Table 1).

Table 1.
Clinical characteristics of the study participants.
3.2. Safety and Continuity
There were no adverse effects during exercise recorded in either Group R or Group B, except for a decrease in blood pressure after using the BEAR system in one patient, which improved with intravenous fluid replacement. Five patients in Group R and four patients in Group B withdrew from the study, and three patients crossed over from Group B to Group R and were excluded from the analysis (Figure 1). The reasons for withdrawal from Group R were interruptions to CR due to work (n = 2), a refusal to continue with the program (n = 1), back pain (n = 1), and difficulties getting to the hospital (n = 1). The reasons for switching from Group B to Group R were an inability to follow instructions due to cognitive decline (n = 1), knee pain (n = 1), and visual impairment due to glaucoma resulting in screen blindness.
The reasons for withdrawal from Group B were difficulties getting to the hospital (n = 1), interrupted hospital attendance due to cognitive decline (n = 1), hospitalization for gastrointestinal disease (n = 1), and rehospitalization due to exacerbation of heart failure (n = 1).
3.3. Intra- and Intergroup Comparisons
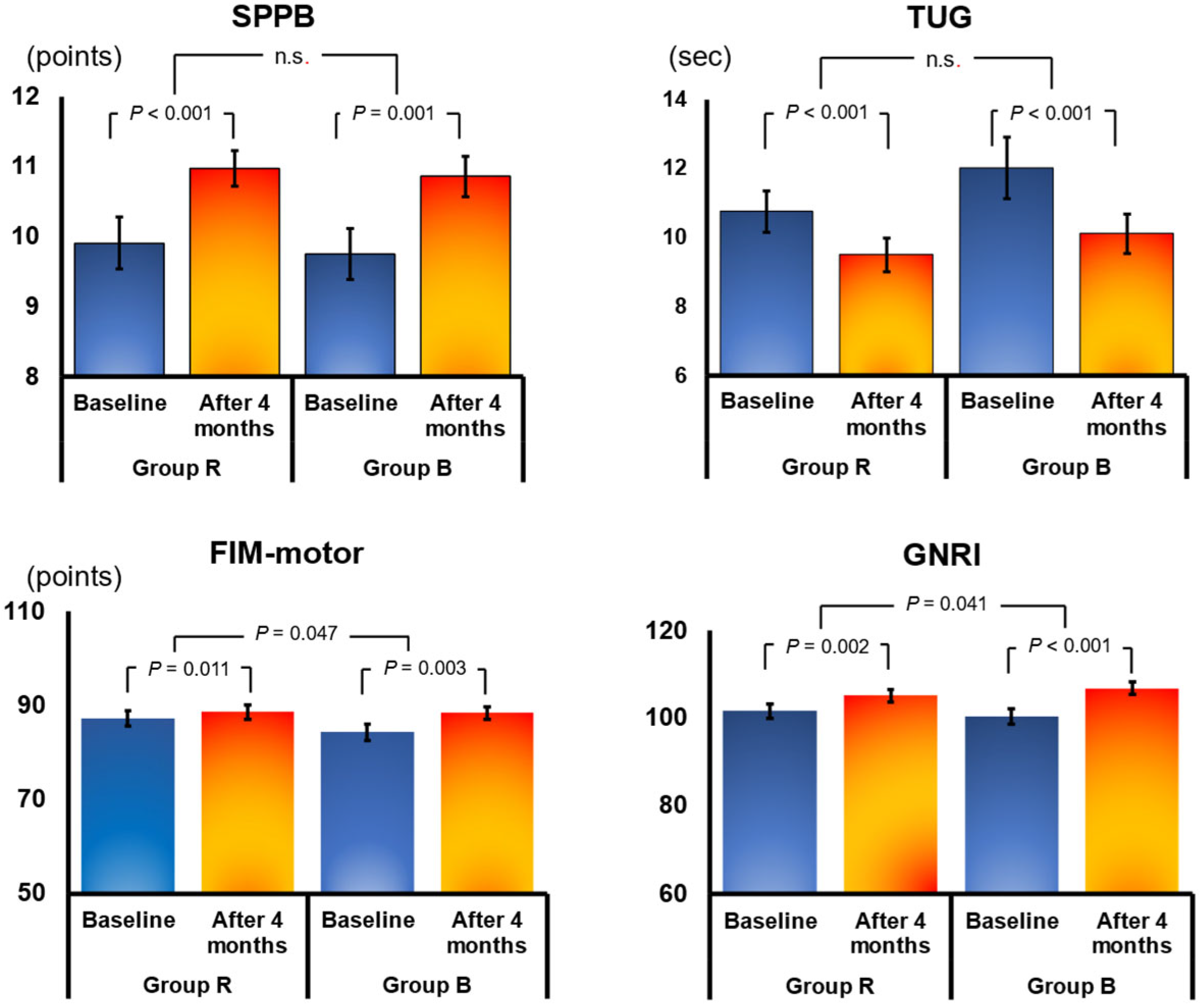
In all, 78 patients were included in the final analysis (39 each in Group B and Group R). The mean age of these 78 patients was 78 years, and 41% were male. Changes in parameters from discharge to 4 months and comparisons between the two groups are presented in Table 2. The Geriatric Nutritional Risk Index (GNRI), a nutritional index for older adults calculated from