Novel Computed Tomography Angiography Parameter Is Associated with Low Cardiac Index in Patients with Chronic Thromboembolic Pulmonary Hypertension: A Retrospective Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. CT Acquisition and Measurements
2.3. Statistical Analysis
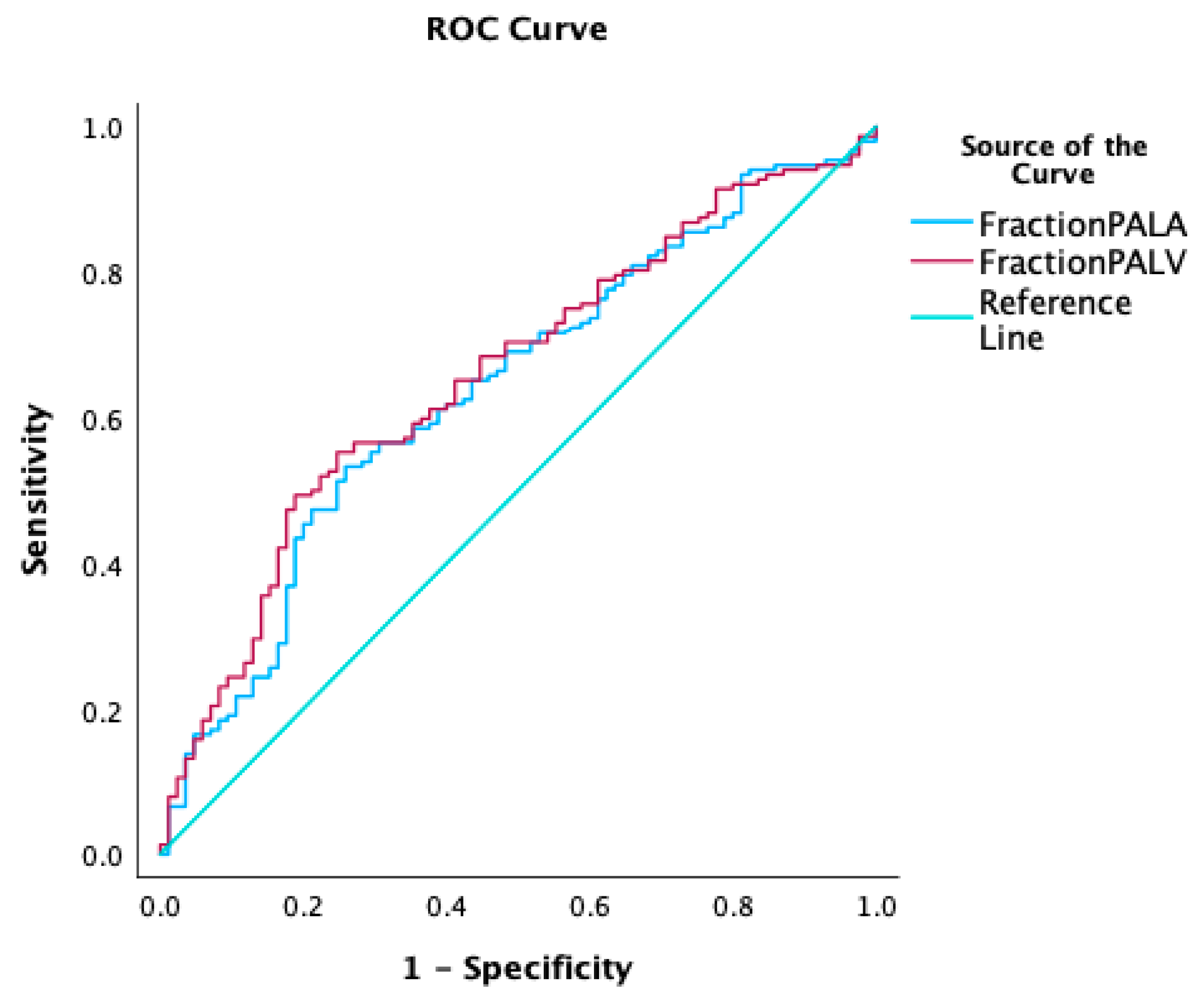
3. Results
3.1. Basic Demographics, Comorbidities, and Hemodynamics of the CTEPH Cohort
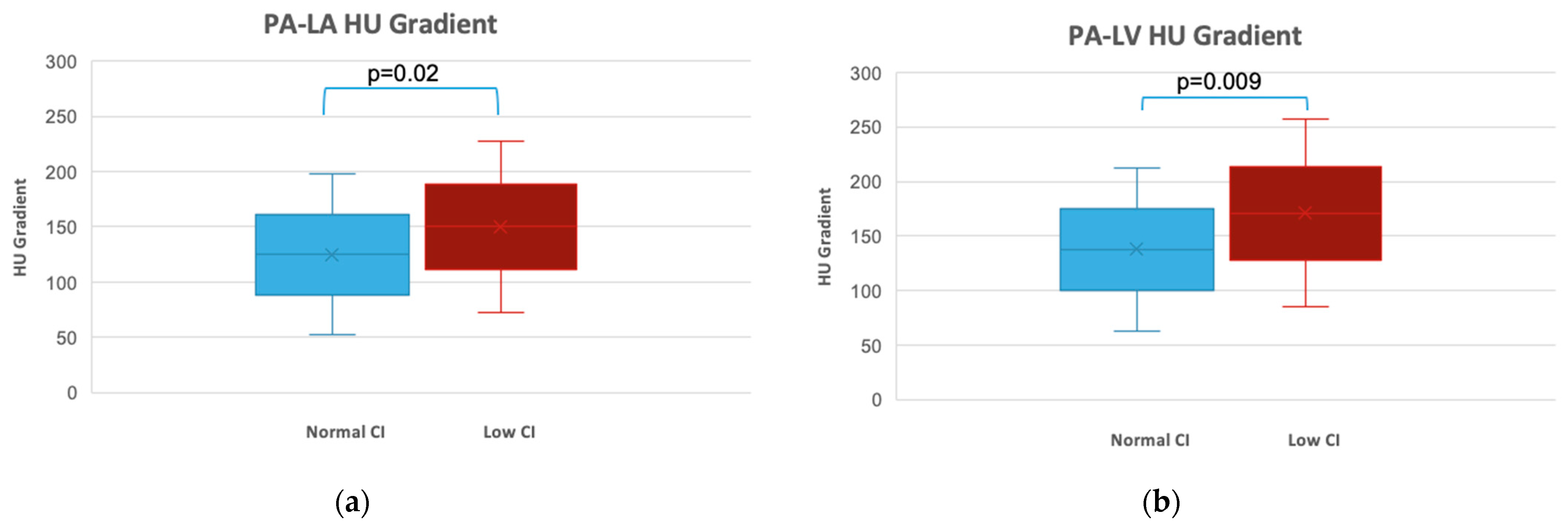
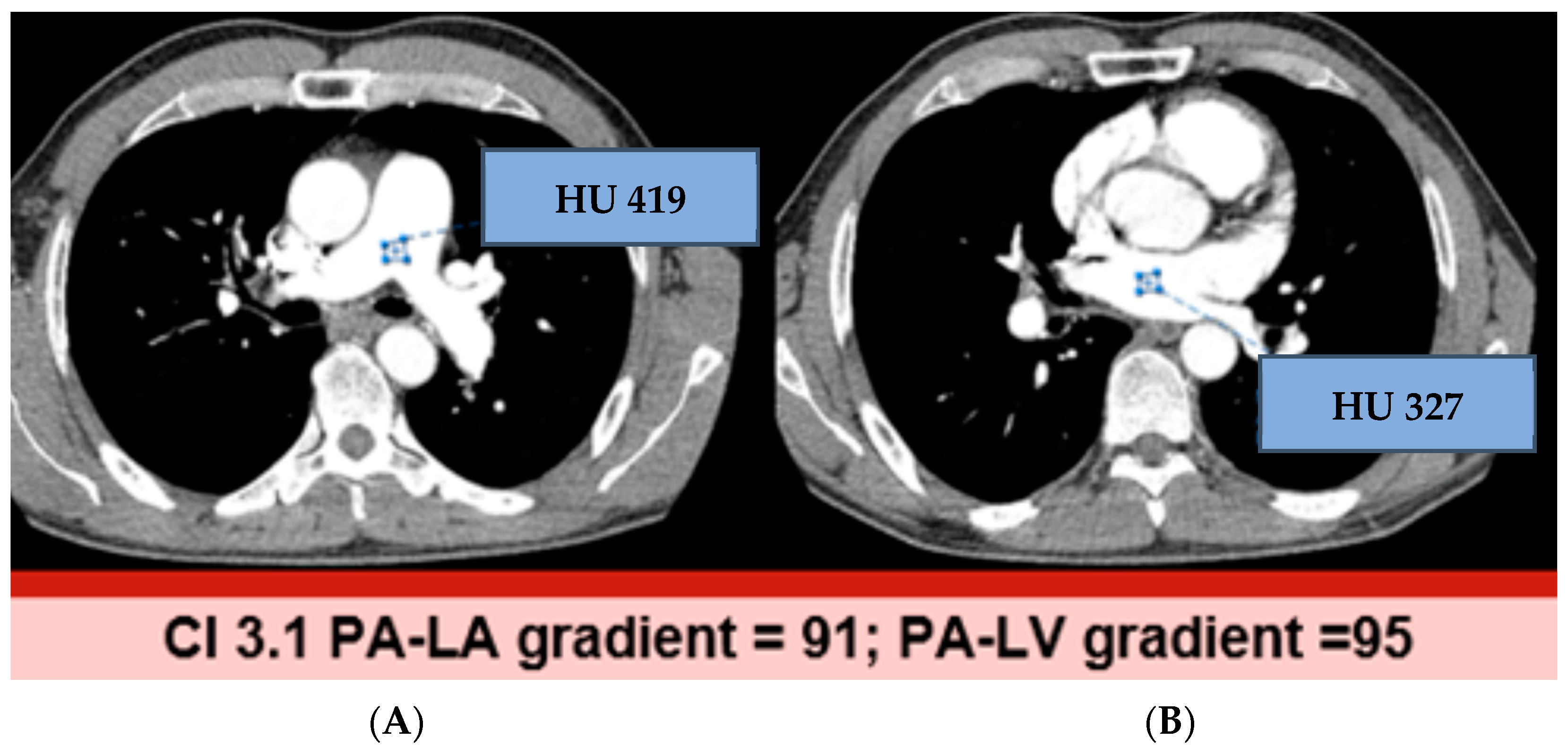
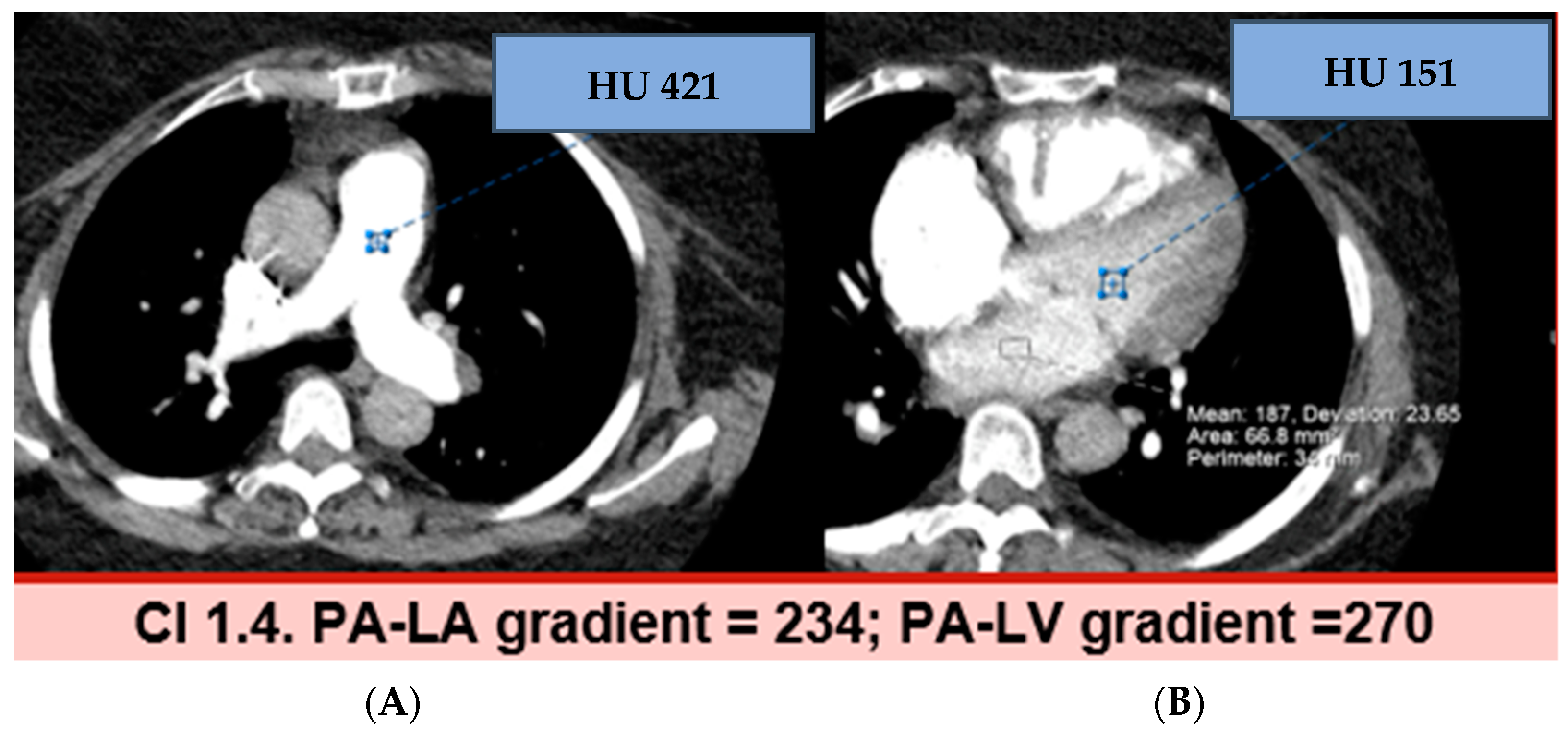
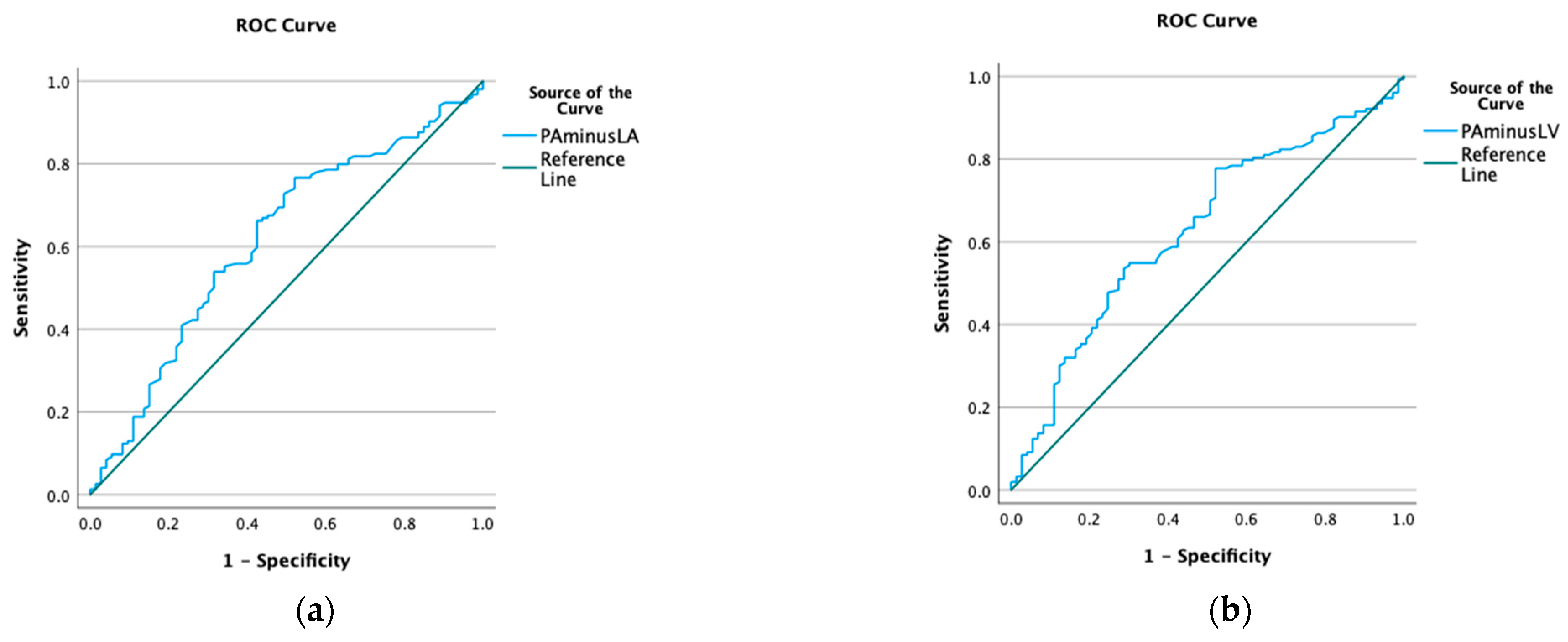
