Improved Interpretation of Pulmonary Artery Wedge Pressures through Left Atrial Volumetry—A Cardiac Magnetic Resonance Imaging Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Definition of Heart Failure and Pulmonary Hypertension
2.3. Data Analysis
3. Results
3.1. Baseline Characteristics
3.1.1. Hemodynamic Differences in Patients with and without PAWP Elevation
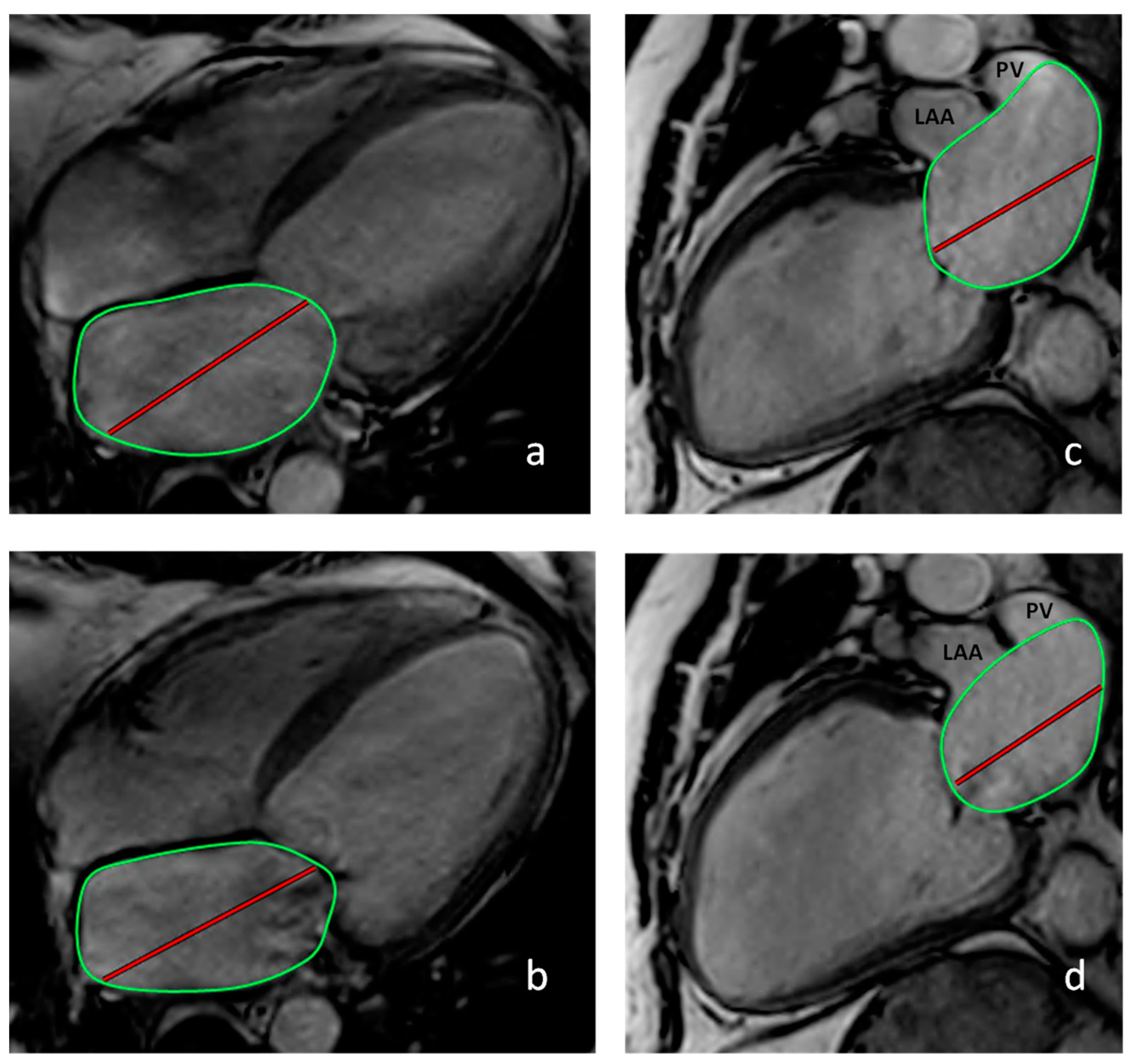
3.1.2. Cardiac Magnetic Resonance Imaging
3.2. Predictors of an Increased PAWP
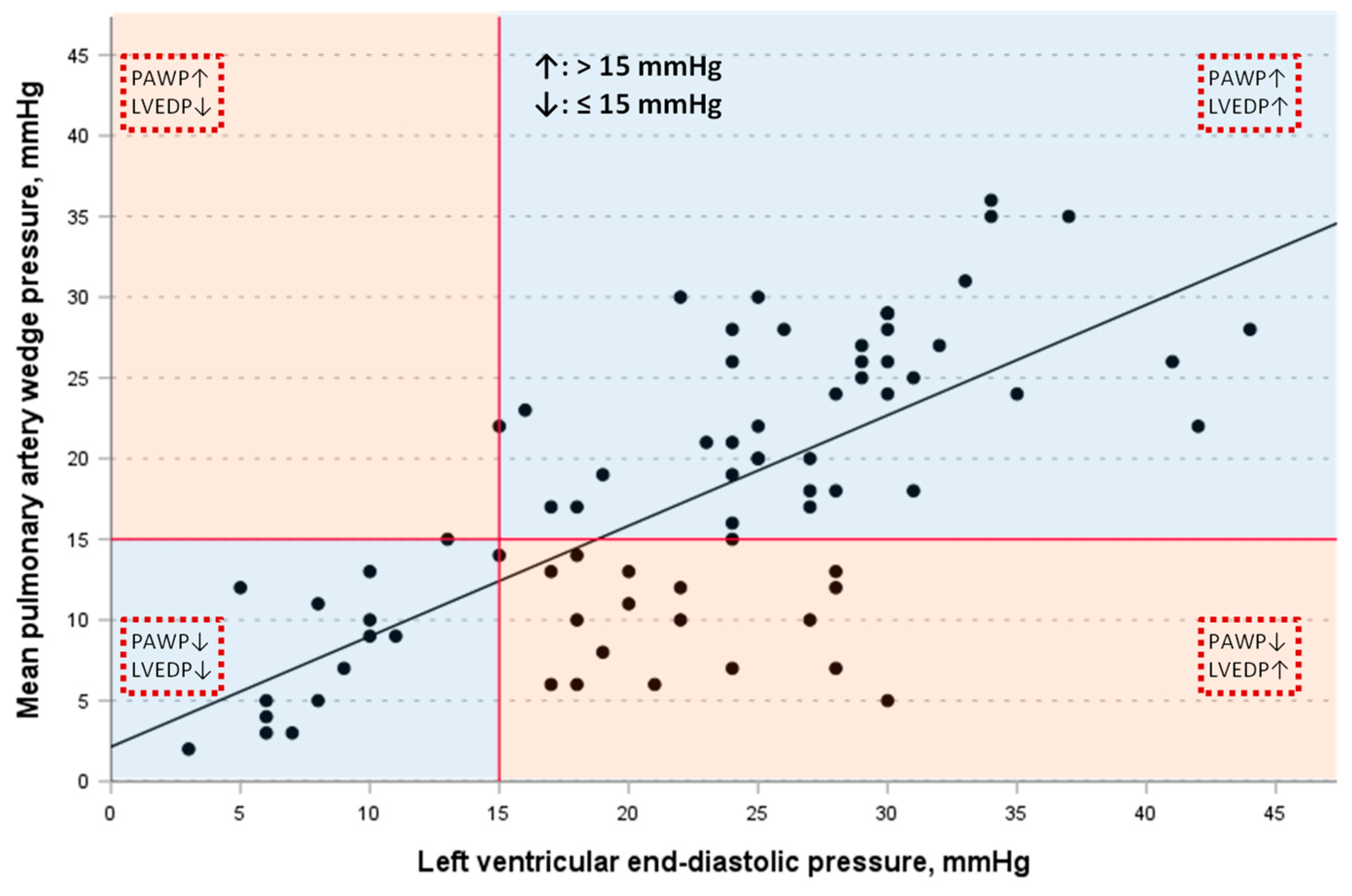
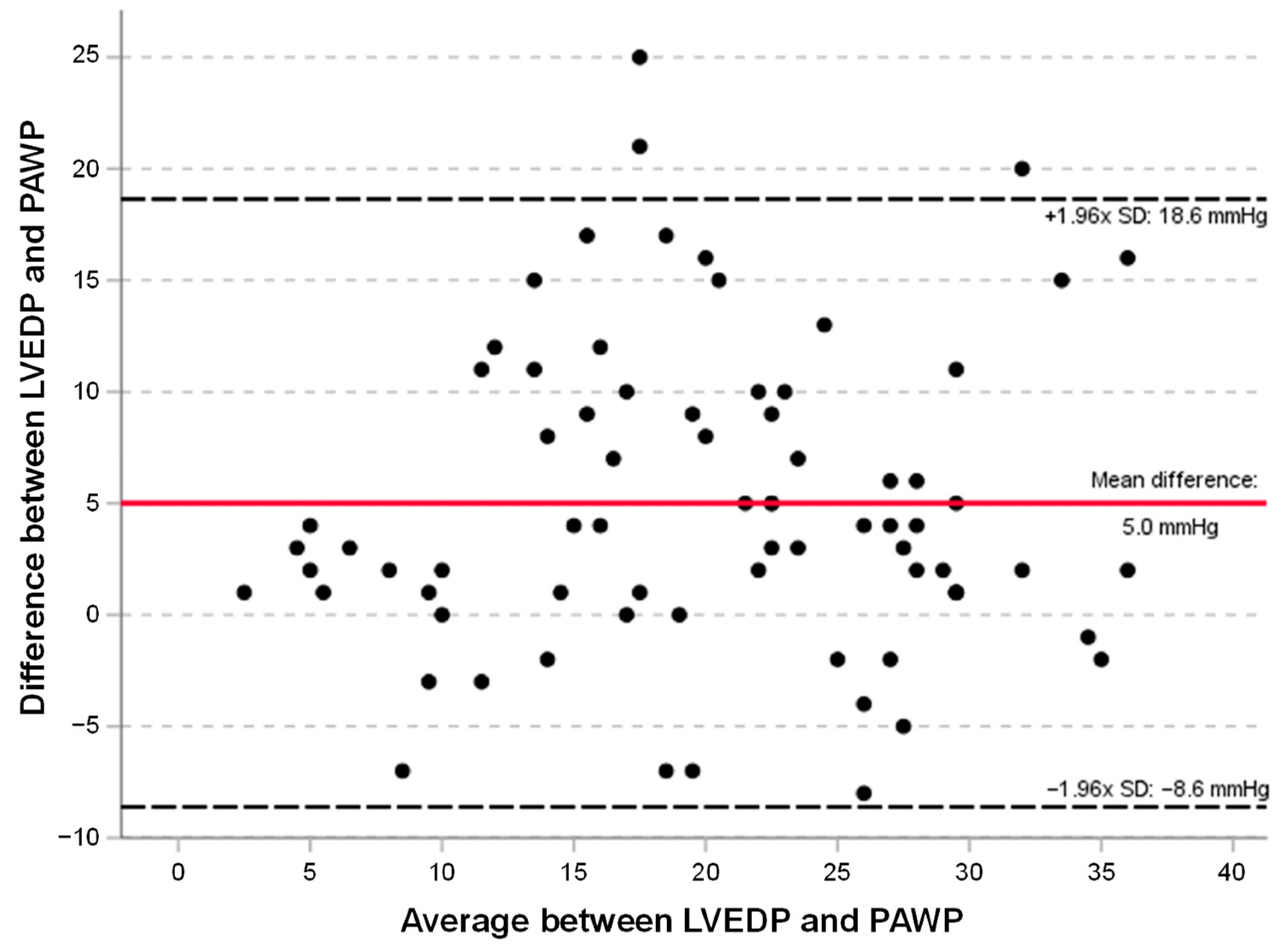
3.3. Correlation of PAWP with LVEDP
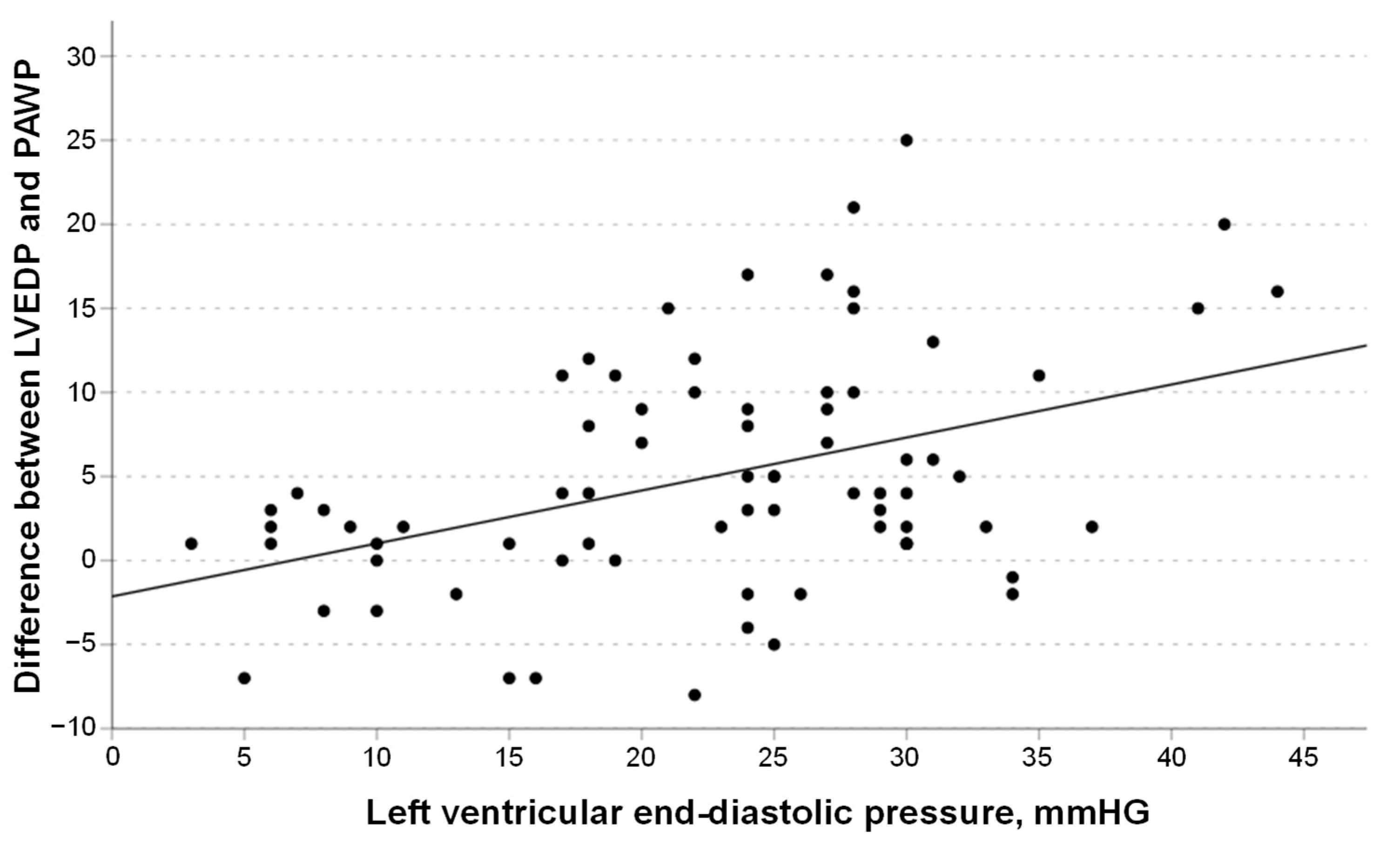
3.4. Linear Regression Models
3.5. Characteristics of Patients with Elevated LVEDP
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Little, W.C.; Downes, T.R. Clinical evaluation of left ventricular diastolic performance. Prog. Cardiovasc. Dis. 1990, 32, 273–290. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Tschope, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2020, 22, 391–412. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Flores, E.D.; Lange, R.A.; Hillis, L.D. Relation of mean pulmonary arterial wedge pressure and left ventricular end-diastolic pressure. Am. J. Cardiol. 1990, 66, 1532–1533. [Google Scholar] [CrossRef] [PubMed]
- Bitar, A.; Selej, M.; Bolad, I.; Lahm, T. Poor agreement between pulmonary capillary wedge pressure and left ventricular end-diastolic pressure in a veteran population. PLoS ONE 2014, 9, e87304. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.T.; Weber, L.; Seidl, S.; Weilenmann, D.; Hochholzer, D.; Joerg, L.; Chronis, J.; Rigger, J.; Haager, P.K.; Rickli, H. Wedge Pressure vs Left Ventricular End-Diastolic Pressure for Pulmonary Hypertension Classification and Prognostication in Severe Aortic Stenosis. CJC Open 2021, 3, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; El-Sabbagh, A.; Nishimura, R.A. Comparing Pulmonary Arterial Wedge Pressure and Left Ventricular End Diastolic Pressure for Assessment of Left-Sided Filling Pressures. JAMA Cardiol. 2018, 3, 453–454. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, M.G.; Lam, C.S.; Rienstra, M.; Vonck, T.E.; Hummel, Y.M.; Voors, A.A.; Hoendermis, E.S. Atrial fibrillation modifies the association between pulmonary artery wedge pressure and left ventricular end-diastolic pressure. Eur. J. Heart Fail. 2017, 19, 1483–1490. [Google Scholar] [CrossRef]
