Arrhythmic Risk Stratification in Cardiac Amyloidosis: A Review of the Current Literature
Abstract
:1. Introduction
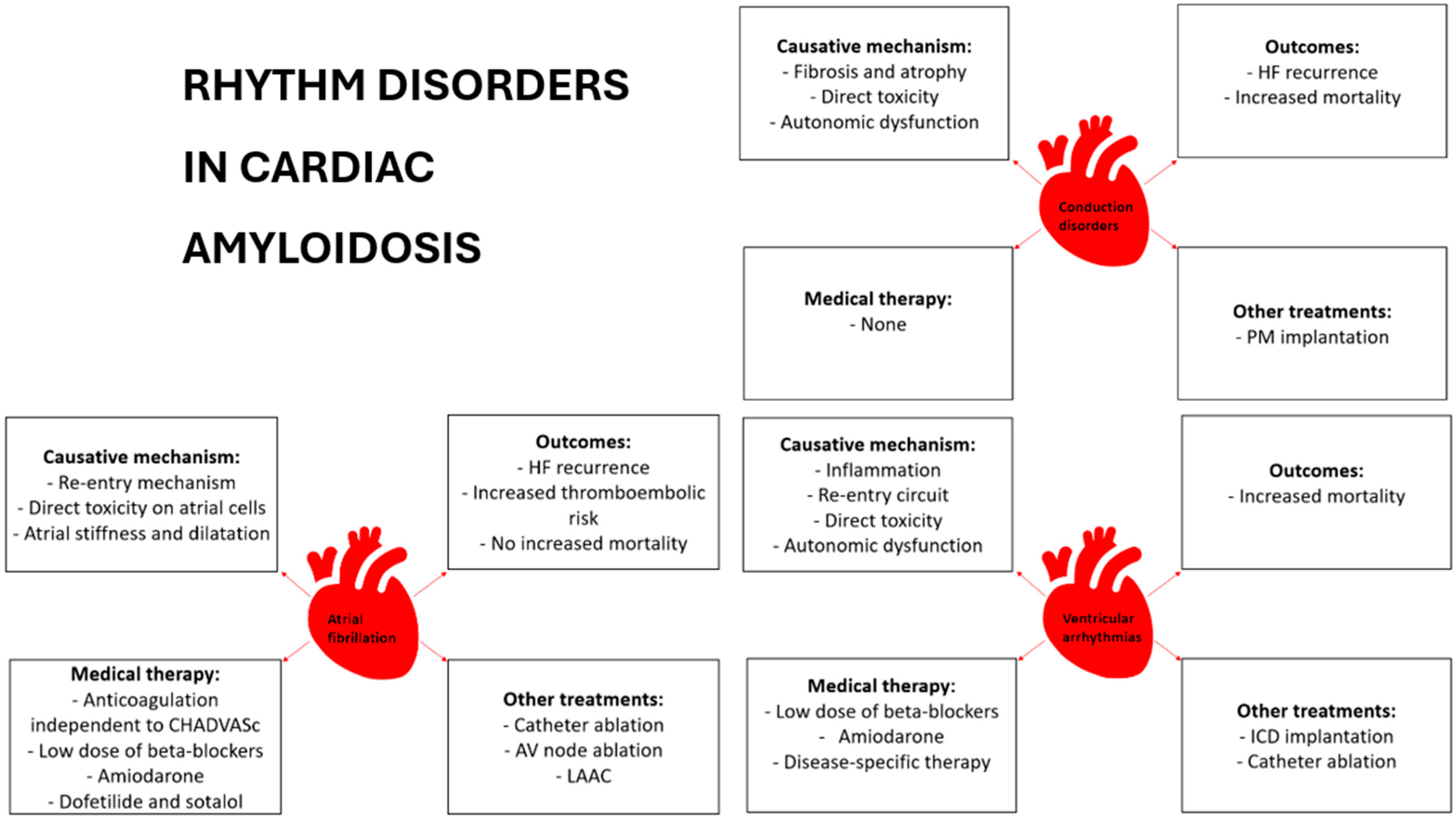
2. Conduction System Disease
2.1. Epidemiology and Risk Stratification
2.2. Anti-Bradycardia Pacing and Cardiac Resynchronization Therapy
3. Atrial Fibrillation
3.1. Epidemiology and Risk Stratification
3.2. Pharmacological Therapy
3.3. Electrical Cardioversion
3.4. Catheter Ablation
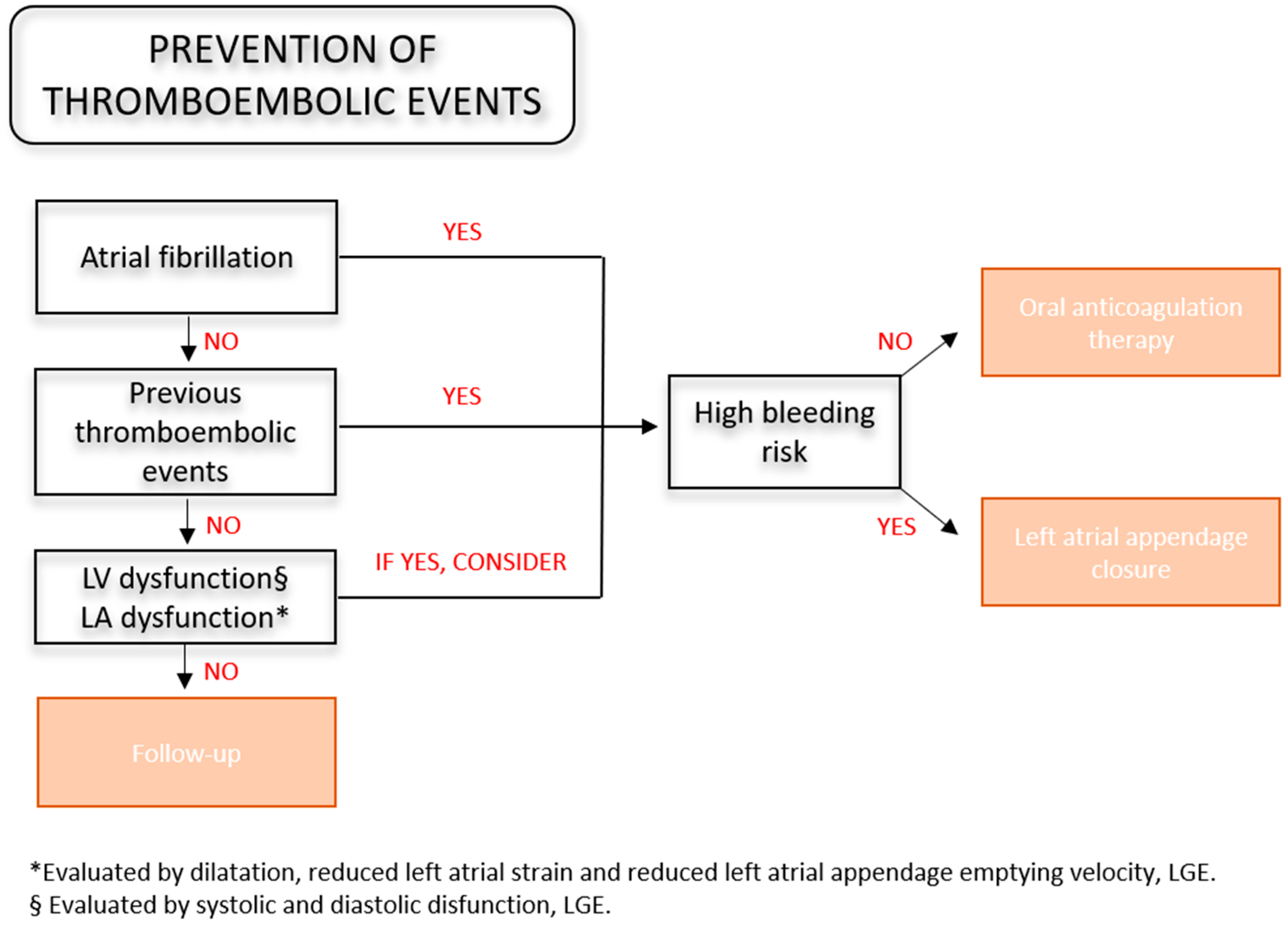
3.5. Thromboembolic Risk Assessment
3.6. Left Atrial Appendage Closure
4. Ventricular Arrythmias
4.1. Etiopathogenesis and Epidemiology
4.2. Pharmacological Therapy
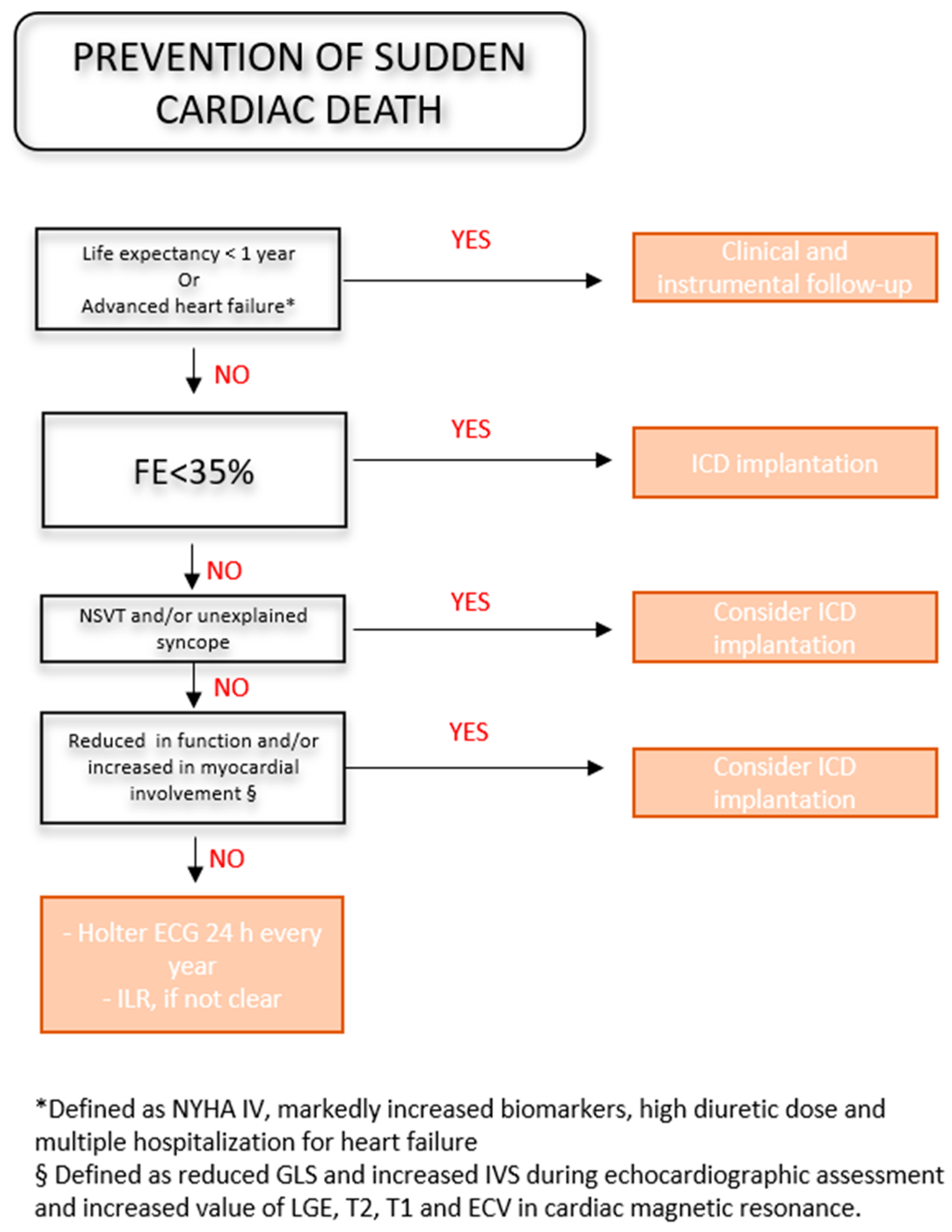
4.3. Prevention of Sudden Cardiac Death
4.4. Resynchronization Therapy
5. Limitations and Future Perspectives
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia-Pavia, P.; Rapezzi, C.; Adler, Y.; Arad, M.; Basso, C.; Brucato, A.; Burazor, I.; Caforio, A.L.P.; Damy, T.; Eriksson, U.; et al. Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2021, 42, 1554–1568. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Varrà, G.G.; Porcari, A.; Saro, R.; Pagura, L.; Lalario, A.; Dore, F.; Bussani, R.; Sinagra, G.; Merlo, M. Re-Definition of the Epidemiology of Cardiac Amyloidosis. Biomedicines 2022, 10, 1566. [Google Scholar] [CrossRef] [PubMed]
- Gilstrap, L.G.; Dominici, F.; Wang, Y.; El-Sady, M.S.; Singh, A.; Di Carli, M.F.; Falk, R.H.; Dorbala, S. Epidemiology of Cardiac Amyloidosis–Associated Heart Failure Hospitalizations Among Fee-for-Service Medicare Beneficiaries in the United States. Circ. Heart Fail. 2019, 12, e005407. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Merlo, M.; Porcari, A.; Georgiopoulos, G.; Pagura, L.; Vergaro, G.; Sinagra, G.; Emdin, M.; Rapezzi, C. Redefining the epidemiology of cardiac amyloidosis. A systematic review and meta-analysis of screening studies. Eur. J. Heart Fail. 2022, 24, 2342–2351. [Google Scholar] [CrossRef] [PubMed]
- Oghina, S.; Bougouin, W.; Kharoubi, M.; Bonnefous, L.; Galat, A.; Guendouz, S.; Bezard, M.; Le Bras, F.; Deux, J.-F.; Itti, E.; et al. Echocardiographic Patterns of Left Ventricular Diastolic Function in Cardiac Amyloidosis: An Updated Evaluation. J. Clin. Med. 2021, 10, 4888. [Google Scholar] [CrossRef] [PubMed]
- Rapezzi, C.; Aimo, A.; Barison, A.; Emdin, M.; Porcari, A.; Linhart, A.; Keren, A.; Merlo, M.; Sinagra, G. Restrictive cardiomyopathy: Definition and diagnosis. Eur. Heart J. 2022, 43, 4679–4693. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, E.T.; Jorge, A.J.L.; Souza Junior, C.V.; de Andrade, T.R. Cardiac Amyloidosis and its New Clinical Phenotype: Heart Failure with Preserved Ejection Fraction. Arq. Bras. Cardiol. 2017, 109, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Matteo, S.; Anna, C.; Federico, S.; Daniele, M.; Gioele, F.; Beatrice, D.P.; Rita, P.; Elisabetta, T.; Giulia, P.; Claudio, R.; et al. Stroke volume and myocardial contraction fraction in transthyretin amyloidosis cardiomyopathy: A systematic review. Front. Cardiovasc. Med. 2023, 10, 1085824. [Google Scholar] [CrossRef] [PubMed]
- Wali, E.; Gruca, M.; Singulane, C.; Cotella, J.; Guile, B.; Johnson, R.; Mor-Avi, V.; Addetia, K.; Lang, R.M. How Often Does Apical Sparing of Longitudinal Strain Indicate the Presence of Cardiac Amyloidosis? Am. J. Cardiol. 2023, 202, 12–16. [Google Scholar] [CrossRef]
- Bandera, F.; Martone, R.; Chacko, L.; Ganesananthan, S.; Gilbertson, J.A.; Ponticos, M.; Lane, T.; Martinez-Naharro, A.; Whelan, C.; Quarta, C.; et al. Clinical Importance of Left Atrial Infiltration in Cardiac Transthyretin Amyloidosis. JACC Cardiovasc. Imaging 2022, 15, 17–29. [Google Scholar] [CrossRef]
- Fontana, M.; Patel, R.K.; Martinez-Naharro, A. Atrial Involvement in Cardiac Amyloidosis: Beyond Dilatation. JACC CardioOncol 2020, 2, 732–734. [Google Scholar] [CrossRef] [PubMed]
- Ternacle, J.; Krapf, L.; Mohty, D.; Magne, J.; Nguyen, A.; Galat, A.; Gallet, R.; Teiger, E.; Côté, N.; Clavel, M.-A.; et al. Aortic Stenosis and Cardiac Amyloidosis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 2638–2651. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.L.F.; Lim, Y.C.; Evangelista, L.K.M.; Wong, R.C.C.; Chai, P.; Sia, C.H.; Loi, H.Y.; Yeo, T.C.; Lin, W. Utility and pitfalls of the electrocardiogram in the evaluation of cardiac amyloidosis. Ann. Noninvasive Electrocardiol. 2022, 27, e12967. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, S.; Patel, H.P.; Chowdhury, M.; Patel, K.; Kumar, A.; Arora, S.; Zahid, S.; Goel, M.; Barssoum, K.; Jain, V.; et al. Impact of Arrhythmias on Hospitalizations in Patients With Cardiac Amyloidosis. Am. J. Cardiol. 2021, 143, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Argirò, A.; Del Franco, A.; Mazzoni, C.; Allinovi, M.; Tomberli, A.; Tarquini, R.; Di Mario, C.; Perfetto, F.; Cappelli, F.; Zampieri, M. Arrhythmic Burden in Cardiac Amyloidosis: What We Know and What We Do Not. Biomedicines 2022, 10, 2888. [Google Scholar] [CrossRef] [PubMed]
- Escher, F.; Senoner, M.; Doerler, J.; Zaruba, M.M.; Messner, M.; Mussner-Seeber, C.; Ebert, M.; Ensinger, C.; Mair, A.; Kroiss, A.; et al. When and how do patients with cardiac amyloidosis die? Clin. Res. Cardiol. 2020, 109, 78–88. [Google Scholar] [CrossRef] [PubMed]
- D’Errico, S.; Mazzanti, A.; Baldari, B.; Maiese, A.; Frati, P.; Fineschi, V. Sudden death in lambda light chain AL cardiac amyloidosis: A review of literature and update for clinicians and pathologists. Int. J. Clin. Exp. Pathol. 2020, 13, 1474–1482. [Google Scholar]
