Compromised Cerebral Arterial Perfusion, Altered Brain Tissue Integrity, and Cognitive Impairment in Adolescents with Complex Congenital Heart Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Sample and Setting
2.2. Measurements
2.2.1. Montreal Cognitive Assessment (MoCA)
2.2.2. Wide Range Assessment of Memory and Learning (WRAML2)
2.2.3. Demographic and Clinical Data
2.3. Data Acquisition
2.3.1. Flow-Encoded Arterial Spin Tagging (FEAST) Imaging
2.3.2. Diffusion Tensor Imaging
2.3.3. High-Resolution T1-Weighted Imaging
2.3.4. Proton Density and T2-Weighted Imaging
2.4. MRI Data Processing
2.4.1. Calculation of ATT Maps and Global Mean ATT Values
2.4.2. Calculation of MD Maps
2.4.3. Realignment, Normalization, and Smoothing of MD and ATT Maps
2.5. MRI Data Processing
3. Results
3.1. Demographic and Characteristics
3.2. Cognitive Scores, MD, and ATT Values between Groups
3.3. Partial Correlations between ATT Values and Cognition
3.4. Partial Correlations between MD Values and Cognition
3.5. Structural Brain MRI Findings
4. Discussions
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cassidy, A.R.; White, M.T.; DeMaso, D.R.; Newburger, J.W.; Bellinger, D.C. Executive function deficits in children and adolescents with critical cyanotic congenital heart disease. Cardiol. Young 2015, 25, 1238–1246. [Google Scholar] [CrossRef]
- Licht, D.J.; Shera, D.M.; Clancy, R.R.; Wernovsky, G.; Montenegro, L.M.; Nicolson, S.C.; Zimmerman, R.A.; Spray, T.L.; Gaynor, J.W.; Vossough, A. Brain maturation is delayed in infants with complex congenital heart defects. J. Thorac. Cardiovasc. Surg. 2009, 137, 529–536. [Google Scholar] [CrossRef]
- von Rhein, M.; Scheer, I.; Loenneker, T.; Huber, R.; Knirsch, W.; Latal, B. Structural brain lesions in adolescents with congenital heart disease. J. Pediatr. 2011, 158, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Galli, K.K.; Zimmerman, R.A.; Jarvik, G.P.; Wernovsky, G.; Kuypers, M.K.; Clancy, R.R.; Montenegro, L.M.; Mahle, W.T.; Newman, M.F.; Saunders, A.M.; et al. Periventricular leukomalacia is common after neonatal cardiac surgery. J. Thorac. Cardiovasc. Surg. 2004, 127, 692–704. [Google Scholar] [CrossRef]
- Block, A.J.; McQuillen, P.S.; Chau, V.; Glass, H.; Poskitt, K.J.; Barkovich, A.J.; Esch, M.; Soulikias, W.; Azakie, A.; Campbell, A.; et al. Clinically silent preoperative brain injuries do not worsen with surgery in neonates with congenital heart disease. J. Thorac. Cardiovasc. Surg. 2010, 140, 550–557. [Google Scholar] [CrossRef]