- Kaspar, M.; Fette, G.; Hanke, M.; Ertl, M.; Puppe, F.; Stork, S. Automated provision of clinical routine data for a complex clinical follow-up study: A data warehouse solution. Health Inform. J. 2022, 28, 14604582211058081. [Google Scholar] [CrossRef]
- Reiter, T.; Kerzner, J.; Fette, G.; Frantz, S.; Voelker, W.; Ertl, G.; Bauer, W.; Morbach, C.; Stork, S.; Guder, G. Accuracy of VO2 estimation according to the widely used Krakau formula for the prediction of cardiac output. Herz 2023, 49, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, M.; Fette, G.; Guder, G.; Seidlmayer, L.; Ertl, M.; Dietrich, G.; Greger, H.; Puppe, F.; Stork, S. Underestimated prevalence of heart failure in hospital inpatients: A comparison of ICD codes and discharge letter information. Clin. Res. Cardiol. 2018, 107, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.M.; Barkhausen, J.; Bucciarelli-Ducci, C.; Flamm, S.D.; Kim, R.J.; Nagel, E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J. Cardiovasc. Magn. Reson. 2020, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Rodevan, O.; Bjornerheim, R.; Ljosland, M.; Maehle, J.; Smith, H.J.; Ihlen, H. Left atrial volumes assessed by three- and two-dimensional echocardiography compared to MRI estimates. Int. J. Card. Imaging 1999, 15, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.; Samiei, S.; Ambale Venkatesh, B.; Opdahl, A.; Helle-Valle, T.M.; Zareian, M.; Almeida, A.L.; Choi, E.Y.; Wu, C.; Alonso, A.; et al. Cardiac Magnetic Resonance-Measured Left Atrial Volume and Function and Incident Atrial Fibrillation: Results From MESA (Multi-Ethnic Study of Atherosclerosis). Circ. Cardiovasc. Imaging 2016, 9, 8. [Google Scholar] [CrossRef]
- Pezel, T.; Venkatesh, B.A.; De Vasconcellos, H.D.; Kato, Y.; Shabani, M.; Xie, E.; Heckbert, S.R.; Post, W.S.; Shea, S.J.; Allen, N.B.; et al. Left Atrioventricular Coupling Index as a Prognostic Marker of Cardiovascular Events: The MESA Study. Hypertension 2021, 78, 661–671. [Google Scholar] [CrossRef]
- Rosenkranz, S.; Preston, I.R. Right heart catheterization: Best practice and pitfalls in pulmonary hypertension. Eur. Respir. Rev. 2015, 24, 642–652. [Google Scholar] [CrossRef]
- Argueta, E.E.; Paniagua, D. Thermodilution Cardiac Output: A Concept over 250 Years in the Making. Cardiol. Rev. 2019, 27, 138–144. [Google Scholar] [CrossRef]
- Dubois, D.; Dubois, E.F. Nutrition Metabolism Classic—A Formula to Estimate the Approximate Surface-Area If Height and Weight Be Known. Nutrition 1989, 5, 303–311, Reprinted from Arch. Intern. Med. 1916, 17, 863. [Google Scholar]
