Computed Tomography Angiography in the Catheterization Laboratory: A Guide Towards Optimizing Coronary Interventions
Abstract
:1. Introduction
2. Anatomical Evaluation of Coronary Arteries
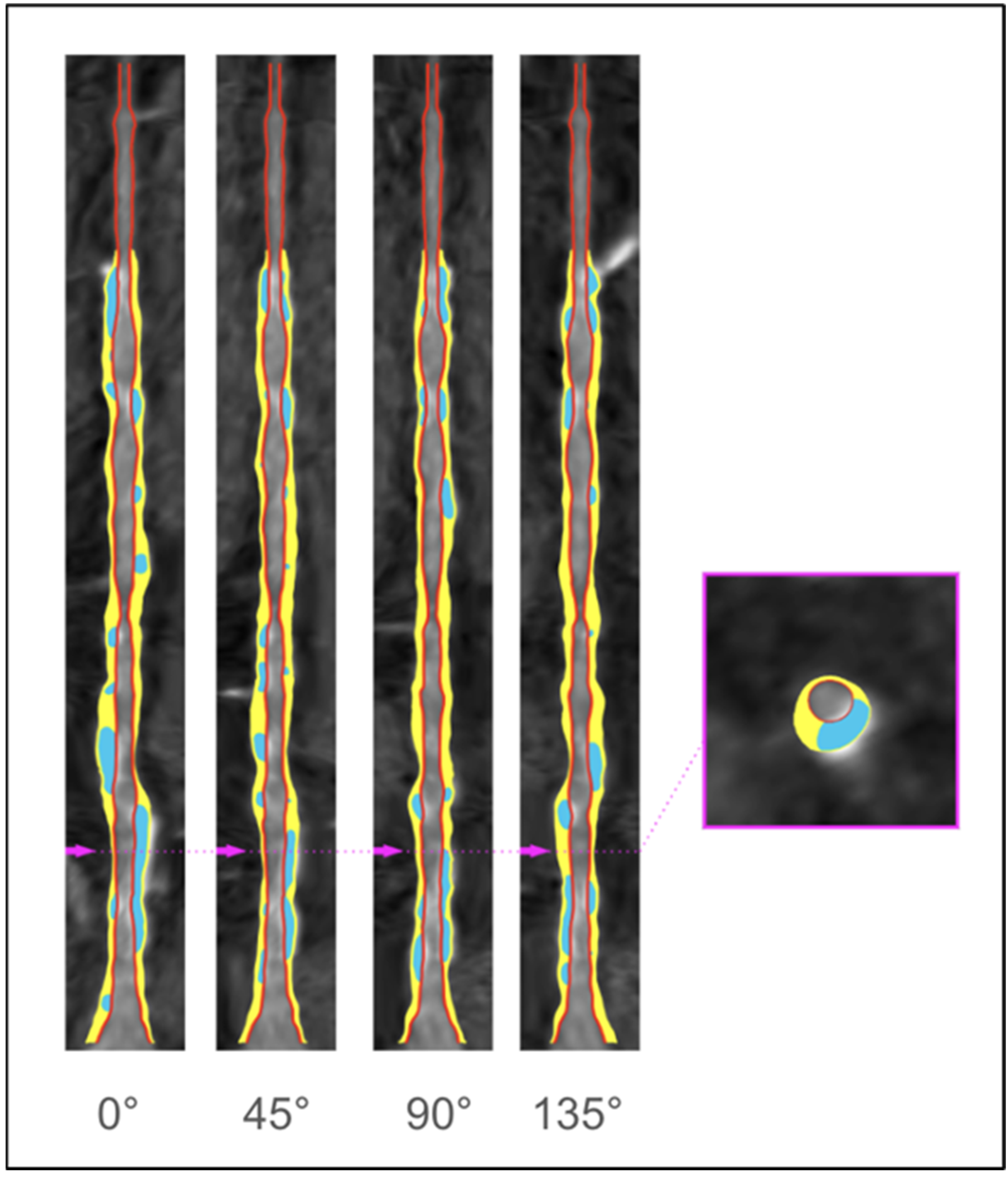
3. Coronary Plaque Assessment
Identifying High-Risk Plaque Features of CAD
4. Functional Evaluation of Coronary Stenoses
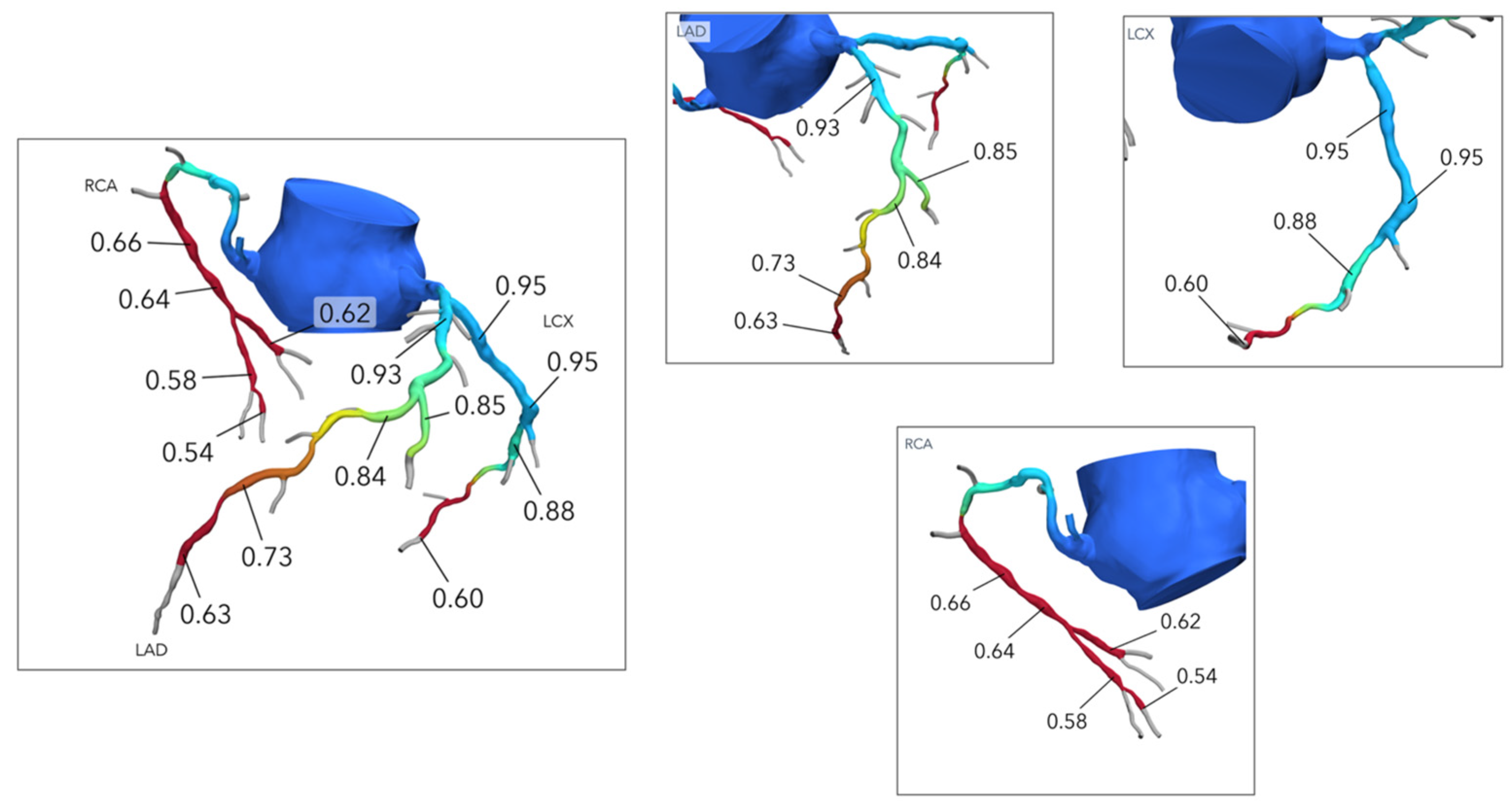
5. Cardiac Computed Tomography-Derived Coronary Artery Volume to Myocardial Mass
