Immediate Increase in the Root Mean Square of Successive Differences after Three Bouts of Remote Ischemic Preconditioning: A Randomized Controlled Trial
Abstract
1. Introduction
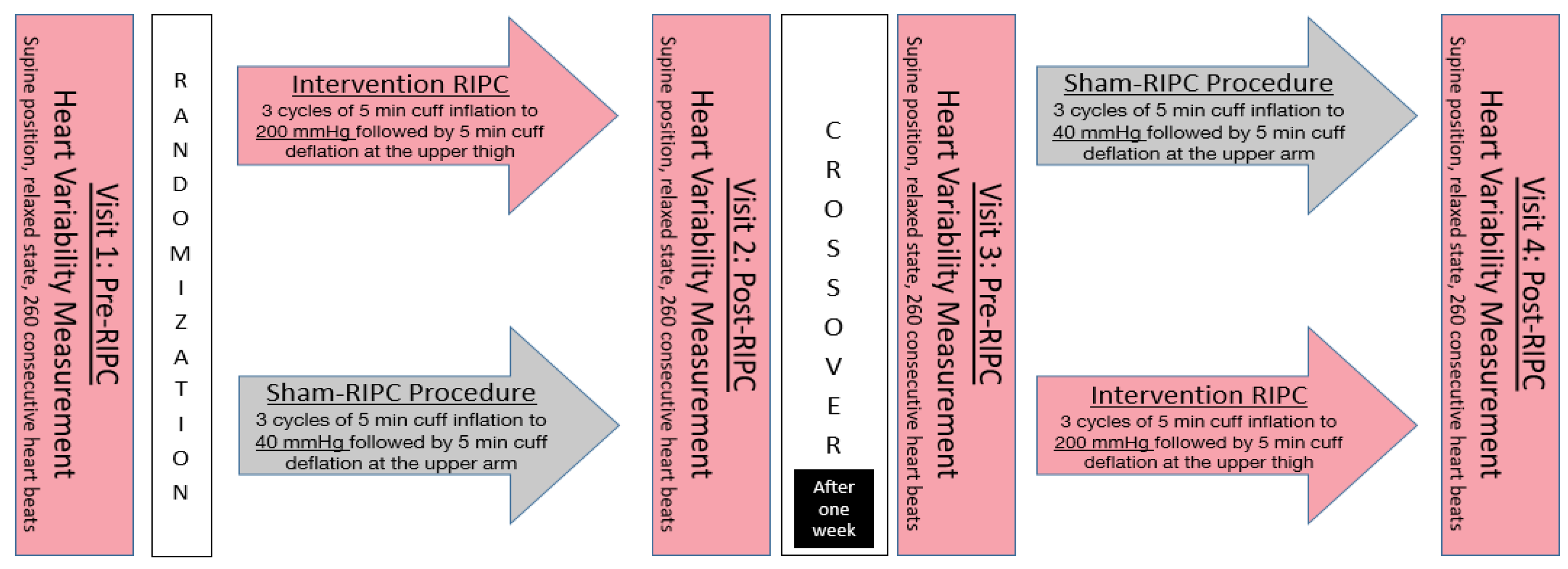
2. Materials and Methods
2.1. Study Subjects
2.2. Assessment of the Autonomous Nervous System
2.3. Intervention
2.4. Data Analyses
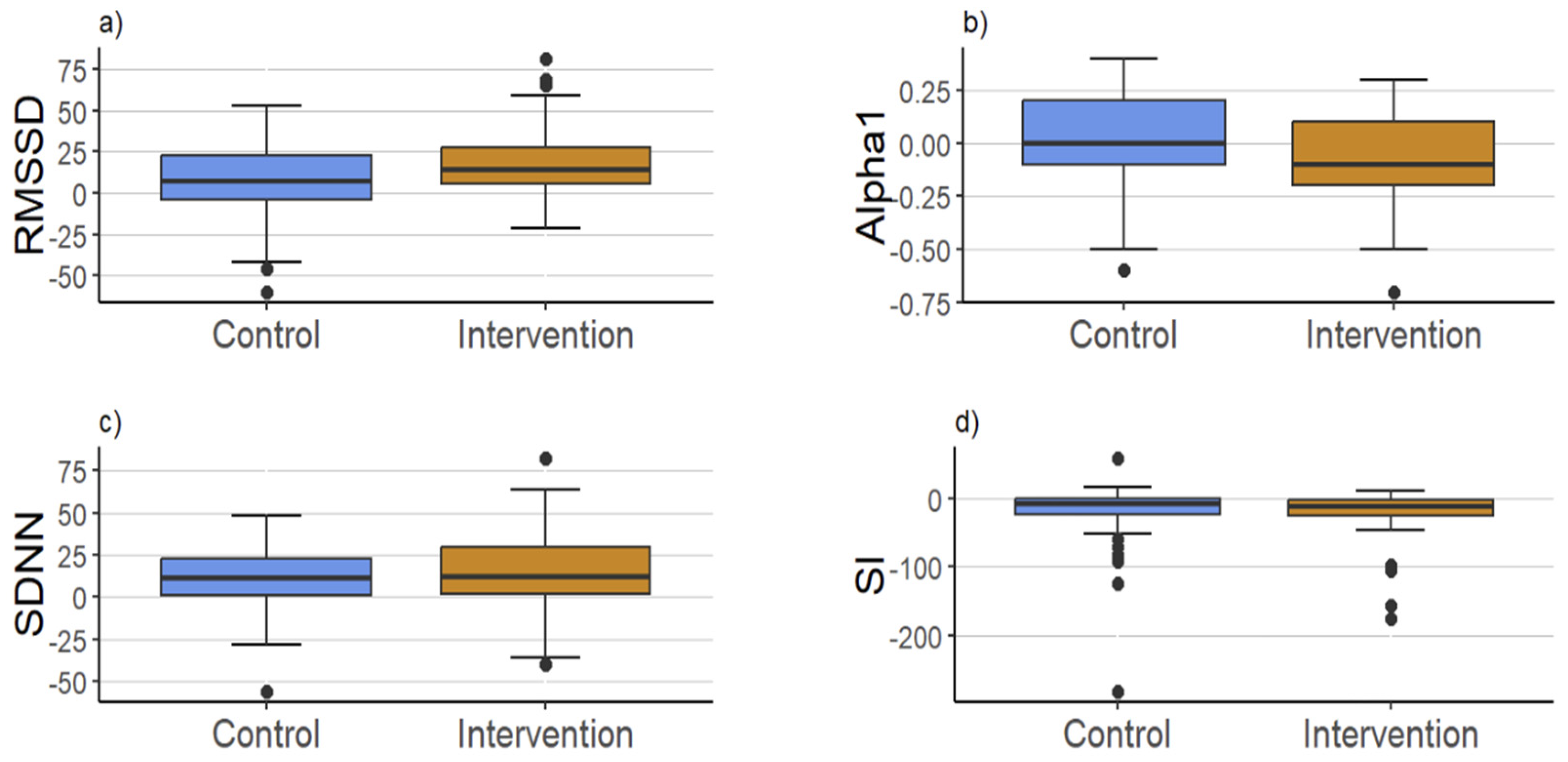
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heusch, G.; Bøtker, H.E.; Przyklenk, K.; Redington, A.; Yellon, D. Remote ischemic conditioning. J. Am. Coll. Cardiol. 2015, 65, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, M. Ischemic preconditioning: Potential impact on exercise performance and underlying mechanisms. J. Phys. Fit. Sports Med. 2017, 6, 15–23. [Google Scholar] [CrossRef][Green Version]
- Vasdekis, S.N.; Athanasiadis, D.; Lazaris, A.; Martikos, G.; Katsanos, A.H.; Tsivgoulis, G.; Machairas, A.; Liakakos, T. The role of remote ischemic preconditioning in the treatment of atherosclerotic diseases. Brain Behav. 2013, 3, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pinzón, M.A.; Xu, G.-P.; Dietrich, W.D.; Rosenthal, M.; Sick, T.J. Rapid preconditioning protects rats against ischemic neuronal damage after 3 but not 7 days of reperfusion following global cerebral ischemia. J. Cereb. Blood Flow Metab. 1997, 17, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.C.; Blauenfeldt, R.A.; Andersen, G.; Hougaard, K.D.; Hoda, M.N.; Ding, Y.; Ji, X. Remote ischaemic conditioning—A new paradigm of self-protection in the brain. Nat. Rev. Neurol. 2015, 11, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Taebling, M.; Oberhoffer, R. Remote Ischemic Preconditioning Has No Short Term Effect on Blood Pressure, Heart Rate, and Arterial Stiffness in Healthy Young Adults. Front. Physiol. 