- Guder, G.; Reiter, T.; Fette, G.; Hundertmark, M.; Frantz, S.; Morbach, C.; Stork, S.; Held, M. Diagnosing post-capillary hypertension in patients with left heart disease: Impact of new guidelines. Clin. Res. Cardiol. 2023, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Hemnes, A.R.; Opotowsky, A.R.; Assad, T.R.; Xu, M.; Doss, L.N.; Farber-Eger, E.; Wells, Q.S.; Brittain, E.L. Features Associated With Discordance Between Pulmonary Arterial Wedge Pressure and Left Ventricular End Diastolic Pressure in Clinical Practice: Implications for Pulmonary Hypertension Classification. Chest 2018, 154, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Gosling, R.; Swoboda, P.; Jones, R.; Rothman, A.; Wild, J.M.; Kiely, D.G.; Condliffe, R.; Alabed, S.; Swift, A.J. Cardiac magnetic resonance identifies raised left ventricular filling pressure: Prognostic implications. Eur. Heart J. 2022, 43, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Grafton-Clarke, C.; Matthews, G.; Swoboda, P.; Zhong, L.; Aung, N.; Thomson, R.; Alabed, S.; Demirkiran, A.; Vassiliou, V.S.; et al. Sex-specific cardiac magnetic resonance pulmonary capillary wedge pressure. Eur. Heart J. Open 2024, 4, oeae038. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Javed, W.; Assadi, H.; Alabed, S.; Grafton-Clarke, C.; Swift, A.J.; Williams, G.; Al-Mohammad, A.; Sawh, C.; Vassiliou, V.S.; et al. An acute increase in Left Atrial volume and left ventricular filling pressure during Adenosine administered myocardial hyperaemia: CMR First-Pass Perfusion Study. BMC Cardiovasc. Disord. 2023, 23, 246. [Google Scholar] [CrossRef]
- Varadarajan, V.; Ambale-Venkatesh, B.; Hong, S.Y.; Habibi, M.; Ashikaga, H.; Wu, C.O.; Chen, L.Y.; Heckbert, S.R.; Bluemke, D.A.; Lima, J.A.C. Association of Longitudinal Changes in NT-proBNP With Changes in Left Atrial Volume and Function: MESA. Am. J. Hypertens. 2021, 34, 626–635. [Google Scholar] [CrossRef]
- Hunt, P.J.; Richards, A.M.; Nicholls, M.G.; Yandle, T.G.; Doughty, R.N.; Espiner, E.A. Immunoreactive amino-terminal pro-brain natriuretic peptide (NT-PROBNP): A new marker of cardiac impairment. Clin. Endocrinol. 1997, 47, 287–296. [Google Scholar] [CrossRef]
- Pezel, T.; Ambale Venkatesh, B.; Kato, Y.; De Vasconcellos, H.D.; Heckbert, S.R.; Wu, C.O.; Post, W.S.; Bluemke, D.A.; Cohen-Solal, A.; Henry, P.; et al. Left Atrioventricular Coupling Index to Predict Incident Heart Failure: The Multi-Ethnic Study of Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 704611. [Google Scholar] [CrossRef]