3.2. Hounsfield Units and Gradients
3.3. Fractional Reduction of HU
3.4. Pulmonary Vein Sign
3.5. Interobserver Variability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.; Brida, M.; Carlsen, J.; Coats, A.J.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [PubMed]
- Mahmud, E.; Behnamfar, O.; Ang, L.; Patel, M.P.; Poch, D.; Kim, N.H. Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension. Interv. Cardiol. Clin. 2018, 7, 103–117. [Google Scholar] [PubMed]
- Mahammedi, A.; Oshmyansky, A.; Hassoun, P.M.; Thiemann, D.R.; Siegelman, S.S. Pulmonary artery measurements in pulmonary hypertension: The role of computed tomography. J. Thorac. Imaging 2013, 28, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Brailovsky, Y.; Masic, D.; Allen, S.; Lakhter, V.; Bashir, R.; Forfia, P.; Bechara, C.F.; Leya, F.S.; Lopez, J.J.; Lewis, B.E.; et al. Novel CT-derived parameter is associated with low cardiac index in acute pulmonary embolism. Thromb. Res. 2021, 202, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, D.; Riley, J.Y.J.; Leong, K.; Alsanjari, S.; Auger, W.; Lindholm, P. Computed Tomography Pulmonary Angiography Prediction of Adverse Long-Term Outcomes in Chronic Thromboembolic Pulmonary Hypertension: Correlation with Hemodynamic Measurements Pre- and Post-Pulmonary Endarterectomy. Tomography 2023, 9, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Bae, K.T. Intravenous contrast medium administration and scan timing at CT: Considerations and approaches. Radiology 2010, 256, 32–61. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.S.; Diamond, G.; Chatterjee, K.; Swan, H.J. Medical therapy of acute myocardial infarction by application of hemodynamic subsets (first of two parts). N. Engl. J. Med. 1976, 295, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Nohria, A.; Tsang, S.W.; Fang, J.C.; Lewis, E.F.; Jarcho, J.A.; Mudge, G.H.; Stevenson, L.W. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J. Am. Coll. Cardiol. 2003, 41, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- University of Wisconsin-Madison. GE CT Protocol Partnership. Protocol Manuals. Available online: https://uwgect.wiscweb.wisc.edu/protocol-manuals/ (accessed on 5 July 2024).