2019, 10, 479281. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, S.; Fleishman, A.N.; Khaliulin, I.; Meloni, M.; Angelini, G.D.; Suleiman, M.S. Remote ischemic preconditioning triggers changes in autonomic nervous system activity: Implications for cardioprotection. Physiol. Rep. 2017, 5, e13085. [Google Scholar] [CrossRef] [PubMed]
- Pomeranz, B.; Macaulay, R.J.; Caudill, M.A.; Kutz, I.; Adam, D.; Gordon, D.; Kilborn, K.M.; Barger, A.C.; Shannon, D.C.; Cohen, R.J.; et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am. J. Physiol.-Heart Circ. Physiol. 1985, 248, H151–H153. [Google Scholar] [CrossRef] [PubMed]
- Jose, A.D.; Collison, D. The normal range and determinants of the intrinsic heart rate in man1. Cardiovasc. Res. 1970, 4, 160–167. [Google Scholar] [CrossRef]
- Malik, M.; Hnatkova, K.; Huikuri, H.V.; Lombardi, F.; Schmidt, G.; Zabel, M. CrossTalk proposal: Heart rate variability is a valid measure of cardiac autonomic responsiveness. J. Physiol. 2019, 597, 2595–2598. [Google Scholar] [CrossRef]
- Gho, B.C.; Schoemaker, R.G.; van den Doel, M.A.; Duncker, D.J.; Verdouw, P.D. Myocardial protection by brief ischemia in noncardiac tissue. Circulation 1996, 94, 2193–2200. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Naggar, I.; Stewart, M.; Rosenbaum, D.M. Neurogenic pathway mediated remote preconditioning protects the brain from transient focal ischemic injury. Brain Res. 2011, 1386, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Ren, C.; Chen, X.; Zhao, H. The chronic protective effects of limb remote preconditioning and the underlying mechanisms involved in inflammatory factors in rat stroke. PLoS ONE 2012, 7, e30892. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J.M.J.; Burke, N.; Davidson, S.M.; Yellon, D.M. Intrinsic cardiac ganglia and acetylcholine are important in the mechanism of ischaemic preconditioning. Basic Res. Cardiol. 2017, 112, 11. [Google Scholar] [CrossRef]