- Pezel, T.; Ambale-Venkatesh, B.; Quinaglia, T.; Heckbert, S.R.; Kato, Y.; de Vasconcellos, H.D.; Wu, C.O.; Post, W.S.; Henry, P.; Bluemke, D.A.; et al. Change in Left Atrioventricular Coupling Index to Predict Incident Atrial Fibrillation: The Multi-Ethnic Study of Atherosclerosis (MESA). Radiology 2022, 303, 317–326. [Google Scholar] [CrossRef]
- Lange, T.; Backhaus, S.J.; Schulz, A.; Evertz, R.; Kowallick, J.T.; Bigalke, B.; Hasenfuss, G.; Thiele, H.; Stiermaier, T.; Eitel, I.; et al. Cardiovascular magnetic resonance-derived left atrioventricular coupling index and major adverse cardiac events in patients following acute myocardial infarction. J. Cardiovasc. Magn. Reson. 2023, 25, 24. [Google Scholar] [CrossRef] [PubMed]
- Halpern, S.D.; Taichman, D.B. Misclassification of pulmonary hypertension due to reliance on pulmonary capillary wedge pressure rather than left ventricular end-diastolic pressure. Chest 2009, 136, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Kerkhof, P.L. Characterizing heart failure in the ventricular volume domain. Clin. Med. Insights Cardiol. 2015, 9, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Triposkiadis, F.; Pieske, B.; Butler, J.; Parissis, J.; Giamouzis, G.; Skoularigis, J.; Brutsaert, D.; Boudoulas, H. Global left atrial failure in heart failure. Eur. J. Heart Fail. 2016, 18, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Proff, J.; et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018, 378, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Sohns, C.; Fox, H.; Marrouche, N.F.; Crijns, H.; Costard-Jaeckle, A.; Bergau, L.; Hindricks, G.; Dagres, N.; Sossalla, S.; Schramm, R.; et al. Catheter Ablation in End-Stage Heart Failure with Atrial Fibrillation. N. Engl. J. Med. 2023, 389, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Kowey, P.R. Building Castles in the Sky: Catheter Ablation in Patients With Atrial Fibrillation and Chronic Heart Failure. Circulation 2018, 138, 751–753. [Google Scholar] [CrossRef] [PubMed]
- Aalders, M.; Kok, W. Comparison of Hemodynamic Factors Predicting Prognosis in Heart Failure: A Systematic Review. J. Clin. Med. 2019, 8, 1757. [Google Scholar] [CrossRef]
- Grafton-Clarke, C.; Garg, P.; Swift, A.J.; Alabed, S.; Thomson, R.; Aung, N.; Chambers, B.; Klassen, J.; Levelt, E.; Farley, J.; et al. Cardiac magnetic resonance left ventricular filling pressure is linked to symptoms, signs and prognosis in heart failure. ESC Heart Fail. 2023, 10, 3067–3076. [Google Scholar] [CrossRef] [PubMed]