albumin, improved significantly from discharge to 4 months in both Group B (from 100.8 ± 11.1 to 107.3 ± 8.9; change 5.9 ± 6.4; p < 0.001) and Group R (from 102.5 ± 10.3 to 105.4 ± 9.9; change 3.3 ± 6.0; p = 0.001; Figure 3; Table 2). However, the change in GNRI was significantly better in Group B than in Group R (p = 0.041). Similarly, FIM motor improved significantly in both groups at 4 months, but the improvement was significantly better in Group B (p = 0.047; Figure 3; Table 2). There were no significant differences between Groups R and B in other parameters. There were no significant improvements at 4 months in B-type natriuretic peptide, estimated glomerular filtration rate (eGFR), echocardiography, and CPX VO2peak in either group (Table 2). Conversely, significant improvements were noted in knee extensor strength, 10-m comfortable gate speed, TUG (a comprehensive test of gait and mobility), and SPPB (a measure of balance) at 4 months in both groups; however, there were no significant differences in the magnitude of changes between the two groups (Figure 3; Table 2).

Table 2.
Changes in parameters from baseline to after 4 months of cardiac rehabilitation.
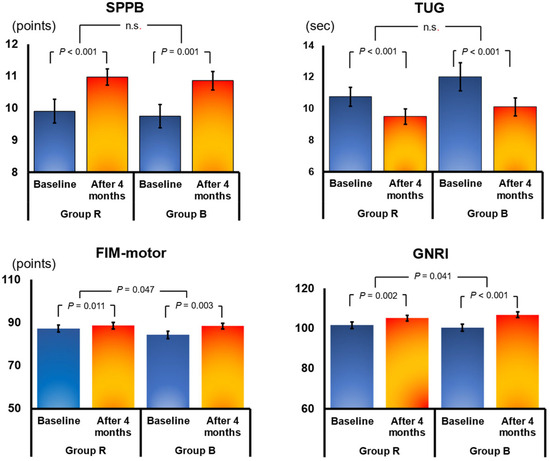
Figure 3.
Effects of 4 months of cardiac rehabilitation (CR) in the resistance training group (Group R) and the group using the balance exercise assist robot (BEAR) system (Group B). Data show mean ± SE values at baseline and after 4 months. FIM motor, motor domain of the Functional Independence Measure; GNRI, Geriatric Nutritional Risk Index; SPPB, Short Physical Performance Battery; TUG, timed up-and-go test. n.s., no significant.
