The Right Coronary Anatomy and Operative Topography of the Tricuspid Valve Annulus
Abstract
1. Introduction
1.1. Anatomy of the Annulus of the Tricuspid Valve
1.2. Clinical Significance of the Region of the Tricuspid Valve Annulus
1.3. Indications and Complications of Tricuspid Valve Surgery
1.4. The Aim of the Study
2. Materials and Methods
2.1. Study Population
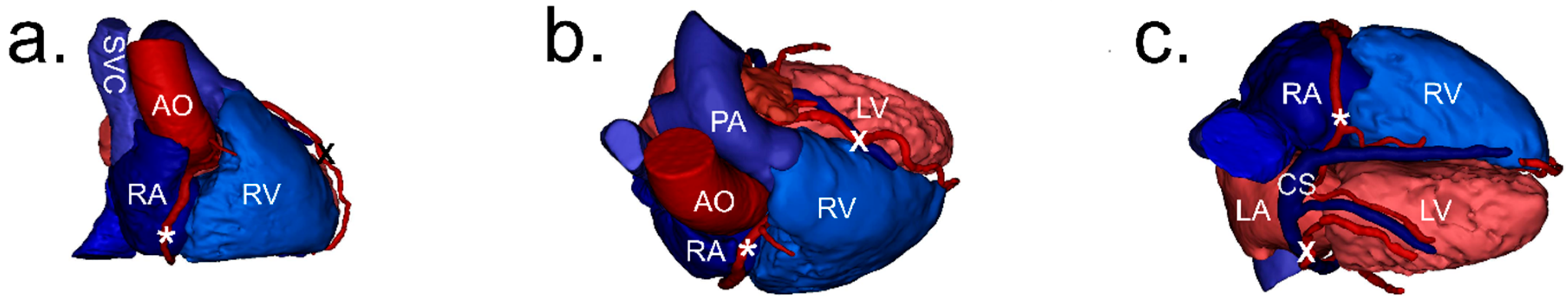
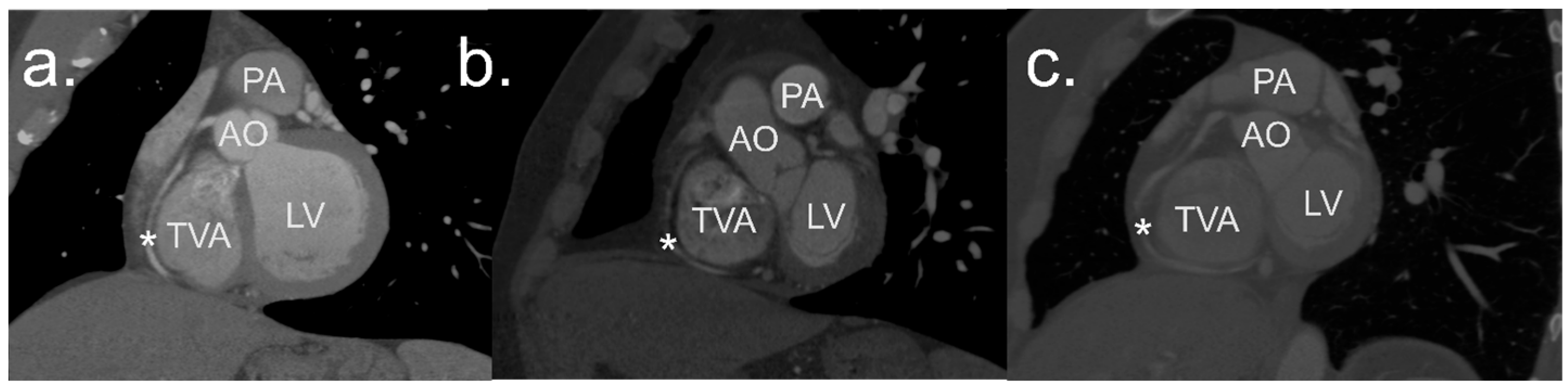
2.2. Image Processing and Analysis
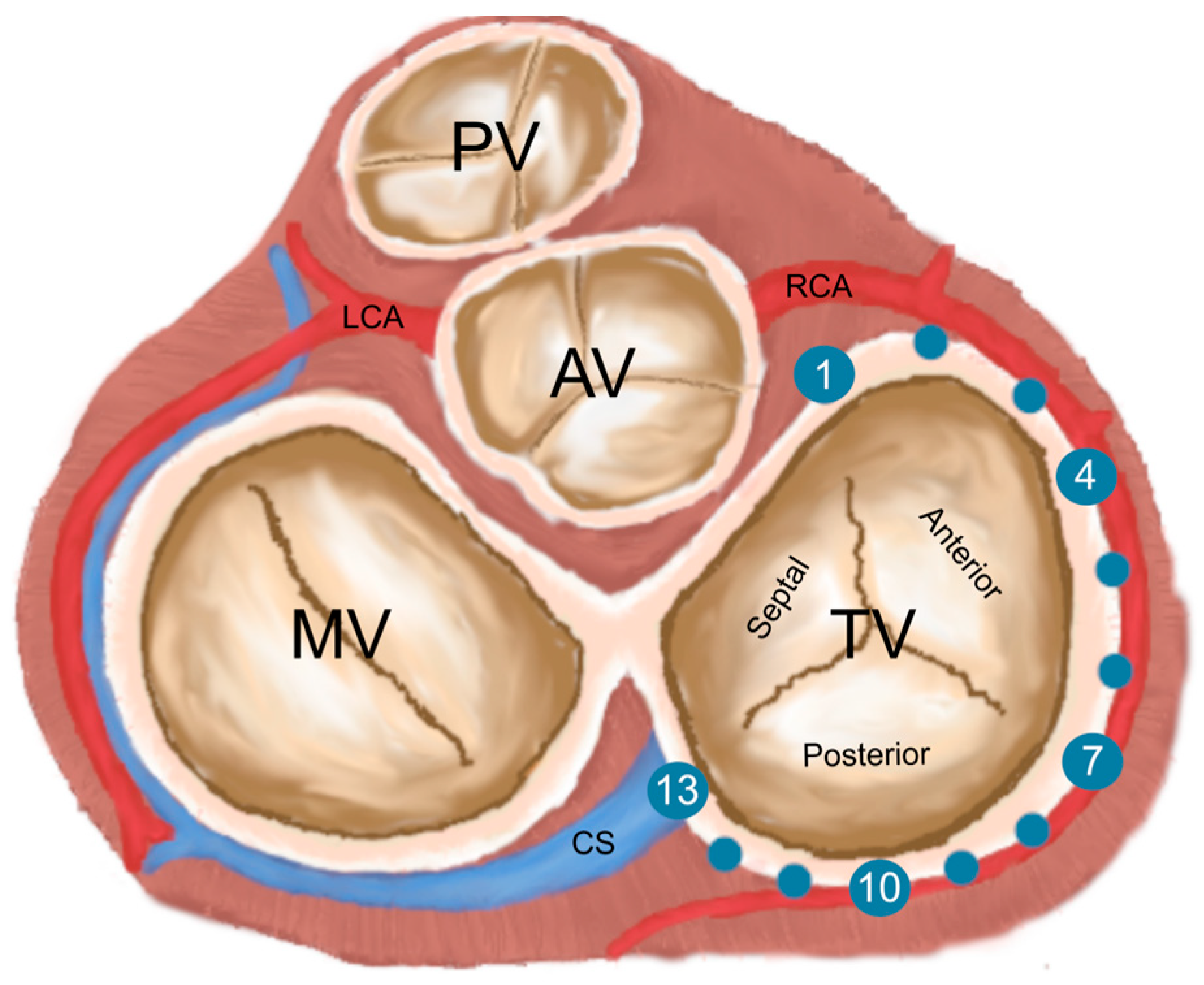
2.3. Definitions
2.4. Measurements
2.5. Statistical Analysis
3. Results
3.1. TVA Dimensions
3.2. TVA and RCA Distances
3.3. RCA Deviation
3.4. Danger Zones
3.5. RCA Branches
3.6. Gender-Specific Differences
4. Discussion
4.1. The Dangerous Distances—Should We Be Aware of Them?
4.2. Tricuspid Annular Points
4.3. Tricuspid Valve Diseases, Surgery and Its Complications
4.4. Surgical Topography of the Right Coronary Artery in the Region of the Tricuspid Valve Annulus
4.5. Clinical Implications
4.6. Further Research Direction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yucel, E.; Bertrand, P.B.; Churchill, J.L.; Namasivayam, M. The Tricuspid Valve in Review: Anatomy, Pathophysiology and Echocardiographic Assessment with Focus on Functional Tricuspid Regurgitation. J. Thorac. Dis. 2020, 12, 2945–2954. [Google Scholar] [CrossRef] [PubMed]
- Hołda, J.; Słodowska, K.; Malinowska, K.; Strona, M.; Mazur, M.; Jasińska, K.A.; Matuszyk, A.; Koziej, M.; Walocha, J.A.; Hołda, M.K. Morphology and Position of the Right Atrioventricular Valve in Relation to Right Atrial Structures. Diagnostics 2021, 11, 960. [Google Scholar] [CrossRef] [PubMed]
- Dahou, A.; Levin, D.; Reisman, M.; Hahn, R.T. Anatomy and Physiology of the Tricuspid Valve. JACC Cardiovasc. Imaging 2019, 12, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Karamali, F.; Hosseini, S.; Shojaeifard, M.; Mohammadi, K.; Kaviani, R.; Rezaei, Y.; Samiei, N. Tricuspid Valve Geometry in Patients with Functional Tricuspid Regurgitation: A Three-Dimensional Echocardiographic Study. Echocardiography 2020, 37, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Adamowicz, J.; Szponder, M.; Sokołowska, M.; Sławuta, A.; Gajek, J.; Zyśko, D. Ablacja Cieśni Trójdzielno-Żylnej Wśród Pacjentów z Przetrwałym Migotaniem Przedsionków Jako Terapia Pomostowa Celem Utrzymania Rytmu Zatokowego—Badanie Pilotażowe. Folia Cardiol. 2017, 12, 239–244. [Google Scholar] [CrossRef]