- Porcari, A.; Rossi, M.; Cappelli, F.; Canepa, M.; Musumeci, B.; Cipriani, A.; Tini, G.; Barbati, G.; Varrà, G.G.; Morelli, C.; et al. Incidence and risk factors for pacemaker implantation in light-chain and transthyretin cardiac amyloidosis. Eur. J. Heart Fail. 2022, 24, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Longhi, S.; Quarta, C.C.; Milandri, A.; Lorenzini, M.; Gagliardi, C.; Manuzzi, L.; Bacchi-Reggiani, M.L.; Leone, O.; Ferlini, A.; Russo, A.; et al. Atrial fibrillation in amyloidotic cardiomyopathy: Prevalence, incidence, risk factors and prognostic role. Amyloid 2015, 22, 147–155. [Google Scholar] [CrossRef]
- Donnellan, E.; Wazni, O.M.; Hanna, M.; Elshazly, M.B.; Puri, R.; Saliba, W.; Kanj, M.; Vakamudi, S.; Patel, D.R.; Baranowski, B.; et al. Atrial Fibrillation in Transthyretin Cardiac Amyloidosis: Predictors, Prevalence, and Efficacy of Rhythm Control Strategies. JACC Clin. Electrophysiol. 2020, 6, 1118–1127. [Google Scholar] [CrossRef]
- Lin, G.; Dispenzieri, A.; Kyle, R.; Grogan, M.; Brady, P.A. Implantable cardioverter defibrillators in patients with cardiac amyloidosis. J. Cardiovasc. Electrophysiol. 2013, 24, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Kuo, M.-J.; Chung, F.-P.; Lin, Y.-J.; Chien, K.-L.; Hsieh, Y.-C.; Chang, S.-L.; Lo, L.-W.; Hu, Y.-F.; Chao, T.-F.; et al. Risks of Ventricular Tachyarrhythmia and Mortality in Patients with Amyloidosis—A Long-Term Cohort Study. Acta Cardiol. Sin. 2022, 38, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.; Kasi, A.; Khan, B. Bradyarrhythmias in Cardiac Amyloidosis and Role of Pacemaker. Curr. Probl. Cardiol. 2023, 48, 101912. [Google Scholar] [CrossRef] [PubMed]
- Kristen, A.V.; Dengler, T.J.; Hegenbart, U.; Schonland, S.O.; Goldschmidt, H.; Sack, F.-U.; Voss, F.; Becker, R.; Katus, H.A.; Bauer, A. Prophylactic implantation of cardioverter-defibrillator in patients with severe cardiac amyloidosis and high risk for sudden cardiac death. Heart Rhythm 2008, 5, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Ridolfi, R.L.; Bulkley, B.H.; Hutchins, G.M. The conduction system in cardiac amyloidosis. Clinical and pathologic features of 23 patients. Am. J. Med. 1977, 62, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Frustaci, A.; Verardo, R.; Russo, M.A.; Caldarulo, M.; Alfarano, M.; Galea, N.; Miraldi, F.; Chimenti, C. Infiltration of Conduction Tissue Is a Major Cause of Electrical Instability in Cardiac Amyloidosis. J. Clin. Med. 2023, 12, 1798. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.A.; Jain, M.; Pimentel, D.R.; Wang, B.; Connors, L.H.; Skinner, M.; Apstein, C.S.; Liao, R. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ. Res. 2004, 94, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.C.; Robinson-Papp, J. Amyloid Neuropathies. Mt. Sinai J. Med. 2012, 79, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.; Karp, K.; Bjerle, P.; Olofsson, B.O. Disturbances of cardiac rhythm and conduction in familial amyloidosis with polyneuropathy. Br. Heart J. 1984, 51, 658–662. [Google Scholar] [CrossRef]
- Algalarrondo, V.; Dinanian, S.; Juin, C.; Chemla, D.; Bennani, S.L.; Sebag, C.; Planté, V.; Le Guludec, D.; Samuel, D.; Adams, D.; et al. Prophylactic pacemaker implantation in familial amyloid polyneuropathy. Heart Rhythm 2012, 9, 1069–1075. [Google Scholar] [CrossRef]
- Yamada, S.; Yoshihisa, A.; Hijioka, N.; Kamioka, M.; Kaneshiro, T.; Yokokawa, T.; Misaka, T.; Ishida, T.; Takeishi, Y. Autonomic dysfunction in cardiac amyloidosis assessed by heart rate variability and heart rate turbulence. Ann. Noninvasive Electrocardiol. 2020, 25, e12749. [Google Scholar] [CrossRef] [PubMed]
- Kastritis, E.; Roussou, M.; Fotiou, D.; Ziogas, D.; Gavriatopoulou, M.; Stamatelopoulos, K.; Pamboucas, C.; Papadopoulou, E.; Michas, F.; Lykka, A.; et al. Prospective Evaluation of Blood Pressure Monitoring and Baroreceptor Reflex Sensitivity (BRS) in Patients with AL Amyloidosis: Prognostic and Pathophysiologic Implications. Blood 2015, 126, 3054. [Google Scholar] [CrossRef]
- Reyners, A.K.L.; Hazenberg, B.P.C.; Reitsma, W.D.; Smit, A.J. Heart rate variability as a predictor of mortality in patients with AA and AL amyloidosis. Eur. Heart J. 2002, 23, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Donnellan, E.; Wazni, O.M.; Saliba, W.I.; Hanna, M.; Kanj, M.; Patel, D.R.; Wilner, B.; Kochar, A.; Jaber, W.A. Prevalence, Incidence, and Impact on Mortality of Conduction System Disease in Transthyretin Cardiac Amyloidosis. Am. J. Cardiol. 2020, 128, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Salinaro, F.; Mussinelli, R.; Raimondi, A.; Alogna, A.; Musca, F.; Palladini, G.; Merlini, G.; Perlini, S. Prevalence and Prognostic Value of Conduction Disturbances at the Time of Diagnosis of Cardiac AL Amyloidosis. Ann. Noninvasive Electrocardiol. 2013, 18, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Chastan, N.; Baert-Desurmont, S.; Saugier-Veber, P.; Dérumeaux, G.; Cabot, A.; Frébourg, T.; Hannequin, D. Cardiac conduction alterations in a French family with amyloidosis of the Finnish type with the p.Asp187Tyr mutation in the GSN gene. Muscle Nerve 2006, 33, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, J.; Dubrey, S.W.; Lavalley, M.; Skinner, M.; Falk, R.H. Electrophysiologic abnormalities in AL (primary) amyloidosis with cardiac involvement. J. Am. Coll. Cardiol. 1997, 30, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Rapezzi, C.; Merlini, G.; Quarta, C.C.; Riva, L.; Longhi, S.; Leone, O.; Salvi, F.; Ciliberti, P.; Pastorelli, F.; Biagini, E.; et al. Systemic cardiac amyloidoses: Disease profiles and clinical courses of the 3 main types. Circulation 2009, 120, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Barbhaiya, C.R.; Kumar, S.; Baldinger, S.H.; Michaud, G.F.; Stevenson, W.G.; Falk, R.; John, R.M. Electrophysiologic assessment of conduction abnormalities and atrial arrhythmias associated with amyloid cardiomyopathy. Heart Rhythm 2016, 13, 383–390. [Google Scholar] [CrossRef]