- Gopalan, D.; Nordgren-Rogberg, A.; Le, E.P.V.; Pavey, H.; Tarkin, J.; Nyrén, S.; Auger, W.; Lindholm, P. Abnormal Pulmonary Venous Filling: An Adjunct Feature in the Computed Tomography Pulmonary Angiogram Assessment of Chronic Thromboembolic Pulmonary Hypertension. J. Am. Heart Assoc. 2020, 9, e018075. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, D.; Kamaya, A. Optimal vascular and parenchymal contrast enhancement: The current state of the art. Radiol. Clin. N. Am. 2009, 47, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Parker, A. Image Reconstruction in Radiology, 1st ed.; CRC-Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Riedel, M.; Stanek, V.; Widimsky, J.; Prerovsky, I. Longterm follow-up of patients with pulmonary thromboembolism: Late prognosis and evolution of hemodynamic and respiratory data. Chest 1982, 81, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, S.W.; Kapelanski, D.P.; Sakakibara, N.; Manecke, G.R.; Thistlethwaite, P.A.; Kerr, K.M.; Channick, R.N.; Fedullo, P.F.; Auger, W.R. Pulmonary endarterectomy: Experience and lessons learned in 1500 cases. Ann. Thorac. Surg. 2003, 76, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.; Jenkins, D.; Lindner, J.; D’armini, A.; Kloek, J.; Meyns, B.; Ilkjaer, L.B.; Klepetko, W.; Delcroix, M.; Lang, I.; et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: Results from an international prospective registry. J. Thorac. Cardiovasc. Surg. 2011, 141, 702–710. [Google Scholar] [CrossRef] [PubMed]
| Variable | All (n = 237) n(%) or Mean ± SD | Normal CI n = 88 n(%) or Mean ± SD | Low CI n = 149 n(%) or Mean ± SD | p Value |
|---|---|---|---|---|
| Female | 119 (50.2%) | 58 (65.9%) | 61 (40.9%) | 0.001 |
| Race/Ethnicity | ||||
| White | 126 (53.2%) | 46 (52.3%) | 80 (53.7%) | 0.372 |
| Black | 87 (36.7%) | 29 (34.1%) | 58 (39.5%) | |
| Latinx | 17 (7.2%) | 10 (11.4%) | 7 (4.7%) | |
| Age (years) | 60 ± 14.5 | 59 ± 16 | 60 ± 14 | 0.776 |
| BMI (kg/m2) | 30.1 ± 8.1 | 31.4 ± 7.3 | 31.2 ± 8.4 | 0.642 |
| WHO FC | ||||
| I | 16 (6.8%) | 6 (6.8%) | 10 (6.7%) | 0.607 |
| II | 71 (30%) | 29 (33%) | 42 (28%) | |
| III | 120 (50.6%) | 45 (51.1%) | 75 (50.3%) | |
| IV | 30 (12.7%) | 8 (9.1%) | 22 (14.8%) | |
| Comorbidities | ||||
| Atrial fibrillation | 32 (13.5%) | 14 (17%) | 18 (18.5%) | 0.358 |
| Diabetes mellitus | 45 (19%) | 15 (17.4%) | 30 (20.1%) | 0.613 |
| History of coagulopathy | 54 (21.8%) | 19 (22.6%) | 31 (20.9%) | 0.766 |
| History of DVT | 121 (51.1%) | 42 (51.2%) | 79 (53.7%) | 0.714 |
| History of PE | 185 (78.1%) | 63 (74%) | 122 (83%) | 0.105 |
| Sleep Disorder | 55 (23.2%) | 14 (16.7%) | 41 (27.7%) | 0.057 |
| PH medication | 82 (23.2%) | 34 (38.6%) | 48 (32.2%) | 0.218 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveros, E.; Ibrahim, M.; Romero, C.M.; Navo, P.; Otero Valdes, P.; Brailovsky, Y.; Darki, A.; Bashir, R.; Vaidya, A.; Forfia, P.; et al. Novel Computed Tomography Angiography Parameter Is Associated with Low Cardiac Index in Patients with Chronic Thromboembolic Pulmonary Hypertension: A Retrospective Analysis. J. Cardiovasc. Dev. Dis. 2024, 11, 281. https://doi.org/10.3390/jcdd11090281
Oliveros E, Ibrahim M, Romero CM, Navo P, Otero Valdes P, Brailovsky Y, Darki A, Bashir R, Vaidya A, Forfia P, et al. Novel Computed Tomography Angiography Parameter Is Associated with Low Cardiac Index in Patients with Chronic Thromboembolic Pulmonary Hypertension: A Retrospective Analysis. Journal of Cardiovascular Development and Disease. 2024; 11(9):281. https://doi.org/10.3390/jcdd11090281
Chicago/Turabian StyleOliveros, Estefania, Michel Ibrahim, Carlos Manuel Romero, Paul Navo, Patricia Otero Valdes, Yevgeniy Brailovsky, Amir Darki, Riyaz Bashir, Anjali Vaidya, Paul Forfia, and et al. 2024. "Novel Computed Tomography Angiography Parameter Is Associated with Low Cardiac Index in Patients with Chronic Thromboembolic Pulmonary Hypertension: A Retrospective Analysis" Journal of Cardiovascular Development and Disease 11, no. 9: 281. https://doi.org/10.3390/jcdd11090281