- Rogers, J.H.; Bolling, S.F. The Tricuspid Valve: Current Perspective and Evolving Management of Tricuspid Regurgitation. Circulation 2009, 119, 2718–2725. [Google Scholar] [CrossRef]
- Mangieri, A.; Popolo Rubbio, A.; San Donato Polyclinic, I.; Alberto Guido Pozzoli, I.; Regionale di Lugano, O.; Avanzas, P. Transcatheter Tricuspid Valve Interventions: Current Status and Future Perspectives. Front. Cardiovasc. Med. 2022, 9, 994502. [Google Scholar]
- Saran, N.; Dearani, J.A. Strategies for Tricuspid Valve Repair. Indian J. Thorac. Cardiovasc. Surg. 2020, 36, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Rdzanek, A.; Szymański, P.; Gackowski, A.; Scisło, P.; Prȩgowski, J.; Pietrasik, A.; Trȩbacz, J.; Zbroński, K.; Kochman, J.; Witkowski, A.; et al. Percutaneous Tricuspid Edge-to-Edge Repair—Patient Selection, Imaging Considerations, and the Procedural Technique. Expert Opinion of the Working Group on Echocardiography and Association of Cardiovascular Interventions of the Polish Cardiac Society. Kardiol. Pol. 2021, 79, 1178–1191. [Google Scholar] [CrossRef]
- Sala, A.; Hahn, R.T.; Kodali, S.K.; Mack, M.J.; Maisano, F. Tricuspid Valve Regurgitation: Current Understanding and Novel Treatment Options. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 101041. [Google Scholar] [CrossRef]
- Klimek-Piotrowska, W.; Hołda, M.K.; Koziej, M.; Sałapa, K.; Piątek, K.; Hołda, J. Geometry of Koch’s Triangle. Europace 2017, 19, 452–457. [Google Scholar] [CrossRef][Green Version]
- Inoue, S.; Becker, A.E. Koch’s Triangle Sized Up: Anatomical Landmarks in Perspective of Catheter Ablation Procedures. Pacing Clin. Electrophysiol. 1998, 21, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Al Aloul, B.; Sigurdsson, G.; Can, I.; Li, J.M.; Dykoski, R.; Tholakanahalli, V.N. Proximity of Right Coronary Artery to Cavotricuspid Isthmus as Determined by Computed Tomography. PACE—Pacing Clin. Electrophysiol. 2010, 33, 1319–1323. [Google Scholar] [CrossRef]