- Saturi, G.; De Frutos, F.; Sguazzotti, M.; Gonzalez-Lopez, E.; Nardi, E.; Domínguez, F.; Ponziani, A.; Cabrera, E.; Caponetti, A.G.; Lozano, S.; et al. Predictors and outcomes of pacemaker implantation in patients with cardiac amyloidosis. Heart 2023, 110, 40–48. [Google Scholar] [CrossRef]
- Pinney, J.H.; Whelan, C.J.; Petrie, A.; Dungu, J.; Banypersad, S.M.; Sattianayagam, P.; Wechalekar, A.; Gibbs, S.D.J.; Venner, C.P.; Wassef, N.; et al. Senile Systemic Amyloidosis: Clinical Features at Presentation and Outcome. J. Am. Heart Assoc. 2013, 2, e000098. [Google Scholar] [CrossRef] [PubMed]
- Sayed, R.H.; Rogers, D.; Khan, F.; Wechalekar, A.D.; Lachmann, H.J.; Fontana, M.; Mahmood, S.; Sachchithanantham, S.; Patel, K.; Hawkins, P.N.; et al. A study of implanted cardiac rhythm recorders in advanced cardiac AL amyloidosis. Eur. Heart J. 2015, 36, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Rehorn, M.R.; Loungani, R.S.; Black-Maier, E.; Coniglio, A.C.; Karra, R.; Pokorney, S.D.; Khouri, M.G. Cardiac Implantable Electronic Devices: A Window Into the Evolution of Conduction Disease in Cardiac Amyloidosis. JACC Clin. Electrophysiol. 2020, 6, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.S.-Y.; Kor, Q.; Kong, W.K.; Lim, Y.C.; Chan, M.Y.-Y.; Syn, N.L.; Ngiam, J.N.; Chew, N.W.; Yeo, T.-C.; Chai, P.; et al. Prevalence and outcomes of concomitant cardiac amyloidosis and aortic stenosis: A systematic review and meta-analysis. Hell. J. Cardiol. 2022, 64, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Towbin, J.A.; McKenna, W.J.; Abrams, D.J.; Ackerman, M.J.; Calkins, H.; Darrieux, F.C.C.; Daubert, J.P.; de Chillou, C.; DePasquale, E.C.; Desai, M.Y.; et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm 2019, 16, e301–e372. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: Developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) With the special contribution of the European Heart Rhythm Association (EHRA). Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef] [PubMed]
- Dias De Frias, A.F.; Rodrigues, P.; Trepa, M.; Fontes-Oliveira, M.; Costa, R.; Campinas, A.; Hipolito-Reis, A.; Torres, S. Hereditary transthyretin amyloidosis: Predictors of conduction disease. Eur. Heart J. 2020, 41, ehaa946.2111. [Google Scholar] [CrossRef]
- Orini, M.; Graham, A.J.; Martinez-Naharro, A.; Andrews, C.M.; de Marvao, A.; Statton, B.; Cook, S.A.; O’Regan, D.P.; Hawkins, P.N.; Rudy, Y.; et al. Noninvasive Mapping of the Electrophysiological Substrate in Cardiac Amyloidosis and Its Relationship to Structural Abnormalities. J. Am. Heart Assoc. 2019, 8, e012097. [Google Scholar] [CrossRef]
- Fischer, K.; Lellouche, N.; Damy, T.; Martins, R.; Clementy, N.; Bisson, A.; Lesaffre, F.; Espinosa, M.; Garcia, R.; Degand, B.; et al. Cardiovascular outcomes after cardiac resynchronization therapy in cardiac amyloidosis. ESC Heart Fail. 2021, 9, 740–750. [Google Scholar] [CrossRef]
- Papathanasiou, M.; Jakstaite, A.-M.; Oubari, S.; Siebermair, J.; Wakili, R.; Hoffmann, J.; Carpinteiro, A.; Hagenacker, T.; Thimm, A.; Rischpler, C.; et al. Clinical features and predictors of atrial fibrillation in patients with light-chain or transthyretin cardiac amyloidosis. ESC Heart Fail. 2022, 9, 1740–1748. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Mints, Y.Y.; Doros, G.; Berk, J.L.; Connors, L.H.; Ruberg, F.L. Features of atrial fibrillation in wild-type transthyretin cardiac amyloidosis: A systematic review and clinical experience. ESC Heart Fail. 2018, 5, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, K.; Cariou, E.; Colombat, M.; Ribes, D.; Huart, A.; Cintas, P.; Fournier, P.; Rollin, A.; Carrié, D.; Galinier, M.; et al. Atrial fibrillation and subtype of atrial fibrillation in cardiac amyloidosis: Clinical and echocardiographic features, impact on mortality. Amyloid 2019, 26, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Olanipekun, T.; Khoury, M.; Egbuche, O.; Effoe, V.; Ghali, J. Trends, Associations, and Impact of Atrial Fibrillation in Patients With Light-chain Cardiac Amyloidosis. Crit. Pathw. Cardiol. 2021, 20, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Röcken, C.; Peters, B.; Juenemann, G.; Saeger, W.; Klein, H.U.; Huth, C.; Roessner, A.; Goette, A. Atrial amyloidosis: An arrhythmogenic substrate for persistent atrial fibrillation. Circulation 2002, 106, 2091–2097. [Google Scholar] [CrossRef] [PubMed]
- Ohe, M.; Tahara, N.; Ueda, M.; Fukumoto, Y. P-wave characteristics and atrium voltage mapping in cardiac amyloidosis with paroxysmal atrial fibrillation. Eur. Heart J. Case Rep. 2023, 7, ytad319. [Google Scholar] [CrossRef] [PubMed]
- Nochioka, K.; Quarta, C.C.; Claggett, B.; Roca, G.Q.; Rapezzi, C.; Falk, R.H.; Solomon, S.D. Left atrial structure and function in cardiac amyloidosis. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, G.; Minutoli, F.; Madaffari, A.; Mazzeo, A.; Russo, M.; Donato, R.; Zito, C.; Aquaro, G.D.; Piccione, M.C.; Pedri, S.; et al. Left atrial function in cardiac amyloidosis. J. Cardiovasc. Med. 2016, 17, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Henein, M.Y.; Suhr, O.B.; Arvidsson, S.; Pilebro, B.; Westermark, P.; Hörnsten, R.; Lindqvist, P. Reduced left atrial myocardial deformation irrespective of cavity size: A potential cause for atrial arrhythmia in hereditary transthyretin amyloidosis. Amyloid 2018, 25, 46–53. [Google Scholar] [CrossRef]