- Mascherbauer, J.; Zotter-Tufaro, C.; Duca, F.; Binder, C.; Koschutnik, M.; Kammerlander, A.A.; Aschauer, S.; Bonderman, D. Wedge Pressure Rather Than Left Ventricular End-Diastolic Pressure Predicts Outcome in Heart Failure with Preserved Ejection Fraction. JACC Heart Fail. 2017, 5, 795–801. [Google Scholar] [CrossRef]
| n | All | n | PAWP ≤ 15 mmHg | n | PAWP > 15 mmHg | p | |
|---|---|---|---|---|---|---|---|
| Age, years | 121 | 63 (55; 74) | 57 | 62 (54; 74) | 64 | 64 (56; 75) | 0.58 |
| Men, n (%) | 121 | 87 (71.9%) | 57 | 37 (64.9%) | 64 | 50 (78.1%) | 0.16 |
| HFrEF, n (%) | 121 | 89 (74%) | 57 | 39 (68%) | 64 | 50 (78%) | 0.30 |
| NYHA class ≥III, n (%) | 121 | 62 (51.2%) | 57 | 27 (47.4%) | 64 | 35 (54.7%) | 0.47 |
| BMI, kg/m2 | 121 | 26.9 (23.7; 29.9) | 57 | 25.8 (23.4; 29.6) | 64 | 27.7 (24.0; 30.3) | 0.13 |
| DCM, % | 121 | 58 (48%) | 57 | 27 (47%) | 64 | 31 (53%) | 0.98 |
| CAD, % | 121 | 61 (50.4%) | 57 | 27 (47.4%) | 64 | 34 (53.1%) | 0.59 |
| Atrial fibrillation, n (%) | 121 | 32 (26.4%) | 57 | 15 (26.3%) | 64 | 17 (26.6%) | 1.00 |
| Medication | |||||||
| Betablocker, n (%) | 121 | 112 (92.6%) | 57 | 52 (91.2%) | 64 | 60 (93.8%) | 0.73 |
| ACEi/ARB/ARNI, n (%) | 121 | 113 (93.4%) | 57 | 55 (96.5%) | 64 | 58 (90.6%) | 0.28 |
| MRA, n (%) | 121 | 78 (64.5%) | 57 | 36 (63.2%) | 64 | 42 (65.6%) | 0.85 |
| Loop diuretics, n (%) | 121 | 93 (76.9%) | 57 | 38 (66.7%) | 64 | 55 (85.9%) | 0.017 |
| Laboratory | |||||||
| eGFR, mL/min/1.73 m2 | 121 | 69 (55; 81) | 57 | 72 (60; 86) | 64 | 67 (49; 78) | 0.042 |
| Hemoglobin, g/dL | 121 | 14.0 (12.6; 14.9) | 57 | 14.1 (12.8; 15.6) | 64 | 13.9 (12.5; 14.8) | 0.16 |
| NT-proBNP, pg/mL | 98 | 3994 (1218; 8379) | 46 | 1733 (910; 5088) | 52 | 7104 (2276; 14,073) | <0.001 |
| Echocardiography | |||||||
| Aortic stenosis °III, n (%) | 119 | 16 (13.3%) | 55 | 4 (7.3%) | 64 | 12 (18.8%) | 0.07 |
| Mitral regurgitation °III, n (%) | 119 | 16 (13.4%) | 55 | 6 (10.7%) | 64 | 10 (15.6%) | 0.59 |
| TAPSE, mm | 114 | 17 (14; 20) | 51 | 18 (15; 21) | 63 | 15 (12; 19) | 0.002 |
| Right heart catheterization | |||||||
| Cardiac output, L/min | 121 | 4.9 (4.1; 5.8) | 57 | 4.9 (4.4; 5.8) | 64 | 5.0 (3.7; 5.9) | 0.43 |
| Cardiac index, L/min/m2 | 121 | 2.6 (2.3; 3.0) | 57 | 2.7 (2.4; 3.1) | 64 | 2.5 (1.9; 2.9) | 0.066 |
| PVR, Wood units | 121 | 2.0 (1.3; 2.9) | 57 | 1.6 (1.3; 2.3) | 61 | 2.3 (1.3; 3.9) | 0.027 |
| LVEDP, mmHG | 75 | 24 (17; 29) | 33 | 17 (9; 22) | 42 | 28 (24; 31) | <0.001 |
| mPAWP, mmHg | 121 | 17 (9; 25) | 57 | 9 (6; 12) | 64 | 24 (20; 28) | <0.001 |
| mPAP, mmHG | 121 | 25 (18; 37) | 57 | 17 (14; 22) | 64 | 37 (30; 41) | <0.001 |
| mRAP, mmHG | 118 | 7 (4; 12) | 56 | 4 (2; 7) | 62 | 10 (8; 13) | <0.001 |