- Abdelbar, A.; Kenawy, A.; Zacharias, J. Minimally Invasive Tricuspid Valve Surgery. J. Thorac. Dis. 2021, 13, 1982–1992. [Google Scholar] [CrossRef]
- Díez-Villanueva, P.; Gutiérrez-Ibañes, E.; Cuerpo-Caballero, G.P.; Sanz-Ruiz, R.; Abeytua, M.; Soriano, J.; Sarnago, F.; Elízaga, J.; González-Pinto, A.; Fernández-Avilés, F. Direct Injury to Right Coronary Artery in Patients Undergoing Tricuspid Annuloplasty. Ann. Thorac. Surg. 2014, 97, 1300–1305. [Google Scholar] [CrossRef]
- Batko, J.; Rams, D.; Filip, G.; Bartoszcze, A.; Kapelak, B.; Bartuś, K.; Litwinowicz, R. Left Atrial Appendage Morphology and Course of the Circumflex Artery: Anatomical Implications for Left Atrial Appendage Occlusion Procedures. Innovations 2022, 17, 424–429. [Google Scholar] [CrossRef]
- Hinzpeter, R.; Eberhard, M.; Pozzoli, A.; Manka, R.; Tanner, F.C.; Taramasso, M.; Maisano, F.; Alkadhi, H. Dynamic Anatomic Relationship of Coronary Arteries to the Valves. Part 2: Tricuspid Annulus and Right Coronary Artery. EuroIntervention 2019, 15, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Chang, C.Y.; Wei, J. Anatomic Consideration of Stitch Depth in Tricuspid Valve Annuloplasty. Acta Cardiol. Sin. 2015, 31, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Zhingre Sanchez, J.D.; Iaizzo, P.A. Computationally Assessed 3D Anatomical Proximities and Spatial Relationships among the Tricuspid Valve Annulus, Right Coronary Artery, and Triangle of Koch: Implications for Transcatheter Tricuspid Annuloplasty Repair. Struct. Heart 2022, 6, 100033. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., III; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, E72–E227. [Google Scholar]
- Golamari, R.; Shams, P.; Bhattacharya, P.T. Tricuspid Stenosis; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Singh, J.P.; Evans, J.C.; Levy, D.; Larson, M.G.; Freed, L.A.; Fuller, D.L.; Lehman, B.; Benjamin, E.J. Prevalence and Clinical Determinants of Mitral, Tricuspid, and Aortic Regurgitation (The Framingham Heart Study). Am. J. Cardiol. 1999, 83, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Chodór-Rozwadowska, K.; Sawicka, M.; Morawski, S.; Kalarus, Z.; Kukulski, T. Tricuspid Regurgitation (TR) after Implantation of a Cardiac Implantable Electronic Device (CIED)—One-Year Observation of Patients with or without Left Ventricular Dysfunction. J. Cardiovasc. Dev. Dis. 2023, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Pappano, A.J.; Gil Wier, W. Coronary Circulation. In Cardiovascular Physiology; Elsevier: Philadelphia, PA, USA, 2013; pp. 223–236. [Google Scholar] [CrossRef]