- Soul, J.S.; Robertson, R.L.; Wypij, D.; Bellinger, D.C.; Visconti, K.J.; du Plessis, A.J.; Kussman, B.D.; Scoppettuolo, L.A.; Pigula, F.; Jonas, R.A.; et al. Subtle hemorrhagic brain injury is associated with neurodevelopmental impairment in infants with repaired congenital heart disease. J. Thorac. Cardiovasc. Surg. 2009, 138, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Pike, N.A.; Woo, M.A.; Poulsen, M.K.; Evangelista, W.; Faire, D.; Halnon, N.J.; Lewis, A.B.; Kumar, R. Predictors of Memory Deficits in Adolescents and Young Adults with Congenital Heart Disease Compared to Healthy Controls. Front. Pediatr. 2016, 4, 117. [Google Scholar] [CrossRef]
- Pike, N.A.; Poulsen, M.K.; Woo, M.A. Validity of the Montreal Cognitive Assessment Screener in Adolescents and Young Adults with and without Congenital Heart Disease. Nurs. Res. 2017, 66, 222–230. [Google Scholar] [CrossRef]
- Brewster, R.C.; King, T.Z.; Burns, T.G.; Drossner, D.M.; Mahle, W.T. White matter integrity dissociates verbal memory and auditory attention span in emerging adults with congenital heart disease. J. Int. Neuropsychol. Soc. 2015, 21, 22–33. [Google Scholar] [CrossRef]
- Panigrahy, A.; Schmithorst, V.J.; Wisnowski, J.L.; Watson, C.G.; Bellinger, D.C.; Newburger, J.W.; Rivkin, M.J. Relationship of white matter network topology and cognitive outcome in adolescents with d-transposition of the great arteries. NeuroImage Clin. 2015, 7, 438–448. [Google Scholar] [CrossRef]
- Rollins, C.K.; Watson, C.G.; Asaro, L.A.; Wypij, D.; Vajapeyam, S.; Bellinger, D.C.; DeMaso, D.R.; Robertson, R.L., Jr.; Newburger, J.W.; Rivkin, M.J. White matter microstructure and cognition in adolescents with congenital heart disease. J. Pediatr. 2014, 165, 936–944.e2. [Google Scholar] [CrossRef]
- Bellinger, D.C.; Rivkin, M.J.; DeMaso, D.; Robertson, R.L.; Stopp, C.; Dunbar-Masterson, C.; Wypij, D.; Newburger, J.W. Adolescents with tetralogy of Fallot: Neuropsychological assessment and structural brain imaging. Cardiol. Young 2015, 25, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Marchi, N.; Angelov, L.; Masaryk, T.; Fazio, V.; Granata, T.; Hernandez, N.; Hallene, K.; Diglaw, T.; Franic, L.; Najm, I.; et al. Seizure-promoting effect of blood–brain barrier disruption. Epilepsia 2007, 48, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Goldwaser, E.L.; Acharya, N.K.; Sarkar, A.; Godsey, G.; Nagele, R.G. Breakdown of the cerebrovasculature and blood-brain barrier: A mechanistic link between diabetes mellitus and Alzheimer’s disease. J. Alzheimer’s Dis. 2016, 54, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Minagar, A.; Alexander, J.S. Blood-brain barrier disruption in multiple sclerosis. Mult. Scler. J. 2003, 9, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.S.S.; Owen, D.; Wang, D.J.J. A two-stage approach for measuring vascular water exchange and arterial transit time by diffusion-weighted perfusion MRI. Magn. Reson. Med. 2012, 67, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Basser, P.J.; Pierpaoli, C. A simplified method to measure the diffusion tensor from seven MR images. Magn. Reson. Med. 1998, 39, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D.; Mangin, J.; Poupon, C.; Clark, C.A.; Pappata, S.; Molko, N.; Chabriat, H. Diffusion tensor imaging: Concepts and applications. J. Magn. Reson. Imaging 2001, 13, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Sheslow, D.; Wayne, A. Wide Range Assessment of Memory and Learning, 2nd ed.; Administration and Technical Manual; Psychological Assessment Resources: Lutz, FL, USA, 2003. [Google Scholar]
- Pike, N.A.; Roy, B.; Gupta, R.; Singh, S.; Woo, M.A.; Halnon, N.J.; Lewis, A.B.; Kumar, R. Brain abnormalities in cognition, anxiety, and depression regulatory regions in adolescents with single ventricle heart disease. J. Neurosci. Res. 2018, 96, 1104–1118. [Google Scholar] [CrossRef]
- Wang, J.; Alsop, D.C.; Song, H.K.; Maldjian, J.A.; Tang, K.; Salvucci, A.E.; Detre, J.A. Arterial transit time imaging with flow encoding arterial spin tagging (FEAST). Magn. Reson. Med. 2003, 50, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Ma, S.J.; Casey, M.; D’Orazio, L.; Ringman, J.M.; Wang, D.J. Mapping water exchange across the blood-brain barrier using 3D diffusion-prepared arterial spin labeled perfusion MRI. Magn. Reason. Med. 2019, 81, 3065–3079. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Friston, K.J. Unified segmentation. NeuroImage 2005, 26, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Macey, P.M.; Woo, M.A.; Alger, J.R.; Harper, R.M. Diffusion tensor imaging demonstrates brainstem and cerebellar abnormalities in congenital central hypoventilation syndrome. Pediatr. Res. 2008, 64, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Schmithorst, V.; Badaly, D.; Beers, S.R.; Lee, V.K.; Weinberg, J.; Lo, C.W.; Panigrahy, A. Relationships between Regional Cerebral Blood Flow and Neurocognitive Outcomes in Children and Adolescents with Congenital Heart Disease. Semin. Thorac. Cardiovasc. Surg. 2022, 34, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, U.D.; Evangelou, I.E.; Donofrio, M.T.; Vezina, L.G.; McCarter, R.; du Plessis, A.J.; Limperopoulos, C. Impaired global and regional cerebral perfusion in newborns with complex congenital heart disease. J. Pediatr. 