| mPAP > 20 mmHg | 121 | 76 (62.8%) | 57 | 16 (28.1%) | 64 | 60 (93.8%) | <0.001 |
| Pre-capillary PH, n (%) | 121 | 12 (9.9%) | 57 | 12 (21.1%) | 64 | 0 (0.0%) | 0.020 |
| Post-capillary PH, n (%) | 121 | 62 (50.8%) | 57 | 0 (0.0%) | 64 | 62 (96.9%) | <0.001 |
| Cardiac magnetic resonance imaging | |||||||
| LAEF, % | 105 | 23 (14; 34) | 47 | 31 (22; 40) | 58 | 17 (10; 27) | <0.001 |
| LAVED, mL | 105 | 85 (61; 120) | 47 | 65 (38; 101) | 58 | 95 (72; 132) | 0.001 |
| LAViED, mL/m2 | 105 | 43 (31; 60) | 47 | 34 (21; 56) | 58 | 48 (38; 70) | <0.001 |
| LAVES, mL | 105 | 112 (78; 153) | 47 | 88 (66; 134) | 58 | 114 (100; 161) | 0.005 |
| LAViES, mL/m2 | 105 | 57 (42; 74) | 47 | 47 (36; 72) | 58 | 60 (50; 79) | 0.006 |
| LACiED, % | 105 | 33 (24; 50) | 47 | 31 (20; 46) | 58 | 34 (26; 54) | 0.11 |
| LVEF, % | 121 | 30 (24; 41) | 57 | 34 (27; 44) | 64 | 28 (22; 38) | 0.025 |
| LVEDD, mm | 121 | 66 (60; 73) | 57 | 64 (59; 71) | 64 | 68 (60; 76) | 0.027 |
| LVEDV, mL | 121 | 253 (192; 313) | 57 | 218 (167; 277) | 64 | 276 (208; 346) | 0.002 |
| LVEDVi, mL/m2 | 121 | 131 (100; 158) | 57 | 117 (88; 148) | 64 | 143 (108; 172) | 0.012 |
| LVESV, mL | 121 | 167 (116; 231) | 57 | 149 (106; 201) | 64 | 194 (124; 256) | 0.002 |
| LVESVi, mL/m2 | 121 | 87 (62; 120) | 57 | 78 (55; 107) | 64 | 98 (68; 130) | 0.008 |
| LV stroke volume, mL | 121 | 74 (62; 91) | 57 | 73 (59; 88) | 64 | 77 (64; 94) | 0.28 |
| LVSVi, mL/m2 | 121 | 39 (33; 46) | 57 | 39 (32; 46) | 63 | 39 (33; 48) | 0.83 |
| RVEF, % | 120 | 46 (36; 56) | 56 | 53 (41; 61) | 64 | 42 (34; 52) | 0.002 |
| RA area, mm2 | 120 | 25 (19; 29) | 56 | 22 (18; 27) | 64 | 27 (20; 31) | 0.012 |
| RVEDD, mm | 121 | 33 (29; 38) | 57 | 32 (28; 35) | 64 | 34 (29; 39) | 0.087 |
| RVEDV, mL | 120 | 158 (122; 205) | 56 | 142 (99; 184) | 64 | 184 (141; 236) | 0.001 |
| RVEDVi, mL/m2 | 120 | 84 (63; 105) | 56 | 73 (57; 94) | 64 | 89 (72; 114) | 0.003 |
| RVESV, mL | 120 | 89 (51; 125) | 56 | 61 (42; 110) | 64 | 98 (66; 152) | <0.001 |
| RVESVi, mL/m2 | 120 | 44 (27; 65) | 56 | 33 (21; 60) | 64 | 49 (35; 72) | 0.001 |
| RV stroke volume, mL | 120 | 70 (58; 86) | 56 | 68 (53; 85) | 64 | 72 (61; 87) | 0.28 |
| RVSVi, mL/m2 | 120 | 37 (30; 45) | 56 | 36 (28; 43) | 64 | 37 (31; 45) | 0.54 |
| Predictors of PAWP > 15 mmHg | Univariable | Multivariable |
|---|---|---|
| Loop diuretics, yes vs. no | 3.06 (1.25; 7.47); p = 0.014 | - |
| TAPSE, per mm | 0.86 (0.78; 0.95); p = 0.002 | - |
| NTproBNP, per 1000 pg/mL | 1.23 (1.10; 1.39); p < 0.001 | 1.18 (1.03; 1.36); p = 0.018 |