3.4. Event-Free Survival
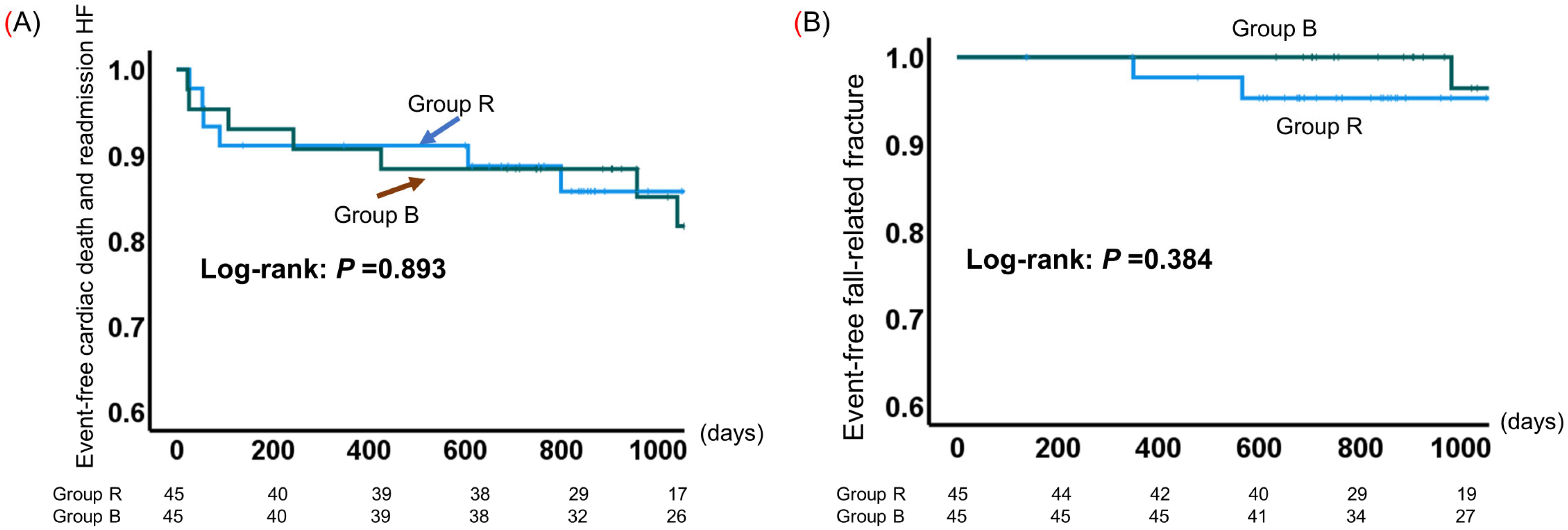
The cumulative probability of event-free survival is shown in Figure 4. All patients were followed for a mean of 867 days (range of 28–1623 days) from the time of enrollment to the time of a cardiac event or the last assessment of survivors. Cardiac deaths were recorded for two patients each in Groups R and B. Nine patients in Group R and eight patients in Group B were rehospitalized for heart failure. The probability of event (i.e., cardiac death and heart failure readmission)-free survival was comparable between Groups R and B (log-rank test p = 0.893; Figure 4A). Although there were no fall-related fractures in Group B over the 2-year follow-up period, three patients in both groups eventually developed fractures; there was no significant difference in the incidence of fall-related fractures between the two groups (Figure 4B).
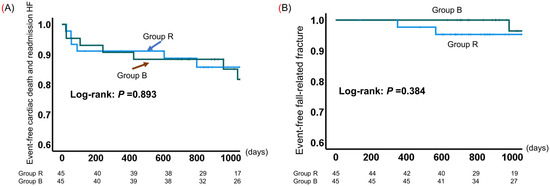
Figure 4.
Kaplan–Meier estimates of event-free survival in older adults with cardiovascular disease (CVD) in the resistance training group (Group R) and the group using the balance exercise assist robot (BEAR) system (Group B). (A) Cardiac death and readmission for heart failure; (B) fall-related fractures.
4. Discussion
To the best of our knowledge, this is the first RCT to show the beneficial effects of CR with BEAR versus conventional CR with established resistance training in older adults with CVD. We also compared the incidence of cardiac events and fall-related fractures between the two groups. The main outcome of this study is the demonstrated safety and benefits of BEAR compared with resistance training in older patients with CVD, specifically concerning the following:
- In terms of safety, there were no accidents during exercise sessions in the BEAR group, and the exercises were safe for patients with heart disease. No fractures due to falls were observed during the follow-up period of up to 2 years.
- The dropout rate over a 4-month CR period was comparable between the BEAR and resistance training groups, so there were no problems with continuity.