- Poon, S.S.; George, J.; Obaid, D.; Kumar, P. Myocardial Infarction and Ventricular Fibrillation Due to Iatrogenic Right Coronary Artery Occlusion Following Tricuspid Valve Annuloplasty: A Case Report. Eur. Heart J. Case Rep. 2020, 4, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Urabe, D.; Ide, M.; Matsuoka, M.; Miyake, R. Iatrogenic Right Coronary Artery Occlusion during Minimally Invasive Cardiac Surgery-Tricuspid Annuloplasty—A Case Report. JA Clin. Rep. 2022, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.B.; Smith, M.M.; Rehfeldt, K.H. Iatrogenic Right Coronary Artery Occlusion Following Tricuspid Valve Repair: Case Report of a Rare but Recognized Complication. A A Case Rep. 2017, 8, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Goldhammer, J.; Clark Hargrove, W.; Vernick, W.J. Right Coronary Artery Occlusion after Tricuspid Ring Annuloplasty. J. Cardiothorac. Vasc. Anesth. 2015, 29, 1606–1609. [Google Scholar] [CrossRef] [PubMed]
- Rams, D.; Batko, J.; Bartuś, K.; Filip, G.; Kowalewski, M.; Litwinowicz, R. Left Internal Mammary Artery Operative Topography for MIDCAB and TECAB Procedures. Innovations 2022, 17, 499–505. [Google Scholar] [CrossRef]
- Burysz, M.; Batko, J.; Olejek, W.; Piotrowski, M.; Litwinowicz, R.; Słomka, A.; Kowalewski, M.; Suwalski, P.; Bartuś, K.; Rams, D. Morphology and Anatomical Classification of Pericardial Cavities: Oblique and Transverse Sinuses. J. Clin. Med. 2023, 12, 4320. [Google Scholar] [CrossRef]
| Measurements, mm | Female | Male | General | p-Value |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Age | 64.8 ± 8.6 | 60 ± 9.5 | 62.1 ± 9.4 | 0.002 |
| Short diameter | 39.4 ± 6.4 | 43.8 ± 6 | 41.8 ± 6.5 | <0.001 |
| Long diameter | 41.2 ± 5.9 | 44.6 ± 7.3 | 43.1 ± 6.9 | 0.012 |
| Area | 1279.2 ± 275.1 | 1527.3 ± 306.7 | 1418.2 ± 317.2 | <0.001 |
| Perimeter | 127.2 ± 13.4 | 139.5 ± 13.7 | 134.1 ± 14.8 | <0.001 |