- Shimizu, M.; Konstantinov, I.E.; Kharbanda, R.K.; Cheung, M.H.; Redington, A.N. Effects of intermittent lower limb ischaemia on coronary blood flow and coronary resistance in pigs. Acta Physiol. 2007, 190, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Noronha Osório, D.; Viana-Soares, R.; Marto, J.P.; Mendonça, M.D.; Silva, H.P.; Quaresma, C.; Viana-Baptista, M.; Gamboa, H.; Vieira, H.L.A. Autonomic nervous system response to remote ischemic conditioning: Heart rate variability assessment. BMC Cardiovasc. Disord. 2019, 19, 211. [Google Scholar] [CrossRef] [PubMed]
- Khaliulin, I.; Fleishman, A.N.; Shumeiko, N.I.; Korablina, T.; Petrovskiy, S.A.; Ascione, R.; Suleiman, M.S. Neuro-autonomic changes induced by remote ischemic preconditioning (RIPC) in healthy young adults: Implications for stress. Neurobiol. Stress 2019, 11, 100189. [Google Scholar] [CrossRef] [PubMed]
- Baxter, G.; Marber, M.; Patel, V.; Yellon, D. Adenosine receptor involvement in a delayed phase of myocardial protection 24 hours after ischemic preconditioning. Circulation 1994, 90, 2993–3000. [Google Scholar] [CrossRef] [PubMed]
- Loukogeorgakis, S.P.; Panagiotidou, A.T.; Broadhead, M.W.; Donald, A.; Deanfield, J.E.; MacAllister, R.J. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: Role of the autonomic nervous system. J. Am. Coll. Cardiol. 2005, 46, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Kumar, M.; Singh, N.; Jaggi, A.S. Late Phases of Cardioprotection During Remote Ischemic Preconditioning and Adenosine Preconditioning Involve Activation of Neurogenic Pathway. J. Cardiovasc. Pharmacol. 2019, 73, 63–69. [Google Scholar] [CrossRef]