2015, 167, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Fogal, M.A.; Li, C.; Elci, O.U.; Pawlowski, T.; Schwab, P.J.; Wilson, F.; Nicolson, S.C.; Montenegro, L.M.; Diaz, L.; Spray, T.L.; et al. Neurologic injury and cerebral blood flow in single ventricles throughout staged surgical reconstruction. Circulation 2017, 135, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Fogal, M.A.; Li, C.; Wilson, F.; Pawlowski, T.; Nicolson, S.C.; Montenegro, L.M.; Diaz, L.; Spray, T.L.; Gaynor, J.W.; Fuller, S.; et al. Relationship between cerebral blood flow to aortic to pulmonary collateral/shunt flow in single ventricles. Heart 2015, 101, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Bracko, O.; Hernández, J.C.C.; Park, L.; Nishimura, N.; Schaffer, C.B. Causes and consequences of baseline cerebral blood flow reductions in Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2021, 41, 1501–1516. [Google Scholar] [CrossRef]
- Morgan, C.A.; Melzer, T.R.; Roberts, R.P.; Wiebels, K.; Mutsaerts, H.J.M.M.; Spriggs, M.J.; Dalrymple-Alford, J.C.; Anderson, T.J.; Cutfield, N.J.; Deib, G.; et al. Spatial variation of perfusion MRI reflects cognitive decline in mild cognitive impairment and early dementia. Sci. Rep. 2021, 11, 23325. [Google Scholar] [CrossRef]
- Bagge, C.N.; Henderson, V.W.; Laursen, H.B.; Adelborg, K.; Olsen, M.; Madsen, N.L. Risk of Dementia in Adults with Congenital Heart Disease: Population-Based Cohort Study. Circulation 2018, 137, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Zhang, L.J.; Zhong, J.; Wang, Z.; Qi, R.; Shi, D.; Lu, G.M. Cerebral blood flow measured by arterial-spin labeling MRI: A useful biomarker for characterization of minimal hepatic encephalopathy in patients with cirrhosis. Eur. J. Radiol. 2013, 82, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Telischak, N.A.; Detre, J.A.; Zaharchuk, G. Arterial spin labeling MRI: Clinical applications in the brain. J. Magn. Reson. Imaging 2015, 41, 1165–1180. [Google Scholar] [CrossRef] [PubMed]