- Lohrmann, G.; Patel, M.A.; Brauneis, D.; Sanchorawala, V.; Sarosiek, S.; Vellanki, N.; Siddiqi, O.K.; Ruberg, F.L.; Gopal, D.M. Left Atrial Mechanics Associates With Paroxysmal Atrial Fibrillation in Light-Chain Amyloidosis Following Stem Cell Transplantation. JACC Cardio Oncol. 2020, 2, 721–731. [Google Scholar] [CrossRef]
- Agarwal, S.K.; Norby, F.L.; Whitsel, E.A.; Soliman, E.Z.; Chen, L.Y.; Loehr, L.R.; Fuster, V.; Heiss, G.; Coresh, J.; Alonso, A. Cardiac Autonomic Dysfunction and Incidence of Atrial Fibrillation: Results From 20 Years Follow-Up. J. Am. Coll. Cardiol. 2017, 69, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Aus dem Siepen, F.; Hein, S.; Bauer, R.; Katus, H.A.; Kristen, A.V. Standard heart failure medication in cardiac transthyretin amyloidosis: Useful or harmful? Amyloid 2017, 24, 132–133. [Google Scholar] [CrossRef]
- Briasoulis, A.; Stamatelopoulos, K.; Petropoulos, I.; Patras, R.; Theodorakakou, F.; Gavriatopoulou, M.; Ntalianis, A.; Dimopoulos, M.-A.; Kastritis, E. Utilization and tolerance of beta-blockers among patients with AL amyloidosis. Amyloid 2022, 29, 31–37. [Google Scholar] [CrossRef]
- Tini, G.; Cappelli, F.; Biagini, E.; Musumeci, B.; Merlo, M.; Crotti, L.; Cameli, M.; Di Bella, G.; Cipriani, A.; Marzo, F.; et al. Current patterns of beta-blocker prescription in cardiac amyloidosis: An Italian nationwide survey. ESC Heart Fail. 2021, 8, 3369–3374. [Google Scholar] [CrossRef] [PubMed]
- Rubinow, A.; Skinner, M.; Cohen, A.S. Digoxin sensitivity in amyloid cardiomyopathy. Circulation 1981, 63, 1285–1288. [Google Scholar] [CrossRef]
- Touboul, O.; Algalarrondo, V.; Oghina, S.; Elbaz, N.; Rouffiac, S.; Hamon, D.; Extramiana, F.; Gandjbakhch, E.; D’Humieres, T.; Marijon, E.; et al. Electrical cardioversion of atrial arrhythmias with cardiac amyloidosis in the era of direct oral anticogulants. ESC Heart Fail. 2022, 9, 3556–3564. [Google Scholar] [CrossRef]
- Gertz, M.A.; Skinner, M.; Connors, L.H.; Falk, R.H.; Cohen, A.S.; Kyle, R.A. Selective binding of nifedipine to amyloid fibrils. Am. J. Cardiol. 1985, 55, 1646. [Google Scholar] [CrossRef] [PubMed]
- Muchtar, E.; Gertz, M.A.; Kumar, S.K.; Lin, G.; Boilson, B.; Clavell, A.; Lacy, M.Q.; Buadi, F.K.; Hayman, S.R.; Kapoor, P.; et al. Digoxin use in systemic light-chain (AL) amyloidosis: Contra-indicated or cautious use? Amyloid 2018, 25, 86–92. [Google Scholar] [CrossRef]
- Ioannou, A.; Massa, P.; Patel, R.K.; Razvi, Y.; Porcari, A.; Rauf, M.U.; Jiang, A.; Cabras, G.; Filisetti, S.; Bolhuis, R.E.; et al. Conventional heart failure therapy in cardiac ATTR amyloidosis. Eur. Heart J. 2023, 44, 2893–2907. [Google Scholar] [CrossRef]
- Aimo, A.; Buda, G.; Fontana, M.; Barison, A.; Vergaro, G.; Emdin, M.; Merlini, G. Therapies for cardiac light chain amyloidosis: An update. Int. J. Cardiol. 2018, 271, 152–160. [Google Scholar] [CrossRef]
- El-Am, E.A.; Dispenzieri, A.; Melduni, R.M.; Ammash, N.M.; White, R.D.; Hodge, D.O.; Noseworthy, P.A.; Lin, G.; Pislaru, S.V.; Egbe, A.C.; et al. Direct Current Cardioversion of Atrial Arrhythmias in Adults With Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2019, 73, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.V.; Blanche, P.; Dalgaard, F.; Gislason, G.H.; Torp-Pedersen, C.; Tønnesen, J.; Ruwald, M.H.; Pallisgaard, J.L.; Hansen, M.L. Electrical cardioversion of atrial fibrillation and the risk of brady-arrhythmic events. Am. Heart J. 2022, 244, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Tzeis, S.; Gerstenfeld, E.P.; Kalman, J.; Saad, E.B.; Sepehri Shamloo, A.; Andrade, J.G.; Barbhaiya, C.R.; Baykaner, T.; Boveda, S.; Calkins, H.; et al. 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society expert consensus statement on catheter and surgical ablation of atrial fibrillation. EP Eur. 2024, 26, euae043. [Google Scholar] [CrossRef]
- Black-Maier, E.; Rehorn, M.; Loungani, R.; Friedman, D.J.; Alenezi, F.; Geurink, K.; Pokorney, S.D.; Daubert, J.P.; Sun, A.Y.; Atwater, B.D.; et al. Catheter ablation of atrial fibrillation in cardiac amyloidosis. Pacing Clin. Electrophysiol. 2020, 43, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Alhassan, H.A.; Kainat, A.; Donohue, J.; Baumgartner, S.J.; Akunor, H.; Saba, S.; Jain, S.; Soman, P. Safety of Catheter Ablation Therapy for Atrial Fibrillation in Cardiac Amyloidosis. J. Am. Heart Assoc. 2023, 12, e029339. [Google Scholar] [CrossRef] [PubMed]
- Seiler, J.; Steven, D.; Roberts-Thomson, K.C.; Inada, K.; Tedrow, U.B.; Michaud, G.F.; Stevenson, W.G. The effect of open-irrigated radiofrequency catheter ablation of atrial fibrillation on left atrial pressure and B-type natriuretic peptide. Pacing Clin. Electrophysiol. 2014, 37, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Donnellan, E.; Wazni, O.; Kanj, M.; Elshazly, M.B.; Hussein, A.; Baranowski, B.; Hanna, M.; Patel, D.; Trulock, K.; Martyn, M.; et al. Atrial fibrillation ablation in patients with transthyretin cardiac amyloidosis. EP Eur. 2020, 22, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.Y.; Mohsin, Y.; Hodge, D.O.; Lacy, M.Q.; Packer, D.L.; Dispenzieri, A.; Grogan, M.; Asirvatham, S.J.; Madhavan, M.; McLeod, C.J. Catheter Ablation for Atrial Arrhythmias in Patients With Cardiac Amyloidosis. J. Cardiovasc. Electrophysiol. 2016, 27, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Ullah, W.; Ruge, M.; Hajduczok, A.G.; Kochar, K.; Frisch, D.R.; Pavri, B.B.; Alvarez, R.; Rajapreyar, I.N.; Brailovsky, Y. Adverse outcomes of atrial fibrillation ablation in heart failure patients with and without cardiac amyloidosis: A Nationwide Readmissions Database analysis (2015–2019). Eur. Heart J. Open 2023, 3, oead026. [Google Scholar] [CrossRef]