- Jones, H.; Hopkins, N.; Bailey, T.G.; Green, D.J.; Cable, N.T.; Thijssen, D.H. Seven-day remote ischemic preconditioning improves local and systemic endothelial function and microcirculation in healthy humans. Am. J. Hypertens. 2014, 27, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Ueda, K.; Goto, C.; Jitsuiki, D.; Nishioka, K.; Umemura, T.; Noma, K.; Yoshizumi, M.; Chayama, K.; Higashi, Y. Repetition of ischemic preconditioning augments endothelium-dependent vasodilation in humans: Role of endothelium-derived nitric oxide and endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Banks, L.; Wells, G.D.; Clarizia, N.A.; Jean-St-Michel, E.; McKillop, A.L.; Redington, A.N.; McCrindle, B.W. Short-term remote ischemic preconditioning is not associated with improved blood pressure and exercise capacity in young adults. Appl. Physiol. Nutr. Metab. 2016, 41, 903–906. [Google Scholar] [CrossRef]
- Ruha, A.; Sallinen, S.; Nissila, S. A real-time microprocessor QRS detector system with a 1-ms timing accuracy for the measurement of ambulatory HRV. IEEE Trans. Biomed. Eng. 1997, 44, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Niskanen, J.P.; Lipponen, J.A.; Ranta-Aho, P.O.; Karjalainen, P.A. Kubios HRV—heart rate variability analysis software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Baevsky, R.M. Methodical Recommendations Use Kardivar System for Determination of the Stress Level and Estimation of the Body Adaptability Standards of Measurements and Physiological Interpretation 2009. Available online: https://api.semanticscholar.org/CorpusID:29215863 (accessed on 21 June 2024).