- Okell, T.W.; Harston, G.W.J.; Chappell, M.A.; Sheerin, F.; Kennedy, J.; Jezzard, P. Measurement of collateral perfusion in acute stroke: A vessel-encoded arterial spin labeling study. Sci. Rep. 2019, 9, 8181. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Duda, J.; Avants, B.; Giannetta, M.; Xie, S.X.; Roberts, T.; Detre, J.A.; Hurt, H.; Wehrli, F.W.; Wang, D.J.J. Longitudinal reproducibility and accuracy of pseudo-continuous arterial spin–labeled perfusion MR imaging in typically developing children. Radiology 2012, 263, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Koziol, L.F.; Budding, D.; Andreasen, N.; D’Arrigo, S.; Bulgheroni, S.; Imamizu, H.; Ito, M.; Manto, M.; Marvel, C.; Parker, K.; et al. Consensus Paper: The Cerebellum’s Role in Movement and Cognition. Cerebellum 2014, 13, 151–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.-H.; Kloth, A.D.; Badura, A. The Cerebellum, Sensitive Periods, and Autism. Neuron 2014, 83, 518–532. [Google Scholar] [CrossRef] [PubMed]
- Sigmon, E.R.; Kelleman, M.; Susi, A.; Nylund, C.M.; Oster, M.E. Congenital Heart Disease and Autism: A Case-Control Study. Pediatrics 2019, 144, e20184114. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.; Becker, N.; Apps, R.; Jones, M. Back to front: Cerebellar connections and interactions with the prefrontal cortex. Front. Syst. Neurosci. 2014, 8, 4. [Google Scholar] [CrossRef]
- Wernovsky, G.; Licht, D.J. Neurodevelopmental Outcomes in children with congenital heart disease—What can we impact? Pediatr. Crit. Care Med. 2016, 17, S232–S242. [Google Scholar] [CrossRef]
- Goldberg, C.S.; Bove, E.L.; Devaney, E.J.; Mollen, E.; Schwartz, E.; Tindall, S.; Nowak, C.; Charpie, J.; Brown, M.B.; Kulik, T.J.; et al. A randomized clinical trial of regional cerebral perfusion versus deep hypothermic circulatory arrest: Outcomes for infants with functional single ventricle. J. Thorac. Cardiovasc. Surg. 2007, 133, 880–887.e1. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, C.S.; Hu, C.; Brosig, C.; Gaynor, J.W.; Mahle, W.T.; Miller, T.; Mussatto, K.A.; Sananes, R.; Uzark, K.; Trachtenberg, F.; et al. Behavior and quality of life at 6 years for children with hypoplastic left heart syndrome. Pediatrics 2019, 144, e20191010. [Google Scholar] [CrossRef] [PubMed]
- International Cardiac Collaborative on Neurodevelopment (ICCON) Investigators. Impact of operative and postoperative factors on neurodevelopmental outcomes after cardiac operations. Ann. Thorac. Surg. 2016, 102, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Newburger, J.W.; Sleeper, L.A.; Bellinger, D.C.; Goldberg, C.S.; Tabbutt, S.; Lu, M.; Mussatto, K.A.; Williams, I.A.; Gustafson, K.E.; Mital, S.; et al. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: The single ventricle reconstruction trial. Circulation 2012, 125, 2081–2091. [Google Scholar] [CrossRef]