- Vilches, S.; Fontana, M.; Gonzalez-Lopez, E.; Mitrani, L.; Saturi, G.; Renju, M.; Griffin, J.M.; Caponetti, A.; Gnanasampanthan, S.; De Los Santos, J.; et al. Systemic embolism in amyloid transthyretin cardiomyopathy. Eur. J. Heart Fail. 2022, 24, 1387–1396. [Google Scholar] [CrossRef]
- Cappelli, F.; Tini, G.; Russo, D.; Emdin, M.; Del Franco, A.; Vergaro, G.; Di Bella, G.; Mazzeo, A.; Canepa, M.; Volpe, M.; et al. Arterial thrombo-embolic events in cardiac amyloidosis: A look beyond atrial fibrillation. Amyloid 2021, 28, 12–18. [Google Scholar] [CrossRef]
- Feng, D.; Edwards, W.D.; Oh, J.K.; Chandrasekaran, K.; Grogan, M.; Martinez, M.W.; Syed, I.I.; Hughes, D.A.; Lust, J.A.; Jaffe, A.S.; et al. Intracardiac Thrombosis and Embolism in Patients With Cardiac Amyloidosis. Circulation 2007, 116, 2420–2426. [Google Scholar] [CrossRef] [PubMed]
- Halligan, C.S.; Lacy, M.Q.; Vincent Rajkumar, S.; Dispenzieri, A.; Witzig, T.E.; Lust, J.A.; Fonseca, R.; Gertz, M.A.; Kyle, R.A.; Pruthi, R.K. Natural history of thromboembolism in AL amyloidosis. Amyloid 2006, 13, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, C.; Tsagalou, E.; Baraboutis, I.; Tampakis, K.; Kastritis, E.; Dimopoulos, M.-A. Cardiac amyloidosis presenting with coronary artery embolization. RCM 2021, 22, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.; Barakat, A.F.; Eisele, Y.S.; Nieves, R.; Jain, S.; Saba, S.; Follansbee, W.P.; Brownell, A.; Soman, P. Prevalence of Atrial Fibrillation and Thromboembolic Risk in Wild-Type Transthyretin Amyloid Cardiomyopathy. Circulation 2021, 143, 1335–1337. [Google Scholar] [CrossRef]
- Selvaraj, S.; Claggett, B.; Minamisawa, M.; Windham, B.G.; Chen, L.Y.; Inciardi, R.M.; Buxbaum, J.N.; Mosley, T.H.; Shah, A.M.; Solomon, S.D. Atrial Fibrillation and Ischemic Stroke With the Amyloidogenic V122I Transthyretin Variant Among Black Americans. J. Am. Coll. Cardiol. 2021, 78, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Naharro, A.; Gonzalez-Lopez, E.; Corovic, A.; Mirelis, J.G.; Baksi, A.J.; Moon, J.C.; Garcia-Pavia, P.; Gillmore, J.D.; Hawkins, P.N.; Fontana, M. High Prevalence of Intracardiac Thrombi in Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2019, 73, 1733–1734. [Google Scholar] [CrossRef] [PubMed]
- Magaud, C.; Harnois, T.; Sebille, S.; Chatelier, A.; Faivre, J.-F.; Bois, P.; Page, G.; Gellen, B. Pro-inflammatory cytokine secretion induced by amyloid transthyretin in human cardiac fibroblasts. Biochem. Biophys. Res. Commun. 2023, 642, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Gamba, G.; Montani, N.; Anesi, E.; Palladini, G.; Lorenzutti, F.; Perfetti, V.; Merlini, G. Abnormalities in thrombin-antithrombin pathway in AL amyloidosis. Amyloid 1999, 6, 273–277. [Google Scholar] [CrossRef]
- Zonder, J.A.; Barlogie, B.; Durie, B.G.M.; McCoy, J.; Crowley, J.; Hussein, M.A. Thrombotic complications in patients with newly diagnosed multiple myeloma treated with lenalidomide and dexamethasone: Benefit of aspirin prophylaxis. Blood 2006, 108, 403. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Donnellan, E.; Elshazly, M.B.; Vakamudi, S.; Wazni, O.M.; Cohen, J.A.; Kanj, M.; Hanna, M.; Baranowski, B.; Saliba, W.; Jaber, W. No Association Between CHADS-VASc Score and Left Atrial Appendage Thrombus in Patients With Transthyretin Amyloidosis. JACC Clin. Electrophysiol. 2019, 5, 1473–1474. [Google Scholar] [CrossRef] [PubMed]
- Akintoye, E.; Majid, M.; Klein, A.L.; Hanna, M. Prognostic Utility of Left Atrial Strain to Predict Thrombotic Events and Mortality in Amyloid Cardiomyopathy. JACC Cardiovasc. Imaging 2023, 16, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Larocca, A.; Cavallo, F.; Bringhen, S.; Di Raimondo, F.; Falanga, A.; Evangelista, A.; Cavalli, M.; Stanevsky, A.; Corradini, P.; Pezzatti, S.; et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood 2012, 119, 933–939, quiz 1093. [Google Scholar] [CrossRef] [PubMed]
- Yood, R.A.; Skinner, M.; Rubinow, A.; Talarico, L.; Cohen, A.S. Bleeding manifestations in 100 patients with amyloidosis. JAMA 1983, 249, 1322–1324. [Google Scholar] [CrossRef] [PubMed]
- Sucker, C.; Hetzel, G.R.; Grabensee, B.; Stockschlaeder, M.; Scharf, R.E. Amyloidosis and bleeding: Pathophysiology, diagnosis, and therapy. Am. J. Kidney Dis. 2006, 47, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Schrutka, L.; Avanzini, N.; Seirer, B.; Rettl, R.; Dachs, T.; Duca, F.; Binder, C.; Dalos, D.; Badr Eslam, R.; Bonderman, D. Bleeding events in patients with cardiac amyloidosis. Eur. Heart J. 2020, 41, ehaa946.2122. [Google Scholar] [CrossRef]
- Lacy, S.C.; Kinno, M.; Joyce, C.; Yu, M.D. Direct Oral Anticoagulants in Patients With Cardiac Amyloidosis: A Systematic Review and Meta-Analysis. Int. J. Heart Fail. 2024, 6, 36–43. [Google Scholar] [CrossRef]
- Mitrani, L.R.; De Los Santos, J.; Driggin, E.; Kogan, R.; Helmke, S.; Goldsmith, J.; Biviano, A.B.; Maurer, M.S. Anticoagulation with warfarin compared to novel oral anticoagulants for atrial fibrillation in adults with transthyretin cardiac amyloidosis: Comparison of thromboembolic events and major bleeding. Amyloid 2021, 28, 30–34. [Google Scholar] [CrossRef]
- Cariou, E.; Sanchis, K.; Rguez, K.; Blanchard, V.; Cazalbou, S.; Fournier, P.; Huart, A.; Roussel, M.; Cintas, P.; Galinier, M.; et al. New Oral Anticoagulants vs. Vitamin K Antagonists Among Patients With Cardiac Amyloidosis: Prognostic Impact. Front. Cardiovasc. Med. 2021, 8, 742428. [Google Scholar] [CrossRef]