- Balaban, S. Stress objektiv messen–der neue holistische Ansatz. In Resilienz für die VUCA-Welt: Individuelle und Organisationale Resilienz Entwickeln; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2018; p. 153. [Google Scholar]
- Maestri, R.; Raczak, G.; Danilowicz-Szymanowicz, L.; Torunski, A.; Sukiennik, A.; Kubica, J.; La Rovere, M.T.; Pinna, G.D. Reliability of heart rate variability measurements in patients with a history of myocardial infarction. Clin. Sci. 2010, 118, 195–201. [Google Scholar] [CrossRef][Green Version]
- Kleiger, R.E.; Stein, P.K.; Bigger, J.T., Jr. Heart Rate Variability: Measurement and Clinical Utility. Ann. Noninvasive Electrocardiol. 2005, 10, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, Q.; Jin, H.; Zhu, K.; Zhi, H.; Chen, Z.; Ma, G. Effects of remote ischaemic conditioning on heart rate variability and cardiac function in patients with mild ischaemic heart failure. Heart Lung Circ. 2018, 27, 477–483. [Google Scholar] [CrossRef]
- Wei, L.; Liang, H.; Mo, M.; Liu, Z.; Ye, R.; Ye, H.; Ouyang, W.; Yu, W.; Zhao, W.; Zhang, X. The effect of remote ischemic postconditioning on autonomic function in patients with acute ischemic stroke: A Randomized Controlled Trail. Complement. Ther. Med. 2020, 54, 102541. [Google Scholar] [CrossRef]
- Qu, Y.; Liu, J.; Guo, Z.N.; Zhang, P.D.; Yan, X.L.; Zhang, P.; Qi, S.; Yang, Y. The Impact of Remote Ischaemic Conditioning on Beat-to-Beat Heart Rate Variability Circadian Rhythm in Healthy Adults. Heart Lung Circ. 2021, 30, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, J.; Pryds, K.; Salman, R.; Løfgren, B.; Kristiansen, S.B.; Bøtker, H.E. The remote ischemic preconditioning algorithm: Effect of number of cycles, cycle duration and effector organ mass on efficacy of protection. Basic Res. Cardiol. 2016, 111, 10. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, R.K.; Peters, M.; Walton, B.; Kattenhorn, M.; Mullen, M.; Klein, N.; Vallance, P.; Deanfield, J.; MacAllister, R. Ischemic preconditioning prevents endothelial injury and systemic neutrophil activation during ischemia-reperfusion in humans in vivo. Circulation 2001, 103, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Bøtker, H.E.; Kharbanda, R.; Schmidt, M.R.; Bøttcher, M.; Kaltoft, A.K.; Terkelsen, C.J.; Munk, K.; Andersen, N.H.; Hansen, T.M.; Trautner, S. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: A randomised trial. Lancet 2010, 375, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Carter, H.H.; Maxwell, J.D.; Hellsten, Y.; Thompson, A.; Thijssen, D.H.; Jones, H. The impact of acute remote ischaemic preconditioning on cerebrovascular function. Eur. J. Appl. Physiol. 2020, 120, 603–612. [Google Scholar] [CrossRef]
- Gardner, R.N.; Sabino-Carvalho, J.L.; Kim, J.; Vianna, L.C.; Lang, J.A. Two weeks of remote ischaemic preconditioning alters sympathovagal balance in healthy humans. Exp. Physiol. 2020, 105, 1500–1506. [Google Scholar] [CrossRef]
- Jensen, R.V.; Zachara, N.E.; Nielsen, P.H.; Kimose, H.H.; Kristiansen, S.B.; Bøtker, H.E. Impact of O-GlcNAc on cardioprotection by remote ischaemic preconditioning in non-diabetic and diabetic patients. Cardiovasc. Res. 2013, 97, 369–378. [Google Scholar] [CrossRef]
- Jang, M.-H.; Kim, D.-H.; Han, J.-H.; Kim, J.; Kim, J.-H. A Single Bout of Remote Ischemic Preconditioning Suppresses Ischemia-Reperfusion Injury in Asian Obese Young Men. Int. J. Environ. Res. Public Health 2023, 20, 3915. [Google Scholar] [CrossRef] [PubMed]