| GFR, per 10 mL/min/1.73 m2 | 0.79 (0.65; 0.95); p = 0.012 | - |
| LAEF, per % | 0.93 (0.89; 0.96); p < 0.001 | 0.93 (0.88; 0.98); p = 0.004 |
| * LAVED, per mL | 1.02 (1.01; 1.03); p = 0.002 | |
| * LAViED, per mL/m2 | 1.03 (1.01; 1.06); p = 0.001 | |
| * LAVES, per mL | 1.01 (1.00; 1.02); p = 0.012 | |
| LAViES, per mL/m2 | 1.02 (1.00; 1.04); p = 0.013 | - |
| LVEF, per % | 0.96 (0.93; 1.00); p = 0.038 | - |
| ** LVEDD, per mm | 1.05 (1.00; 1.09); p = 0.029 | - |
| ** LVEDV, per mL | 1.01 (1.00; 1.01); p = 0.002 | |
| ** LVEDVi, per mL/m2 | 1.01 (1.00; 1.02); p = 0.009 | |
| ** LVESV, per mL | 1.01 (1.00; 1.01); p = 0.002 | |
| LVESVi, per mL/m2 | 1.01 (1.00; 1.02); p = 0.006 | 1.03 (1.00; 1.05); p = 0.036 |
| RA area, per mm2 | 1.06 (1.01; 1.12); p = 0.028 | |
| RVEF, per % | 0.96 (0.93; 0.99); p = 0.003 | |
| *** RVEDV, per mL | 1.01 (1.00; 1.02); p = 0.001 | |
| *** RVEDVi, per mL/m2 | 1.02 (1.01; 1.03); p = 0.005 | |
| *** RVESV, per mL | 1.01 (1.01; 1.02); p < 0.001 | |
| RVESVi, per mL/m2 | 1.03 (1.01; 1.04); p = 0.002 |
| LVEDP (mmHg) | ||||
|---|---|---|---|---|
| ≤15 | >15 | Total | ||
| PAWP (mmHg) | ≤15 | 15 | 18 | 33 (44%) |
| >15 | 1 | 41 | 42 (56%) | |
| Total | 16 (21%) | 59 (79%) | 75 (100%) | |
| n = 18 | LVEDP > 15 mmHg and PAWP ≤ 15 mmHg | n = 41 | LVEDP > 15 mmHg and PAWP > 15 mmHg | p | |
|---|---|---|---|---|---|
| Echocardiography | |||||
| TAPSE, mm | 17 | 20 (15; 24) | 41 | 16 (14; 19) | 0.015 |
| Right heart catheterization | |||||
| CI, L/min/m2 | 18 | 2.9 (2.6; 3.2) | 41 | 2.5 (2.0; 2.8) | 0.015 |
| PVR, Wood units | 18 | 1.7 (1.3; 2.4) | 41 | 2.7 (1.6; 4.6) | 0.030 |
| LVEDP, mmHG | 18 | 22 (18; 27) | 41 | 28 (24; 31) | <0.001 |
| mPAWP, mmHg | 18 | 10 (7; 13) | 41 | 25 (20; 28) | <0.001 |
| mPAP, mmHG | 18 | 19 (16; 22) | 41 | 39 (31; 42) | <0.001 |
| mPAP > 20 mmHg, n (%) | 18 | 7 (38.9%) | 41 | 39 (95.1%) | <0.001 |
| Pre-capillary PH, n (%) | 18 | 5 (27.8%) | 41 | 0 (0.0%) | <0.001 |
| Post-capillary PH, n (%) | 18 | 0 (0.0%) | 41 | 40 (97.6%) | <0.001 |
| mRAP, mmHG | 18 | 5 (3; 7) | 41 | 10 (8; 13) | <0.001 |
| Cardiac magnetic resonance imaging | |||||
| LAEF,% | 15 | 35 (25; 43) | 38 | 16 (10; 25) | <0.001 |
| LAVED, mL | 15 | 76 (38; 101) | 38 | 95 (73; 143) | 0.063 |
| LAViED, mL/m2 | 15 | 38 (20; 58) | 38 | 51 (39; 73) | 0.055 |
| LAVES, mL | 15 | 112 (70; 134) | 38 | 116 (100; 159) | 0.20 |
| LAViES, mL/m2 | 15 | 56 (41; 70) | 38 | 62 (51; 85) | 0.24 |
| LACiED, % | 15 | 33 (14; 44) | 38 | 35 (25; 59) | 0.13 |
| LVEF, % | 18 | 29 (26; 36) | 41 | 28 (24; 41) | 0.88 |