- Mentias, A.; Alvarez, P.; Chaudhury, P.; Nakhla, M.; Moudgil, R.; Kanj, M.; Hanna, M.; Desai, M.Y. Direct Oral Anticoagulants in Cardiac Amyloidosis–Associated Heart Failure and Atrial Fibrillation. Am. J. Cardiol. 2022, 164, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Manning, W.J.; Weintraub, R.M.; Waksmonski, C.A.; Haering, J.M.; Rooney, P.S.; Maslow, A.D.; Johnson, R.G.; Douglas, P.S. Accuracy of transesophageal echocardiography for identifying left atrial thrombi. A prospective, intraoperative study. Ann. Intern. Med. 1995, 123, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Guarracini, F.; Bonvicini, E.; Preda, A.; Martin, M.; Muraglia, S.; Casagranda, G.; Mochen, M.; Coser, A.; Quintarelli, S.; Branzoli, S.; et al. Appropriate use criteria of left atrial appendage closure devices: Latest evidences. Expert. Rev. Med. Devices 2023, 20, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Amat-Santos, I.J.; Delgado-Arana, J.R.; Cruz-González, I.; Gutiérrez, H.; García-Bolao, I.; Millán, X.; Tirado-Conte, G.; Ruiz-Nodar, J.M.; Mohandes, M.; Palazuelos, J.; et al. Cardiac amyloidosis and left atrial appendage closure. The CAMYLAAC study. Rev. Esp. Cardiol. 2023, 76, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Santarone, M.; Corrado, G.; Tagliagambe, L.M.; Manzillo, G.F.; Tadeo, G.; Spata, M.; Longhi, M. Atrial thrombosis in cardiac amyloidosis: Diagnostic contribution of transesophageal echocardiography. J. Am. Soc. Echocardiogr. 1999, 12, 533–536. [Google Scholar] [CrossRef] [PubMed]
- El-Am, E.A.; Grogan, M.; Ahmad, A.; Patlolla, S.H.; Klarich, K.W.; AbouEzzeddine, O.F.; Melduni, R.M.; Maleszewski, J.J.; Dispenzieri, A.; Nkomo, V.T. Persistence of Left Atrial Appendage Thrombus in Patients With Cardiac Amyloidosis. J. Am. Coll. Cardiol. 2021, 77, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Tarantini, G.; D’Amico, G.; Latib, A.; Montorfano, M.; Mazzone, P.; Fassini, G.; Maltagliati, A.; Ronco, F.; Saccà, S.; Cruz-Gonzalez, I.; et al. Percutaneous left atrial appendage occlusion in patients with atrial fibrillation and left appendage thrombus: Feasibility, safety and clinical efficacy. EuroIntervention 2018, 13, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Preda, A.; Baroni, M.; Varrenti, M.; Vargiu, S.; Carbonaro, M.; Giordano, F.; Gigli, L.; Mazzone, P. Left Atrial Appendage Occlusion in Patients with Failure of Antithrombotic Therapy: Good Vibes from Early Studies. J. Clin. Med. 2023, 12, 3859. [Google Scholar] [CrossRef]
- Margonato, D.; Preda, A.; Ingallina, G.; Rizza, V.; Fierro, N.; Radinovic, A.; Ancona, F.; Patti, G.; Agricola, E.; Bella, P.D.; et al. Left atrial appendage occlusion after thromboembolic events or left atrial appendage sludge during anticoagulation therapy: Is two better than one? Real-world experience from a tertiary care hospital. J. Arrhythm. 2023, 39, 395–404. [Google Scholar] [CrossRef]
- Piccini, J.P.; Sievert, H.; Patel, M.R. Left atrial appendage occlusion: Rationale, evidence, devices, and patient selection. Eur. Heart J. 2017, 38, 869–876. [Google Scholar] [CrossRef]
- Sharma, S.P.; Cheng, J.; Turagam, M.K.; Gopinathannair, R.; Horton, R.; Lam, Y.; Tarantini, G.; D’Amico, G.; Freixa Rofastes, X.; Lange, M.; et al. Feasibility of Left Atrial Appendage Occlusion in Left Atrial Appendage Thrombus: A Systematic Review. JACC Clin. Electrophysiol. 2020, 6, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Preda, A.; Montalto, C.; Galasso, M.; Munafò, A.; Garofani, I.; Baroni, M.; Gigli, L.; Vargiu, S.; Varrenti, M.; Colombo, G.; et al. Fighting Cardiac Thromboembolism during Transcatheter Procedures: An Update on the Use of Cerebral Protection Devices in Cath Labs and EP Labs. Life 2023, 13, 1819. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Preda, A.; Fierro, N.; Marzi, A.; Radinovic, A.; Della Bella, P.; Mazzone, P. A Referral Center Experience with Cerebral Protection Devices: Challenging Cardiac Thrombus in the EP Lab. J. Clin. Med. 2023, 12, 1549. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yang, Z.; Wen, L.; Liu, X.; Xu, H.; Zhang, Q.; Guo, Y. Regional myocardial microvascular dysfunction in cardiac amyloid light-chain amyloidosis: Assessment with 3T cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2016, 18, 16. [Google Scholar] [CrossRef] [PubMed]
- Le Bras, F.; Molinier-Frenkel, V.; Guellich, A.; Dupuis, J.; Belhadj, K.; Guendouz, S.; Ayad, K.; Colombat, M.; Benhaiem, N.; Tissot, C.M.; et al. Sequential cyclophosphamide-bortezomib-dexamethasone unmasks the harmful cardiac effect of dexamethasone in primary light-chain cardiac amyloidosis. Eur. J. Cancer 2017, 76, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Dubrey, S.W.; Cha, K.; Anderson, J.; Chamarthi, B.; Reisinger, J.; Skinner, M.; Falk, R.H. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM 1998, 91, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Palladini, G.; Malamani, G.; Cò, F.; Pistorio, A.; Recusani, F.; Anesi, E.; Garini, P.; Merlini, G. Holter monitoring in AL amyloidosis: Prognostic implications. Pacing Clin. Electrophysiol. 2001, 24, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shi, A.; Dong, H.; Laptseva, N.; Chen, F.; Yang, J.; Guo, X.; Duru, F.; Chen, K.; Chen, L. Prognostic implications of premature ventricular contractions and non-sustained ventricular tachycardia in light-chain cardiac amyloidosis. EP Eur. 2024, 26, euae063. [Google Scholar] [CrossRef]
- Varr, B.C.; Zarafshar, S.; Coakley, T.; Liedtke, M.; Lafayette, R.A.; Arai, S.; Schrier, S.L.; Witteles, R.M. Implantable cardioverter-defibrillator placement in patients with cardiac amyloidosis. Heart Rhythm 2014, 11, 158–162. [Google Scholar] [CrossRef]
- Knoll, K.; Fuchs, P.; Weidmann, I.; Altunkas, F.; Voss, S.; Lennerz, C.; Kolb, C.; Kessler, T.; Schunkert, H.; Reinhard, W.; et al. Incidence and Predictors of Ventricular Arrhythmias in Transthyretin Amyloid Cardiomyopathy. J. Clin. Med. 2023, 12, 4624. [Google Scholar] [CrossRef]