- The effects of CR at 4 months were similar in the BEAR and resistance training groups. The improvements in FIM motor and GNRI at 4 months were significantly better in the BEAR than resistance training group.
4.1. Safety of BEAR
No adverse effects of using the BEAR system were seen. Nine percent of patients dropped out of the BEAR group, compared with 11% in the resistance training group. The reasons for dropping out of the BEAR group or switching to the resistance training group included visual or cognitive impairment or hospitalization. The use of the BEAR system appeared to be comparable to the use of conventional CR in terms of safety and adherence.
The risk of falls and trauma during exercise was considered a disadvantage of the BEAR system, but sufficient safety precautions were taken, such as the use of a safety harness while patients were using the BEAR system, so this is no longer considered a problem. There were no reports of adverse events, including falls or device malfunction, by patients using the BEAR system in this study, and we believe that we have demonstrated the safety of the system in patients with cardiac disease as long as their disease is under control.
4.2. Efficacy of BEAR
Although there are numerous reports of the effectiveness of rehabilitation with BEAR [3,5,10,19,20,21], there are none in patients with cardiac disease, except in our previous study, which was a single arm without a control group [4]. In the present study, CR with resistance training and using BEAR resulted in significant improvements in nutrition, physical function, and balance after 4 months, with no significant differences in the magnitude of the changes between the resistance training and BEAR groups, except for FIM motor and GNRI.
4.2.1. Improvements in FIM motor
The improvement in FIM motor after CR for 4 months using the BEAR system was significantly greater than that seen with resistance training, but there was no difference between the groups in terms of improvements in FIM cognitive. Previous studies have reported that older adults with heart failure have reduced physical function (e.g., mobility and balance), reduced exercise tolerance due to complications such as frailty, and a higher incidence of falls [22] and orthopedic fractures [23] than healthy community-dwelling people. Exercise therapy that improves balance and mobility in addition to aerobic and resistance training is considered necessary, but the content of exercise therapy and its effectiveness for older people with heart failure have not been adequately tested.
4.2.2. Improvements in GNRI
In the present study, the improvement in GNRI was significantly greater in the BEAR than the resistance training group. GNRI is a screening tool used to assess nutritional status in older people and is primarily based on serum albumin and BMI [7]. Heart failure with low GNRI is associated with increased all-cause mortality [24]. The systemic proinflammatory state is a major contributor to the cardiac pathology of heart failure, resulting in irreversible cardiac damage [25], although the underlying mechanism remains unclear. According to questionnaire respondents, BEAR is an enjoyable rehabilitation strategy [5], and we speculate that BEAR may improve a patient’s mental state and motivation, thus leading to an improvement in a patient’s diet.
4.3. BEAR vs. Resistance Training
Current clinical guidelines strongly recommend CR in patients with chronic heart failure [26], and the benefits of CR are well established, particularly in older adults with CVD, for whom a combination of aerobic and resistance training is recommended. A recent multicenter retrospective cohort study of patients with heart failure has found that CR is effective in both heart failure with preserved left ventricular ejection fraction and frailty [6]. The use of a BEAR has been shown to improve gait speed, TUG, and knee extension muscle strength in community-dwelling frail and pre-frail older adults [3]. In the present study, in which 34% of patients were frail and 36% had atrial fibrillation, CR with BEAR was demonstrated to be safe and comparable to conventional resistance training for improving balance in older adults with CVD who were medically stable and prescribed standard medications for their CVD.
The physical function and balance outcomes following CR with BEAR were comparable to those for CR with resistance training, demonstrating that exercise training with the BEAR system is safe and effective even for older adults with CVD. In addition, the incidence of cardiac events and fall-related fractures over the longer term (2 years) was comparable between CR with BEAR and CR with established resistance and aerobic training groups. Thus, we have demonstrated that the use of a BEAR system is comparable to conventional resistance training in improving exercise capacity and balance in older adults and frail patients with CVD.