- Sood, E.; Newburger, J.W.; Anixt, J.S.; Cassidy, A.R.; Jackson, J.L.; Jonas, R.A.; Lisanti, A.J.; Lopez, K.N.; Peyvandi, S.; Marino, B.S.; et al. Neurodevelopmental outcomes for individuals with congenital heart disease: Updates in neuroprotection, risk-stratification, evaluation, and management: A scientific statement from the American Heart Association. Circulation 2024, 149, e997–e1022. [Google Scholar] [CrossRef]
| Characteristic | CCHD Group [n = 37] | Control Group [n = 33] | p-Value |
|---|---|---|---|
| Mean [SD] or n [%] | |||
| Age [years] | 15.8 [1.37] | 15.8 [1.21] | 0.881 |
| Gender [female] | 19 [51%] | 19 [58%] | 0.638 |
| Ethnicity | 0.75 | ||
| White | 16 [43%] | 13 [39%] | |
| Hispanic | 17 [46%] | 15 [45%] | |
| Other | 4 [11%] | 5 [15%] | |
| Body mass index (kg/m2) | 23 [6.3] | 24.4 [4.8] | 0.467 |
| Handedness [Right] | 30 [81%] | 30 [91%] | 0.411 |
| Household income [highest] | 0.233 | ||
| <30,000 | 11 [30%] | 5 [15%] | |
| 30,000–50,000 | 5 [13.5%] | 1 [3%] | |
| 50,001–80,000 | 7 [19%] | 7 [21%] | |
| 81,001–100,000 | 2 [6%] | 6 [18%] | |
| >100,000 | 9 [24%] | 13 [40%] | |
| Unsure | 3 [8%] | 1 [3%] | |
| Maternal education [highest] | 0.725 | ||
| <High school | 10 [27%] | 7 [21%] | |
| High school graduate | 11 [30%] | 9 [27%] | |
| College or university degree | 10 [27%] | 10 [31%] | |
| Graduate degree | 6 [16%] | 7 [21%] | |
| CCHD type | N/A | N/A | |
| Tetralogy of Fallot | 14 [38%] | ||
| Single-ventricle/Fontan | 23 [62%] | ||
| Number of surgeries | 3 [0.7] | N/A | N/A |
| Number of years since last surgery | 11 [5.1] | N/A | N/A |
| Oxygen saturation <93% | 8 [22%] | N/A | N/A |
| Variables | CCHD Group [n = 37] | Control Group [n = 33] | p Value |
|---|---|---|---|
| Mean [SD] | |||
| MoCA, Total | 23.1 [4.1] | 28.1 [2.3] | <0.001 |
| Visuospatial/executive function | 3.59 [1.2] | 4.48 [0.9] | 0.002 |
| Naming | 2.95 [0.2] | 3.00 [0.0] | 0.18 |
| Attention | 4.19 [1.4] | 5.45 [1.0] | <0.001 |
| Language | 1.68 [1.0] | 2.64 [0.6] | <0.001 |
| Abstraction | 0.95 [0.7] | 1.88 [0.3] | <0.001 |
| Delayed recall | 2.81 [1.4] | 3.91 [1.1] | <0.001 |
| WRAML2, General Memory Index | 86.89 [15.4] | 110.3 [14.5] | <0.001 |
| Verbal memory | 87.14 [13.3] | 101.21 [13.1] | <0.001 |
| Visual memory | 102.41 [13.3] | 118.94 [14.1] | <0.001 |
| Attention/concentration | 82.70 [15.9] | 102.73 [13.3] | <0.001 |
| WRAML2, General Recognition Index | 95.67 [13.6] | 108.70 [13.6] | <0.001 |
| Working memory | 87.59 [17.5] | 105.36 [19.1] | <0.001 |
| Verbal recognition | 92.62 [13.4] | 100.42 [17.4] | 0.038 |