| T1 | 18.6 ± 5.2 | 20.5 ± 5.7 | 19.6 ± 5.5 | 0.008 |
| T2 | 15.4 ± 4.6 | 15.6 ± 5.4 | 15.5 ± 5.1 | 0.889 |
| T3 | 12.7 ± 4.3 | 11.2 ± 4.0 | 11.9 ± 4.2 | 0.073 |
| T4 | 9.5 ± 4.0 | 8 ± 3.7 | 8.7 ± 3.9 | 0.026 |
| T5 | 7.7 ± 3.1 | 7.1 ± 3.7 | 7.3 ± 3.4 | 0.197 |
| T6 | 7.3 ± 2.7 | 6.9 ± 3.1 | 7.1 ± 2.9 | 0.384 |
| T7 | 7.2 ± 3.3 | 6.3 ± 2.6 | 6.7 ± 2.9 | 0.229 |
| T8 | 6.7 ± 3.0 | 6 ± 2.7 | 6.3 ± 2.9 | 0.379 |
| T9 | 5.6 ± 2.8 | 5.5 ± 3.4 | 5.6 ± 3.1 | 0.648 |
| T10 | 4.3 ± 1.6 | 4.7 ± 3.5 | 4.5 ± 2.8 | 0.783 |
| T11 | 4.4 ± 1.9 | 5.3 ± 3.9 | 4.9 ± 3.2 | 0.462 |
| T12 | 5.9 ± 2.7 | 6.4 ± 4.1 | 6.1 ± 3.6 | 0.683 |
| T13 | 8.4 ± 3.7 | 9 ± 4.2 | 8.8 ± 4.0 | 0.509 |
| RCA Deviation, Direction | Female | Male | General | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| T1–T3 | none | 46 (97.9) | 57 (95) | 103 (96.3) |
| ventricular | 1 (2.1) | 3 (5) | 4 (3.7) | |
| T3–T5 | none | 46 (97.9) | 59 (98.3) | 105 (98.1) |
| atrial | 1 (2.1) | 1 (1.7) | 2 (1.9) | |
| T5–T7 | none | 47 (100) | 60 (100) | 107 (100) |
| T7–T9 | none | 47 (100) | 60 (100) | 107 (100) |
| T9–T11 | none | 46 (97.9) | 59 (98.3) | 105 (98.1) |
| atrial | 1 (2.1) | 1 (1.7) | 2 (1.9) | |
| T11–T13 | none | 41 (87.2) | 56 (93.3) | 97 (90.7) |
| ventricular | 4 (8.5) | 3 (5) | 7 (6.5) | |
| atrial | 2 (4.3) | 1 (1.7) | 3 (2.8) | |
| Point | Male | Female | General | p Value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| T1 | 0 (0) | 0 (0) | 0 (0) | - |
| T2 | 0 (0) | 0 (0) | 0 (0) | - |
| T3 | 0 (0) | 0 (0) | 0 (0) | - |
| T4 | 0 (0) | 1 (2) | 1 (0.9) | 0.114 |
| T5 | 1 (1.6) | 0 (0) | 1 (0.9) | 0.293 |
| T6 | 0 (0) | 0 (0) | 0 (0) | 0.168 |
| T7 | 2 (3.2) | 0 (0) | 2 (1.8) | 0.194 |
| T8 | 4 (6.6) | 0 (0) | 4 (3.6) | 0.686 |
| T9 | 6 (9.8) | 2 (4) | 8 (7.2) | 0.503 |
| T10 | 8 (13.1) | 5 (10) | 13 (11.7) | 0.1 |
| T11 | 6 (9.8) | 4 (8) | 10 (9) | 0.486 |
| T12 | 3 (4.9) | 3 (6) | 6 (5.4) | 0.863 |
| T13 | 0 (0) | 1 (2) | 1 (0.9) | 0.818 |
| Location | Number of Branches of RCA | n (%) |
|---|---|---|
| T1–T3 | 0 | 15 (13.9) |
| 1 | 52 (48.1) | |
| 2 | 35 (32.4) | |
| 3 | 6 (5.6) | |
| T3–T5 | 0 | 6 (5.6) |
| 1 | 52 (48.1) | |
| 2 | 38 (35.2) | |
| 3 | 10 (9.3) | |
| 4 | 2 (1.9) | |
| T5–T7 | 0 | 7 (6.5) |
| 1 | 44 (40.7) | |