- Trachte, T.E.; Hemenway, B.A.; Van Guilder, G.P. Reduced effect of ischemic preconditioning against endothelial ischemia-reperfusion injury with cardiovascular risk factors in humans. J. Hum. Hypertens. 2021, 35, 870–879. [Google Scholar] [CrossRef]
- Enko, K.; Nakamura, K.; Yunoki, K.; Miyoshi, T.; Akagi, S.; Yoshida, M.; Toh, N.; Sangawa, M.; Nishii, N.; Nagase, S.; et al. Intermittent arm ischemia induces vasodilatation of the contralateral upper limb. J. Physiol. Sci. 2011, 61, 507. [Google Scholar] [CrossRef]
- Adamopoulos, S.; Ponikowski, P.; Cerquetani, E.; Piepoli, M.; Rosano, G.; Sleight, P.; Coats, A. Circadian pattern of heart rate variability in chronic heart failure patients Effects of physical training. Eur. Heart J. 1995, 16, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Coats, A.; Adamopoulos, S.; Radaelli, A.; McCance, A.; Meyer, T.; Bernardi, L.; Solda, P.; Davey, P.; Ormerod, O.; Forfar, C. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation 1992, 85, 2119–2131. [Google Scholar] [CrossRef]
- Li, M.; Zheng, C.; Sato, T.; Kawada, T.; Sugimachi, M.; Sunagawa, K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 2004, 109, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E.; Schwartz, P.J.; Stone, H.L. The effects of daily exercise on susceptibility to sudden cardiac death. Circulation 1984, 69, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Vanoli, E.; De Ferrari, G.M.; Stramba-Badiale, M.; Hull, S.S., Jr.; Foreman, R.D.; Schwartz, P.J. Vagal stimulation and prevention of sudden death in conscious dogs with a healed myocardial infarction. Circ. Res. 1991, 68, 1471–1481. [Google Scholar] [CrossRef]
- La Rovere, M.T.; Porta, A.; Schwartz, P.J. Autonomic control of the heart and its clinical impact. A personal perspective. Front. Physiol. 2020, 11, 522559. [Google Scholar] [CrossRef]
| Variable | Median [Q1, Q3] |
|---|---|
| Gender (female) | 29 (56.9%) |
| Age (years) | 24.9 [23.8, 26.4] |
| BMI | 21.9 [20.3, 23.4] |
| Heart rate (beats/min) | 66.8 [59.6, 72.3] |
| RMSSD (ms) | 50.3 [33.6, 84.5] |
| SDNN (ms) | 60.5 [46.7, 83.5] |
| SI | 28.9 [19.4, 54.6] |
| Alpha 1 | 1.00 [0.80, 1.2] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schöneburg, C.; Seyram Amevor, B.; Bauer, T.; Boateng, I.; Nsia-Tawia, B.; Öztürk, N.; Pop, M.-A.; Müller, J. Immediate Increase in the Root Mean Square of Successive Differences after Three Bouts of Remote Ischemic Preconditioning: A Randomized Controlled Trial. J. Cardiovasc. Dev. Dis. 2024, 11, 193. https://doi.org/10.3390/jcdd11070193
Schöneburg C, Seyram Amevor B, Bauer T, Boateng I, Nsia-Tawia B, Öztürk N, Pop M-A, Müller J. Immediate Increase in the Root Mean Square of Successive Differences after Three Bouts of Remote Ischemic Preconditioning: A Randomized Controlled Trial. Journal of Cardiovascular Development and Disease. 2024; 11(7):193. https://doi.org/10.3390/jcdd11070193
Chicago/Turabian StyleSchöneburg, Charlotte, Benedicta Seyram Amevor, Theresa Bauer, Ivy Boateng, Bright Nsia-Tawia, Nehir Öztürk, Maria-Alexandra Pop, and Jan Müller. 2024. "Immediate Increase in the Root Mean Square of Successive Differences after Three Bouts of Remote Ischemic Preconditioning: A Randomized Controlled Trial" Journal of Cardiovascular Development and Disease 11, no. 7: 193. https://doi.org/10.3390/jcdd11070193
APA StyleSchöneburg, C., Seyram Amevor, B., Bauer, T., Boateng, I., Nsia-Tawia, B., Öztürk, N., Pop, M.-A., & Müller, J. (2024). Immediate Increase in the Root Mean Square of Successive Differences after Three Bouts of Remote Ischemic Preconditioning: A Randomized Controlled Trial. Journal of Cardiovascular Development and Disease, 11(7), 193. https://doi.org/10.3390/jcdd11070193