| Visual recognition | 99.94 [13.9] | 108.03 [20.7] | 0.06 |
| Arterial transit time [ATT] [s] | 1.3 [0.13] | 1.22 [0.13] | 0.02 |
| Mean diffusivity values | 0.98 [0.06] | 0.94 [0.06] | 0.006 |
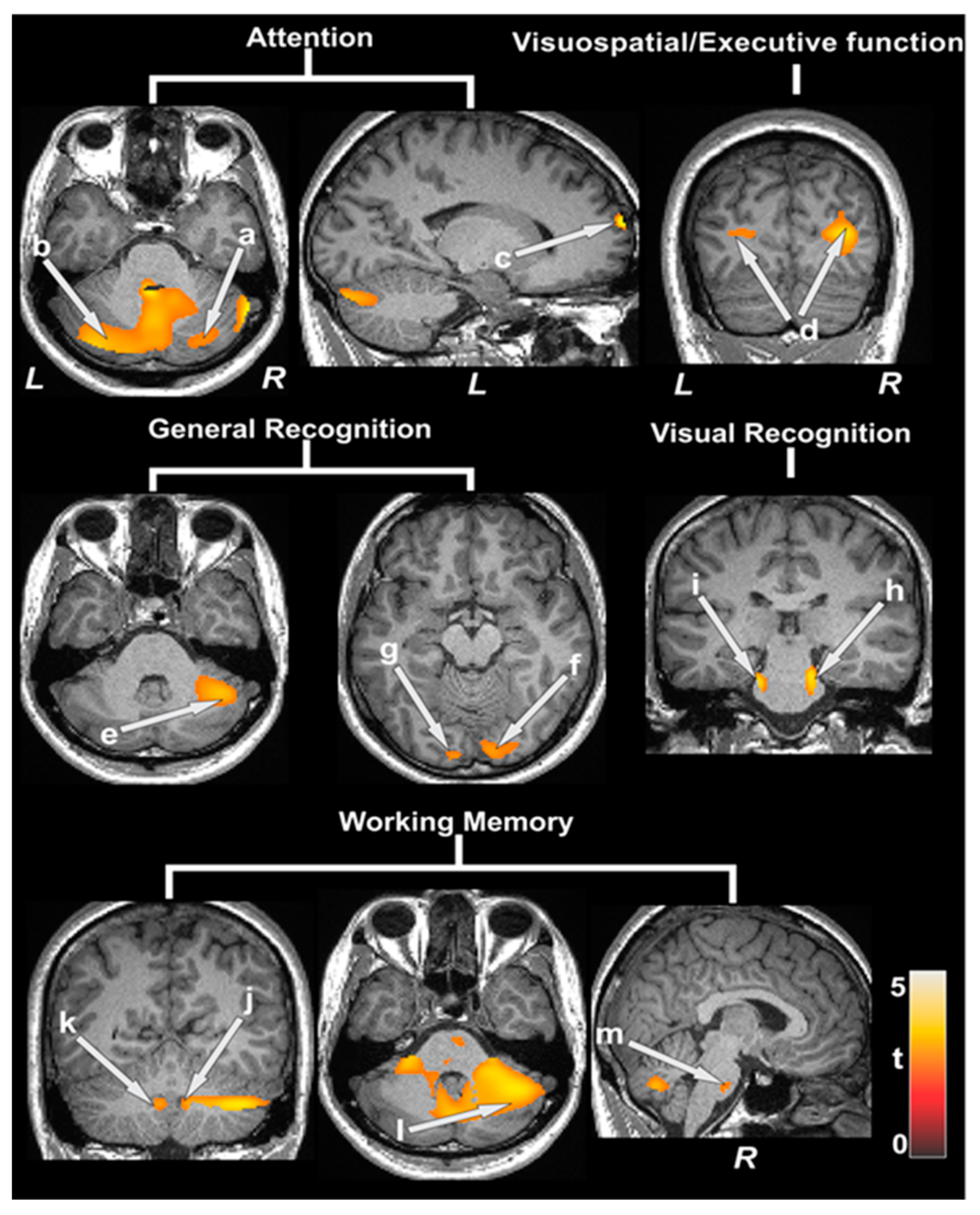
| Cognitive Variables vs. ATT | Brain Regions | r (p-Value) |
|---|---|---|
| MoCA vs. ATT | Right cerebellum | −0.51 (0.006) |
| Left anterior cingulate | −0.50 (0.007) | |
| Right anterior insula | −0.51 (0.006) | |
| Left prefrontal cortices | −0.52 (0.004) | |
| Abstraction vs. ATT | Right thalamus | −0.59 (0.001) |
| Attention vs. ATT | Left prefrontal | −0.51 (0.006) |
| Language vs. ATT | Right cerebellum | −0.53 (0.004) |
| Left anterior cingulate | −0.50 (0.007) | |
| Right lingual gyrus | −0.50 (0.007) | |
| Left mid cingulate | −0.49 (0.008) | |
| Delayed recall vs. ATT | Left prefrontal cortices | −0.49 (0.008) |
| Visuospatial vs. ATT | Left amygdala | −0.52 (0.005) |
| Left hippocampus | −0.54 (0.003) | |
| Left parahippocampal gyrus | −0.51 (0.006) | |
| Right lingual gyrus | −0.51 (0.005) | |
| Left lingual gyrus | −0.53 (0.004) | |
| General memory vs. ATT | Left caudate | −0.50 (0.007) |
| Right anterior insula | −0.54 (0.003) | |
| Left prefrontal cortices | −0.53 (0.004) | |
| General recognition vs. ATT | Left anterior cingulate | −0.50 (0.007) |
| Left mid cingulate | −0.49 (0.008) | |
| Right prefrontal cortices | −0.55 (0.002) | |
| Verbal recognition vs. ATT | Left caudate | −0.52 (0.005) |
| Right anterior insula | −0.56 (0.002) | |
| Right posterior insula | −0.51 (0.006) | |
| Right prefrontal cortices | −0.55 (0.003) | |
| Verbal memory vs. ATT | Right anterior insula | −0.49 (0.006) |
| Visual memory vs. ATT | Right prefrontal cortices | −0.51 (0.006) |
| Working memory vs. ATT | Left prefrontal cortices | −0.50 (0.006) |
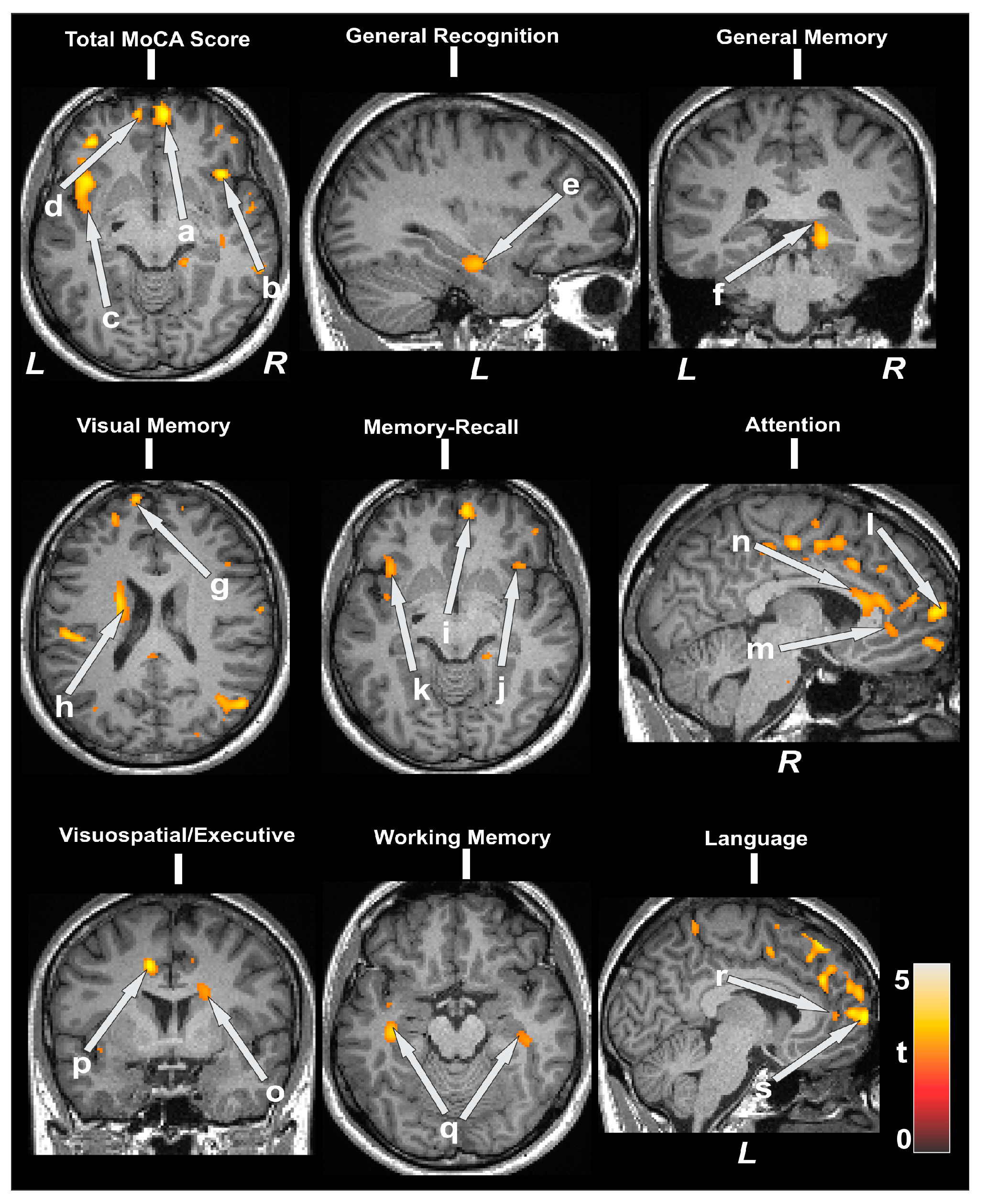
| Cognitive Variables vs. MD | Brain Regions | r (p-Value) |
|---|---|---|
| MoCA total vs. MD | Right prefrontal | −0.47 (0.004) |
| Left prefrontal | −0.52 (0.001) | |
| Right insula | −0.52 (0.001) | |
| Left insula | −0.55 (<0.001) | |
| Right anterior cingulate | −0.55 (<0.001) | |
| Left anterior cingulate | −0.54 (<0.001) | |
| Right mid cingulate | −0.51 (0.002) | |
| Left mid cingulate | −0.54 (<0.001) | |
| Visuospatial/executive function vs. MD | Left prefrontal | −0.48 (0.004) |
| Left insula | −0.50 (0.002) | |
| Right anterior cingulate | −0.53 (0.001) | |
| Left anterior cingulate | −0.50 (0.002) | |
| Right mid cingulate | −0.49 (0.003) | |