4.4. Study Limitations
The limitations of this study include the small number of participants and the fact that it was performed at a single center. To confirm whether BEAR is more effective than resistance training in any respect, a larger number of cases need to be accumulated, along with performing subanalyses (e.g., according to age and sex). In the present study, no adverse events were reported during CR in either group, suggesting that this study can be safely replicated in older adults with heart failure. Further studies are needed to determine whether the effects of CR with BEAR differ in different subgroups.
5. Conclusions
Cardiac rehabilitation with the balance exercise assist robot was safe and without adverse events, but required a certain level of visual acuity and cognitive function. Cardiac rehabilitation that focuses on balance to prevent falls may be as effective as resistance training and may be useful for older adults with cardiovascular disease.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jcdd11050133/s1, Supplementary Video S1. Representative video showing a patient using the balance exercise assist robot (BEAR) system.
Author Contributions
Conceptualization: A.H., K.H., T.K., M.K., K.K., K.S., I.U., N.I., T.M., H.K. and I.K.; Methodology: A.H., K.H., K.K., K.S., I.U. and A.S.; Formal analysis: A.H. and K.H.; Investigation: A.H. and K.H.; Resources: A.H., T.K., M.K. and A.S.; Data curation: A.H. and K.H.; Writing—original draft preparation: A.H.; Writing—review and editing, A.H., H.K., I.K., and A.S.; Visualization: A.H. and K.H.; Supervision: A.S., H.K., I.K. and A.S.; Project administration: A.H., K.H., I.K. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was performed with the support of 2019–2021 geriatrics and gerontology research funds sponsored by the Ministry of Health, Labour and Welfare, Japan.
Institutional Review Board Statement
The study protocol complied with the Declaration of Helsinki, and written informed consent was obtained from each subject. This study was approved by the Nagoya University Certified Clinical Research Review Board (Approval no. 2020) and was registered with the Japan Registry of Clinical Trials (registration no. jRCTs042190086).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We thank the staff members of the National Center for Geriatrics and Gerontology (Obu, Japan), particularly Junpei Sugioka (occupational therapist), Katsunori Hara, Hideki Yanagisawa, Koharu Oya, Shunya Tanioku (physical therapists), Chinatsu Makita, Chieko Hokao, and Kaori Inaguma (technical assistants).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Barbaccia, V.; Bravi, L.; Murmura, F.; Savelli, E.; Viganò, E. Older adults now represent a growing sector of the population since they are living longer, and the world’s population is increasingly ageing. Int. J. Environ. Res. Public. Health. 2022, 19, 7660. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Trisan, R. Balance Problems and Fall Risks in the Elderly. Clin. Geriatr. Med. 2019, 35, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Kondo, I.; Hirano, S.; Kagaya, H.; Saitoh, E.; Osawa, A.; Fujinori, Y. Training with a balance exercise assist robot is more effective than conventional training for frail older adults. Geriatr. Gerontol. Int. 2017, 17, 1982–1990. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Hirashiki, A.; Ozaki, K.; Kawamura, K.; Sugioka, J.; Tanioku, S.; Sato, K.; Ueda, I.; Itoh, N.; Nomoto, K.; et al. Benefits of a Balance Exercise Assist Robot in the Cardiac Rehabilitation of Older Adults with Cardiovascular Disease: A Preliminary Study. J. Cardiovasc. Dev. Dis. 2022, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Kagaya, H.; Hirano, S.; Kondo, I.; Tanabe, S.; Itoh, N.; Saitoh, E.; Fuwa, T.; Murakami, R. Preliminary trial of postural strategy training using a personal transport assistance robot for patients with central nervous system disorder. Arch. Phys. Med. Rehabil. 2013, 94, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, K.; Sato, Y.; Takahashi, T.; Tsuchihashi-Makaya, M.; Kotooka, N.; Ikegame, T.; Takura, T.; Yamamoto, T.; Nagayama, M.; Goto, Y.; et al. Multidisciplinary Cardiac Rehabilitation and Long-Term Prognosis in Patients With Heart Failure. Circ. Heart Fail. 2020, 13, e006798. [Google Scholar] [CrossRef] [PubMed]