- Hamon, D.; Algalarrondo, V.; Gandjbakhch, E.; Extramiana, F.; Marijon, E.; Elbaz, N.; Selhane, D.; Dubois-Rande, J.-L.; Teiger, E.; Plante-Bordeneuve, V.; et al. Outcome and incidence of appropriate implantable cardioverter-defibrillator therapy in patients with cardiac amyloidosis. Int. J. Cardiol. 2016, 222, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, Y.B.; Liu, J.; Chou, J.; Hoffman, J.; Comenzo, R.L.; Steingart, R.M. Frequencies and types of arrhythmias in patients with systemic light-chain amyloidosis with cardiac involvement undergoing stem cell transplantation on telemetry monitoring. Am. J. Cardiol. 2009, 104, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Dale, Z.; Chandrashekar, P.; Al-Rashdan, L.; Kim, M.; Masri, A.; Nazer, B. Management Strategies for Atrial Fibrillation and Flutter in Patients with Transthyretin Cardiac Amyloidosis. Am. J. Cardiol. 2021, 157, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.S.; Schwartz, J.H.; Gundapaneni, B.; Elliott, P.M.; Merlini, G.; Waddington-Cruz, M.; Kristen, A.V.; Grogan, M.; Witteles, R.; Damy, T.; et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N. Engl. J. Med. 2018, 379, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Mlcochova, H.; Saliba, W.I.; Burkhardt, D.J.; Rodriguez, R.E.; Cummings, J.E.; Lakkireddy, D.; Patel, D.; Natale, A. Catheter ablation of ventricular fibrillation storm in patients with infiltrative amyloidosis of the heart. J. Cardiovasc. Electrophysiol. 2006, 17, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.-P.; Lin, Y.-J.; Kuo, L.; Chen, S.-A. Catheter Ablation of Ventricular Tachycardia/Fibrillation in a Patient with Right Ventricular Amyloidosis with Initial Manifestations Mimicking Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Korean Circ. J. 2017, 47, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Compagnucci, P.; Casella, M.; Falanga, U.; Ciliberti, G.; Lofiego, C.; Vagnarelli, F.; Valeri, Y.; Stronati, G.; Principi, S.; Barbarossa, A.; et al. PO-02-213 ELECTROANATOMICAL ASPECTS OF BIOPSY-PROVEN CARDIAC AMYLOIDOSIS. Heart Rhythm 2023, 20, S279–S280. [Google Scholar] [CrossRef]
- Tilz, R.R.; Lenarczyk, R.; Scherr, D.; Haugaa, K.H.; Iliodromitis, K.; Pürerfellner, H.; Kiliszek, M.; Dagres, N. Management of ventricular tachycardia in the ablation era: Results of the European Heart Rhythm Association Survey. EP Eur. 2018, 20, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Donnellan, E.; Wazni, O.M.; Saliba, W.I.; Baranowski, B.; Hanna, M.; Martyn, M.; Patel, D.; Trulock, K.; Menon, V.; Hussein, A.; et al. Cardiac devices in patients with transthyretin amyloidosis: Impact on functional class, left ventricular function, mitral regurgitation, and mortality. J. Cardiovasc. Electrophysiol. 2019, 30, 2427–2432. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Kim, E.-J.; Holmes, B.B.; Huang, S.; Lugo, R.; Al Aboud, A.; Goodman, S.; Hung, R.R.; Slosky, D.; Stevenson, W.G.; Michaud, G.F.; et al. Outcomes in patients with cardiac amyloidosis and implantable cardioverter-defibrillator. EP Eur. 2020, 22, 1216–1223. [Google Scholar] [CrossRef]
- Kumar, S.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Colby, C.; Laumann, K.; Zeldenrust, S.R.; Leung, N.; Dingli, D.; et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J. Clin. Oncol. 2012, 30, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Grogan, M.; Scott, C.G.; Kyle, R.A.; Zeldenrust, S.R.; Gertz, M.A.; Lin, G.; Klarich, K.W.; Miller, W.L.; Maleszewski, J.J.; Dispenzieri, A. Natural History of Wild-Type Transthyretin Cardiac Amyloidosis and Risk Stratification Using a Novel Staging System. J. Am. Coll. Cardiol. 2016, 68, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Saef, J.; Martyn, T.; Ray Dey, A.; Khedraki, R.; Ives, L.; Collier, P.; Jaber, W.A.; Estep, J.D.; Hanna, M.; Tang, W.H.W. Changes in Left Ventricular Ejection Fraction and Clinical Trajectories of Transthyretin Cardiac Amyloidosis with Systolic Dysfunction. J. Clin. Med. 2023, 12, 7250. [Google Scholar] [CrossRef] [PubMed]
- Pagourelias, E.D.; Mirea, O.; Duchenne, J.; Van Cleemput, J.; Delforge, M.; Bogaert, J.; Kuznetsova, T.; Voigt, J.-U. Echo Parameters for Differential Diagnosis in Cardiac Amyloidosis: A Head-to-Head Comparison of Deformation and Nondeformation Parameters. Circ. Cardiovasc. Imaging 2017, 10, e005588. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Johnson, G.; Hellkamp, A.S.; Anderson, J.; Mark, D.B.; Lee, K.L.; Bardy, G.H.; Poole, J.E. Rapid-Rate Nonsustained Ventricular Tachycardia Found on Implantable Cardioverter-Defibrillator Interrogation. J. Am. Coll. Cardiol. 2013, 61, 2161–2168. [Google Scholar] [CrossRef]
- Murtagh, B.; Hammill, S.C.; Gertz, M.A.; Kyle, R.A.; Tajik, A.J.; Grogan, M. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am. J. Cardiol. 2005, 95, 535–537. [Google Scholar] [CrossRef]
- Fontana, M.; Pica, S.; Reant, P.; Abdel-Gadir, A.; Treibel, T.A.; Banypersad, S.M.; Maestrini, V.; Barcella, W.; Rosmini, S.; Bulluck, H.; et al. Prognostic Value of Late Gadolinium Enhancement Cardiovascular Magnetic Resonance in Cardiac Amyloidosis. Circulation 2015, 132, 1570–1579. [Google Scholar] [CrossRef]
- Sharma, A.D.; Rizo-Patron, C.; Hallstrom, A.P.; O’Neill, G.P.; Rothbart, S.; Martins, J.B.; Roelke, M.; Steinberg, J.S.; Greene, H.L. Percent right ventricular pacing predicts outcomes in the DAVID trial. Heart Rhythm 2005, 2, 830–834. [Google Scholar] [CrossRef]
- Barsheshet, A.; Moss, A.J.; McNitt, S.; Jons, C.; Glikson, M.; Klein, H.U.; Huang, D.T.; Steinberg, J.S.; Brown, M.W.; Zareba, W.; et al. Long-term implications of cumulative right ventricular pacing among patients with an implantable cardioverter-defibrillator. Heart Rhythm 2011, 8, 212–218. [Google Scholar] [CrossRef]