| 2 | 44 (40.7) | |
| 3 | 11 (10.2) | |
| 4 | 2 (1.9) | |
| T7–T9 | 0 | 39 (36.4) |
| 1 | 50 (46.7) | |
| 2 | 16 (15) | |
| 3 | 2 (1.9) | |
| T9–T11 | 0 | 75 (70.1) |
| 1 | 32 (29.9) | |
| T11–T13 | 0 | 2 (1.9) |
| 1 | 80 (74.8) | |
| 2 | 24 (22.4) | |
| 3 | 1 (0.9) |
| Case | Age (y), Sex (M/F) | TVA Long Diameter (mm) | Surgery | Injured RCA Segment | Result |
|---|---|---|---|---|---|
| 1 | 78, F | 44 | DeVega | Crux | Died |
| 2 | 76, F | 44 | DeVega | Mid | Good |
| 3 | 68, F | 40 | DeVega | Mid | Good |
| 4 | 69, F | 41 | Ring | Distal | Good |
| 5 | N, N | N | Ring | N | Died |
| 6 | 64, F | N | DeVega | Distal | Good |
| 7 | 38, M | N | Band | Distal | Good |
| 8 | 83, M | N | Band | Beyond RMA | Good |
| 9 | 82, M | N | MI | Distal | Good |
| 10 | 74, F | N | Band | Distal | Good |
| 11 | 72, M | 49 | Ring | Distal | Good |
| 12 | 44, M | N | MI | Distal | Good |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowski, M.; Burysz, M.; Batko, J.; Litwinowicz, R.; Kowalewski, M.; Bartuś, K.; Wróbel, K.; Graczykowski, Ł.; Słomka, A. The Right Coronary Anatomy and Operative Topography of the Tricuspid Valve Annulus. J. Cardiovasc. Dev. Dis. 2024, 11, 159. https://doi.org/10.3390/jcdd11060159
Piotrowski M, Burysz M, Batko J, Litwinowicz R, Kowalewski M, Bartuś K, Wróbel K, Graczykowski Ł, Słomka A. The Right Coronary Anatomy and Operative Topography of the Tricuspid Valve Annulus. Journal of Cardiovascular Development and Disease. 2024; 11(6):159. https://doi.org/10.3390/jcdd11060159
Chicago/Turabian StylePiotrowski, Michał, Marian Burysz, Jakub Batko, Radosław Litwinowicz, Mariusz Kowalewski, Krzysztof Bartuś, Krzysztof Wróbel, Łukasz Graczykowski, and Artur Słomka. 2024. "The Right Coronary Anatomy and Operative Topography of the Tricuspid Valve Annulus" Journal of Cardiovascular Development and Disease 11, no. 6: 159. https://doi.org/10.3390/jcdd11060159
APA StylePiotrowski, M., Burysz, M., Batko, J., Litwinowicz, R., Kowalewski, M., Bartuś, K., Wróbel, K., Graczykowski, Ł., & Słomka, A. (2024). The Right Coronary Anatomy and Operative Topography of the Tricuspid Valve Annulus. Journal of Cardiovascular Development and Disease, 11(6), 159. https://doi.org/10.3390/jcdd11060159