| LVEDD, mm | 18 | 68 (64; 72) | 41 | 69 (58; 78) | 0.58 |
| LVEDV, mL | 18 | 258 (211; 294) | 41 | 276 (195; 353) | 0.54 |
| LVEDVi, mL/mm2 | 18 | 135 (111; 155) | 41 | 140 (100; 174) | 0.73 |
| LVESV, mL | 18 | 179 (140; 206) | 41 | 186 (116; 265) | 0.66 |
| LVESVi mL/mm2 | 18 | 94 (77; 113) | 41 | 99 (66; 131) | 0.77 |
| LV stroke volume | 18 | 74 (60; 87) | 41 | 79 (65; 94) | 0.32 |
| LVSVi, mL/m2 | 18 | 39 (31; 49) | 41 | 40 (35; 47) | 0.68 |
| RVEF, % | 18 | 45 (35; 63) | 41 | 44 (34; 53) | 0.34 |
| RA area, mm2 | 18 | 21 (19; 26) | 41 | 27 (20; 31) | 0.06 |
| RVEDD, mm | 18 | 34 (28; 40) | 41 | 32 (28; 40) | 0.77 |
| RVEDV mL/m2 | 18 | 145 (121; 196) | 41 | 181 (137; 237) | 0.12 |
| RVEDVi, mL/m2 | 18 | 76 (63; 96) | 41 | 90 (75; 116) | 0.11 |
| RVESV, mL | 18 | 74 (49; 130) | 41 | 98 (64; 149) | 0.13 |
| RVESVi, mL | 18 | 40 (24; 64) | 41 | 49 (36; 72) | 0.13 |
| RV stroke volume, mL | 18 | 69 (58; 93) | 41 | 72 (62; 85) | 0.84 |
| RVSVi, mL/m2 | 18 | 36 (29; 45) | 41 | 37 (31; 45) | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güder, G.; Reiter, T.; Drayss, M.; Bauer, W.; Lengenfelder, B.; Nordbeck, P.; Fette, G.; Frantz, S.; Morbach, C.; Störk, S. Improved Interpretation of Pulmonary Artery Wedge Pressures through Left Atrial Volumetry—A Cardiac Magnetic Resonance Imaging Study. J. Cardiovasc. Dev. Dis. 2024, 11, 178. https://doi.org/10.3390/jcdd11060178
Güder G, Reiter T, Drayss M, Bauer W, Lengenfelder B, Nordbeck P, Fette G, Frantz S, Morbach C, Störk S. Improved Interpretation of Pulmonary Artery Wedge Pressures through Left Atrial Volumetry—A Cardiac Magnetic Resonance Imaging Study. Journal of Cardiovascular Development and Disease. 2024; 11(6):178. https://doi.org/10.3390/jcdd11060178
Chicago/Turabian StyleGüder, Gülmisal, Theresa Reiter, Maria Drayss, Wolfgang Bauer, Björn Lengenfelder, Peter Nordbeck, Georg Fette, Stefan Frantz, Caroline Morbach, and Stefan Störk. 2024. "Improved Interpretation of Pulmonary Artery Wedge Pressures through Left Atrial Volumetry—A Cardiac Magnetic Resonance Imaging Study" Journal of Cardiovascular Development and Disease 11, no. 6: 178. https://doi.org/10.3390/jcdd11060178
APA StyleGüder, G., Reiter, T., Drayss, M., Bauer, W., Lengenfelder, B., Nordbeck, P., Fette, G., Frantz, S., Morbach, C., & Störk, S. (2024). Improved Interpretation of Pulmonary Artery Wedge Pressures through Left Atrial Volumetry—A Cardiac Magnetic Resonance Imaging Study. Journal of Cardiovascular Development and Disease, 11(6), 178. https://doi.org/10.3390/jcdd11060178