- Deif, B.; Ballantyne, B.; Almehmadi, F.; Mikhail, M.; McIntyre, W.F.; Manlucu, J.; Yee, R.; Sapp, J.L.; Roberts, J.D.; Healey, J.S.; et al. Cardiac resynchronization is pro-arrhythmic in the absence of reverse ventricular remodelling: A systematic review and meta-analysis. Cardiovasc. Res. 2018, 114, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Baroni, M.; Preda, A.; Varrenti, M.; Vargiu, S.; Carbonaro, M.; Giordano, F.; Gigli, L.; Mazzone, P. Left Bundle Branch Area Pacing over His Bundle Pacing: How Far Have We Come? J. Clin. Med. 2023, 12, 3251. [Google Scholar] [CrossRef] [PubMed]
- Pham-Trung, C.; Veloza-Urrea, D.; Segura-Domínguez, M.; De la Rosa Rojas, Y.; Aguilera-Agudo, C.; García-Izquierdo, E.A.; García-Rodríguez, D.; Jiménez-Sánchez, D.; Lorente-Ros, A.; Mingo-Santos, S.; et al. Feasibility and safety of left bundle branch area pacing in cardiac amyloidosis. A single center experience. Pacing Clin. Electrophysiol. 2024, 47, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Jing-Jing, J.; Ke-Xin, W.; Zhao-Meng, J.; Nan, W.; Lian-Jun, G.; Yun-Long, X.; Ying-Xue, D. Conduction system pacing for ventricular pacing requirement is feasible and effective on patients with hypertrophic cardiomyopathy and cardiac dysfunction. Int. J. Cardiol. Heart Vasc. 2023, 49, 101296. [Google Scholar] [CrossRef] [PubMed]

| Arrythmia | Study | Type of Study | Patients | Major Findings |
|---|---|---|---|---|
| Conduction system defects | Donnellan et al., 2020 [34] | Retrospective study | ATTRwt (261); ATTRv (108) | Incidence and prevalence of AV block |
| Boldrini et al., 2013 [35] | Prospective observational study | AL (344) | Prevalence and prognostic implications of conduction delays | |
| Reisinger et al., 1997 [37] | Prospective observational study | AL (25) | Electrophysiologic abnormalities | |
| Algalarrondo et al., 2012 [30] | Retrospective study | ATTRv (262) | Prophylactic pacemaker implantation | |
| Barbhaiya et al., 2016 [39] | Prospective observational study | 18 | Intracardiac conduction, atrial arrythmias and ablation outcomes | |
| Porcari et al., 2022 [18] | Retrospective study | AL (127); ATTRv (66); ATTRwt (266) | Incidence and risk factors for pacemaker implantation | |
| Saturi et al., 2023 [40] | Retrospective study | AL (216); ATTR (571) | Prevalence, incidence and prognostic implications of pacemaker implantation | |
| Atrial fibrillation | Longhi et al., 2015 [19] | Retrospective study | AL (123); ATTRv (94); ATTRwt (45) | Prevalence, incidence and prognostic implications of atrial fibrillation |
| Papathamasiou et al., 2022 [50] | Retrospective study | AL (71); ATTRv (8); ATTRwt (54) | Prevalence and predictors of atrial fibrillation | |
| Donnellan et al., 2020 [20] | Retrospective study | ATTR (382) | Prevalence and incidence of atrial fibrillation | |
| Sanchis et al., 2019 [53] | Retrospective study | AL (115); ATTR (123) | Prevalence, incidence and prognostic implications of atrial fibrillation | |
| Rőcken et al., 2002 [55] | Prospective observational study | 245 | Role of isolated atrial amyloidosis in atrial fibrillation occurrence | |
| El-Am et al., 2019 [71] | Retrospective study | 58 | Outcomes of electrical cardioversion | |
| Black-Meier et al., 2020 [74] | Retrospective study | 13 | Feasibility of atrial fibrillation catheter ablation | |
| Amat-Santos et al., 2023 [104] | Retrospective study | ATTR (40) | Feasibility of left atrial appendage closure | |
| Ventricular arrhythmias | Chen et al., 2022 [22] | Retrospective study | 1130 | Prevalence, incidence and prognostic implications of ventricular arrhythmias |
| Palladini et al., 2001 [117] | Prospective observational study | AL (51) | Ventricular couplets as predictors of sudden death | |
| Varr et al., 2014 [119] | Retrospective study | 31 | Incidence of ventricular arrhythmias and role of ICD implantation | |
| Mlcochova et al., 2006 [125] | Case report | 2 | Catheter ablation of ventricular fibrillation | |
| Hamon et al., 2016 [121] | Prospective observational study | AL (12); ATTRv (27); ATTRwt (6) | Appropriate ICD therapy and survival predictors | |
| Kim et al., 2020 [131] | Retrospective study | AL (49); ATTR (41); Other (1) | Survival with and without a primary prevention ICD | |
| Lin et al., 2013 [21] | Retrospective study | AL (33); ATTRv (9); ATTRwt (10); Other (1) | Benefits of ICD therapy | |
| Donnellan et al., 2019 [129] | Retrospective study | ATTR (78) | Outcomes of implantable devices |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonvicini, E.; Preda, A.; Tognola, C.; Falco, R.; Gidiucci, R.; Leo, G.; Vargiu, S.; Varrenti, M.; Gigli, L.; Baroni, M.; et al. Arrhythmic Risk Stratification in Cardiac Amyloidosis: A Review of the Current Literature. J. Cardiovasc. Dev. Dis. 2024, 11, 222. https://doi.org/10.3390/jcdd11070222
Bonvicini E, Preda A, Tognola C, Falco R, Gidiucci R, Leo G, Vargiu S, Varrenti M, Gigli L, Baroni M, et al. Arrhythmic Risk Stratification in Cardiac Amyloidosis: A Review of the Current Literature. Journal of Cardiovascular Development and Disease. 2024; 11(7):222. https://doi.org/10.3390/jcdd11070222
Chicago/Turabian StyleBonvicini, Eleonora, Alberto Preda, Chiara Tognola, Raffaele Falco, Roberto Gidiucci, Giulio Leo, Sara Vargiu, Marisa Varrenti, Lorenzo Gigli, Matteo Baroni, and et al. 2024. "Arrhythmic Risk Stratification in Cardiac Amyloidosis: A Review of the Current Literature" Journal of Cardiovascular Development and Disease 11, no. 7: 222. https://doi.org/10.3390/jcdd11070222