| Left mid cingulate | −0.51 (0.002) | |
| Caudate | −0.48 (0.004) | |
| Attention vs. MD | Right prefrontal | −0.48 (0.004) |
| Left prefrontal | −0.53 (0.001) | |
| Right insula | −0.48 (0.004) | |
| Left insula | −0.52 (0.002) | |
| Right anterior cingulate | −0.54 (<0.001) | |
| Left anterior cingulate | −0.55 (<0.001) | |
| Right mid cingulate | −0.51 (0.002) | |
| Left mid cingulate | −0.54 (<0.001) | |
| Delayed memory recall vs. MD | Right prefrontal | −0.50 (0.002) |
| Right insula | −0.47 (0.004) | |
| Left insula | −0.48 (0.003) | |
| Language vs. MD | Right prefrontal | −0.50 (0.002) |
| Left prefrontal | −0.57 (<0.001) | |
| Left insula | −0.45 (0.006) | |
| Left anterior cingulate | −0.49 (0.003) | |
| Right mid cingulate | −0.49 (0.003) | |
| Left mid cingulate | −0.46 (0.006) | |
| General memory vs. MD | Hippocampus | −0.48 (0.003) |
| Prefrontal | −0.43 (0.01) | |
| Caudate | −0.44 (0.008) | |
| General recognition vs. MD | Hippocampus | −0.52 (0.002) |
| Working memory vs. MD | Hippocampus | −0.44 (0.008) |
| Visual memory vs. MD | Hippocampus | −0.48 (0.004) |
| Prefrontal | −0.49 (0.003) | |
| Caudate | −0.46 (0.005) |
| Brain MRI Findings | CCHD [n = 37] | Controls [n = 33] |
|---|---|---|
| Any abnormality n [%] | 12 [32%] | 2 [5%] |
| Focal or multifocal abnormality | ||
| Focal infarction or atrophy | 7 [22%] | 0 [0%] |
| Developmental abnormality Minor a | 4 [14%] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pike, N.A.; Roy, B.; Cabrera-Mino, C.; Halnon, N.J.; Lewis, A.B.; Shao, X.; Wang, D.J.J.; Kumar, R. Compromised Cerebral Arterial Perfusion, Altered Brain Tissue Integrity, and Cognitive Impairment in Adolescents with Complex Congenital Heart Disease. J. Cardiovasc. Dev. Dis. 2024, 11, 236. https://doi.org/10.3390/jcdd11080236
Pike NA, Roy B, Cabrera-Mino C, Halnon NJ, Lewis AB, Shao X, Wang DJJ, Kumar R. Compromised Cerebral Arterial Perfusion, Altered Brain Tissue Integrity, and Cognitive Impairment in Adolescents with Complex Congenital Heart Disease. Journal of Cardiovascular Development and Disease. 2024; 11(8):236. https://doi.org/10.3390/jcdd11080236
Chicago/Turabian StylePike, Nancy A., Bhaswati Roy, Cristina Cabrera-Mino, Nancy J. Halnon, Alan B. Lewis, Xingfeng Shao, Danny J. J. Wang, and Rajesh Kumar. 2024. "Compromised Cerebral Arterial Perfusion, Altered Brain Tissue Integrity, and Cognitive Impairment in Adolescents with Complex Congenital Heart Disease" Journal of Cardiovascular Development and Disease 11, no. 8: 236. https://doi.org/10.3390/jcdd11080236