- Sze, S.; Pellicori, P.; Kazmi, S.; Rigby, A.; Cleland, J.G.F.; Wong, K.; Clark, A.L. Prevalence and Prognostic Significance of Malnutrition Using 3 Scoring Systems Among Outpatients With Heart Failure: A Comparison With Body Mass Index. JACC Heart Fail. 2018, 6, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, R.G.; Semsarian, C.; Macdonald, P. Dilated cardiomyopathy. Lancet 2017, 390, 400–414. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, e521–e643. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Bohm, M.; Butler, J.; et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J. Card. Fail. 2021, 27, 387–413. [Google Scholar] [CrossRef]
- Group, J.C.S.J.W. Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012). Circ. J. 2014, 78, 2022–2093. [Google Scholar] [CrossRef]
- Inoue, S.; Otaka, Y.; Kumagai, M.; Sugasawa, M.; Mori, N.; Kondo, K. Effects of Balance Exercise Assist Robot training for patients with hemiparetic stroke: A randomized controlled trial. J. Neuroeng. Rehabil. 2022, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Satake, S.; Shimada, H.; Yamada, M.; Kim, H.; Yoshida, H.; Gondo, Y.; Matsubayashi, K.; Matsushita, E.; Kuzuya, M.; Kozaki, K.; et al. Prevalence of frailty among community-dwellers and outpatients in Japan as defined by the Japanese version of the Cardiovascular Health Study criteria. Geriatr. Gerontol. Int. 2017, 17, 2629–2634. [Google Scholar] [CrossRef]
- Ross, R.M. ATS/ACCP statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 1451, author reply 1451. [Google Scholar] [CrossRef]
- Graham, J.E.; Ostir, G.V.; Fisher, S.R.; Ottenbacher, K.J. Assessing walking speed in clinical research: A systematic review. J. Eval. Clin. Pract. 2008, 14, 552–562. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Mijnarends, D.M.; Meijers, J.M.; Halfens, R.J.; ter Borg, S.; Luiking, Y.C.; Verlaan, S.; Schoberer, D.; Cruz Jentoft, A.J.; van Loon, L.J.; Schols, J.M. Validity and reliability of tools to measure muscle mass, strength, and physical performance in community-dwelling older people: A systematic review. J. Am. Med. Dir. Assoc. 2013, 14, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Bohannon, R.W. Reference Values for Knee Extension Strength Obtained by Hand-Held Dynamometry from Apparently Healthy Older Adults: A Meta-Analysis. J. Frailty Aging 2017, 6, 199–201. [Google Scholar] [CrossRef]
- Ottenbacher, K.J.; Hsu, Y.; Granger, C.V.; Fiedler, R.C. The reliability of the functional independence measure: A quantitative review. Arch. Phys. Med. Rehabil. 1996, 77, 1226–1232. [Google Scholar] [CrossRef]
- Massari, F.; Mastropasqua, F.; Guida, P.; De Tommasi, E.; Rizzon, B.; Pontraldolfo, G.; Pitzalis, M.V.; Rizzon, P. Whole-body bioelectrical impedance analysis in patients with chronic heart failure: Reproducibility of the method and effects of body side. Ital. Heart J. 2001, 2, 594–598. [Google Scholar] [PubMed]
- Lee, K.; Davis, M.A.; Marcotte, J.E.; Pressler, S.J.; Liang, J.; Gallagher, N.A.; Titler, M.G. Falls in community-dwelling older adults with heart failure: A retrospective cohort study. Heart Lung 2020, 49, 238–250. [Google Scholar] [CrossRef] [PubMed]
- van Diepen, S.; Majumdar, S.R.; Bakal, J.A.; McAlister, F.A.; Ezekowitz, J.A. Heart failure is a risk factor for orthopedic fracture: A population-based analysis of 16,294 patients. Circulation. 2008, 118, 1946–1952. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cen, K.; Sun, W.; Feng, B. Prognostic value of geriatric nutritional risk index in elderly patients with heart failure: A meta-analysis. Aging Clin. Exp. Res. 2021, 33, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Reina-Couto, M.; Pereira-Terra, P.; Quelhas-Santos, J.; Silva-Pereira, C.; Albino-Teixeira, A.; Sousa, T. Inflammation in Human Heart Failure: Major Mediators and Therapeutic Targets. Front Physiol. 2021, 12, 746494. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).