6. Cardiac Computed Tomography-Derived Myocardial Viability
7. Cardiac Computed Tomography in Special Clinical Scenarios
7.1. Coronary Anomalies
7.2. Coronary Fistula
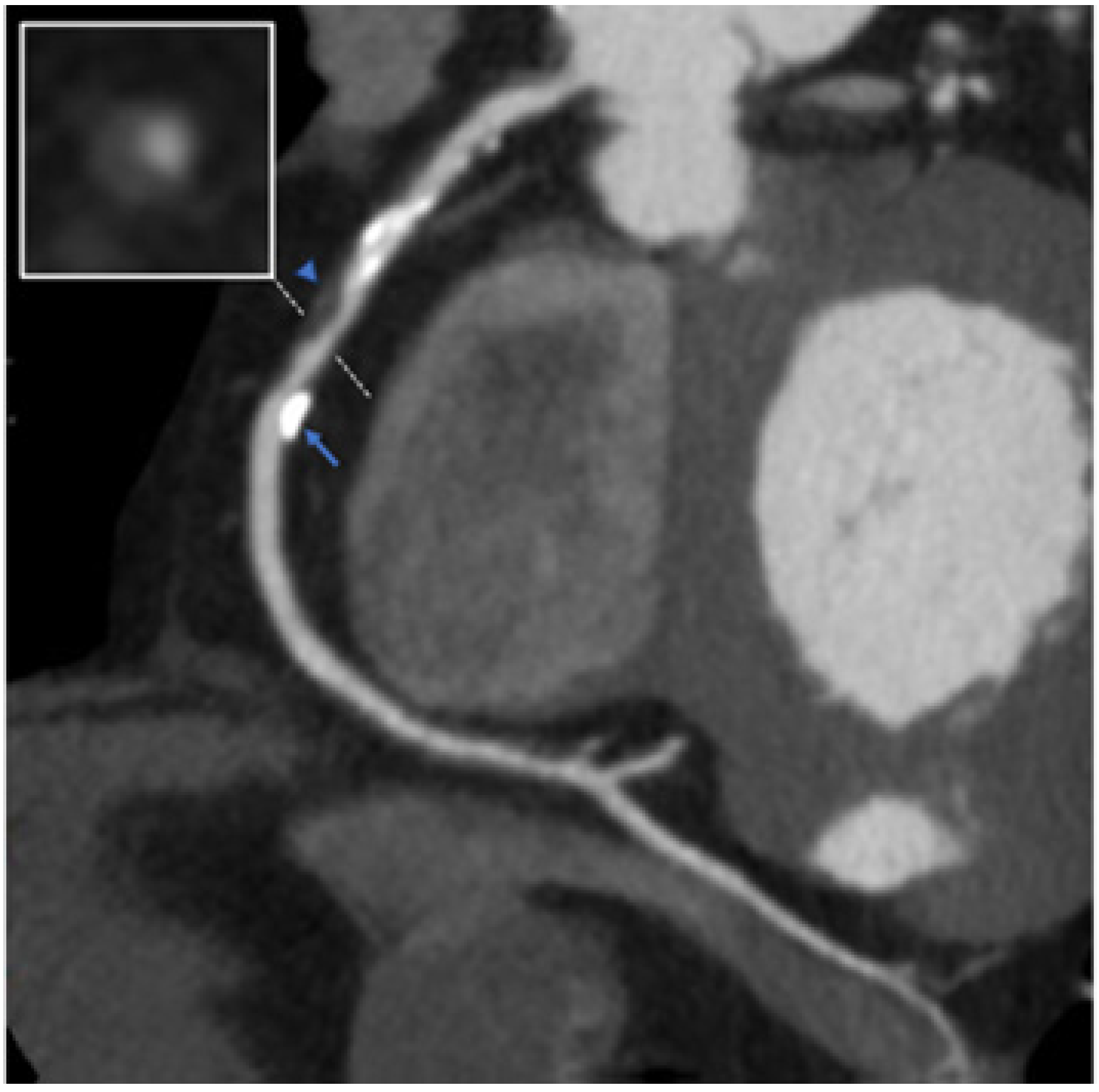
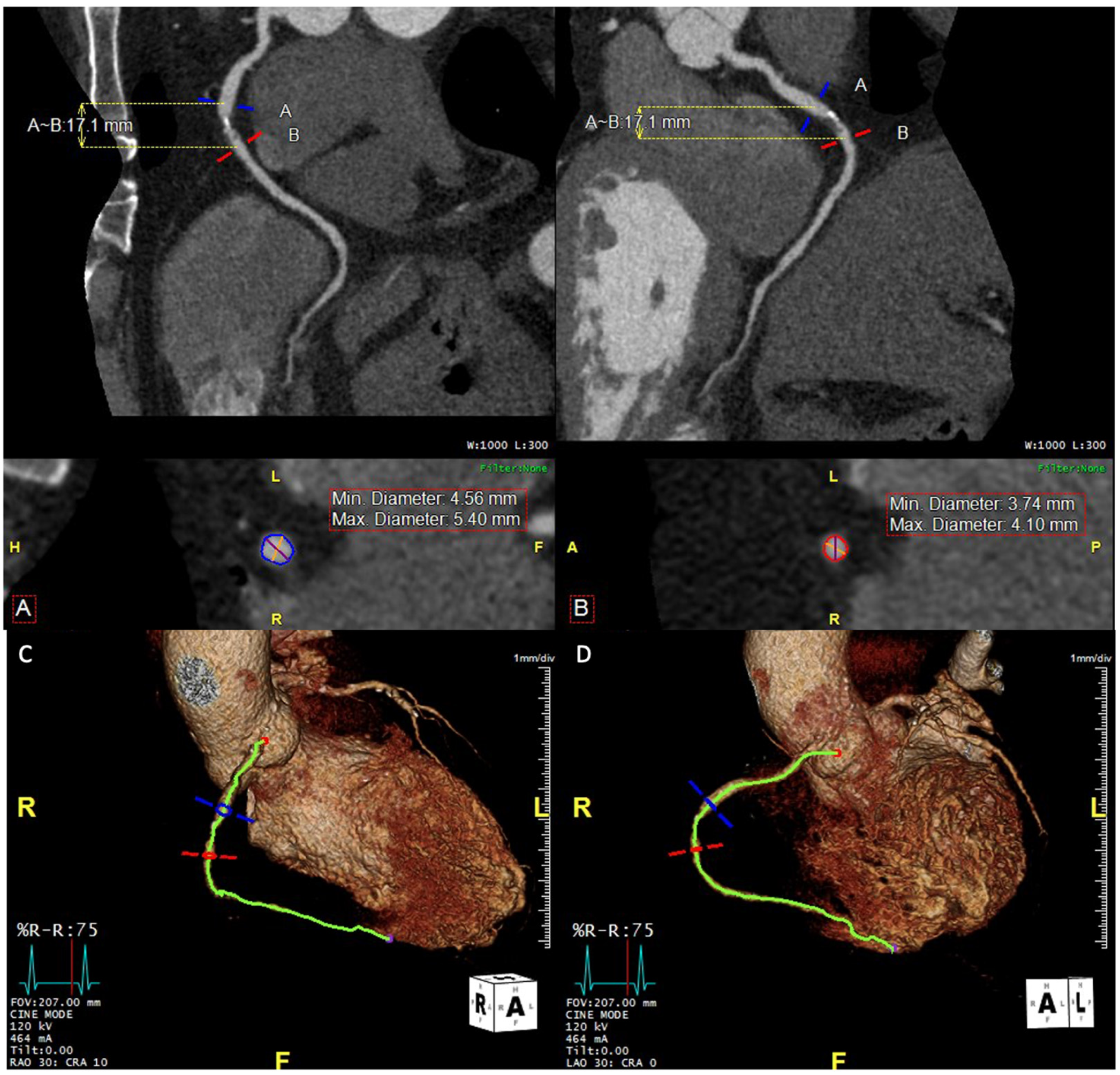
7.3. Coronary Aneurysm
7.4. Post-CABG
7.5. Chronic Total Occlusion (CTO)
8. Diagnostic Advances and Limitations of CCTA in Obstructive CAD
9. Future Directions
9.1. Artificial Intelligence, Machine Learning, and Radiomics
9.2. Advancements in CT Technology for PCI Guidance
9.3. The P4 Study and Beyond
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Linde, J.J.; Kelbæk, H.; Hansen, T.F.; Sigvardsen, P.E.; Torp-Pedersen, C.; Bech, J.; Heitmann, M.; Nielsen, O.W.; Høfsten, D.; Kühl, J.T.; et al. Coronary CT angiography in patients with non-ST-segment elevation acute coronary syndrome. J. Am. Coll. Cardiol. 2020, 75, 453–463. [Google Scholar] [CrossRef]
- Vrints, C.; Andreotti, F.; Koskinas, K.C.; Rossello, X.; Adamo, M.; Ainslie, J.; Banning, A.P.; Budaj, A.; Buechel, R.R.; Chiariello, G.A.; et al. 2024 ESC Guidelines for the management of chronic coronary syndromes: Developed by the task force for the management of chronic coronary syndromes of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3415–3537. [Google Scholar] [CrossRef]
- Kočka, V.; Thériault-Lauzier, P.; Xiong, T.Y.; Ben-Shoshan, J.; Petr, R.; Laboš, M.; Buithieu, J.; Mousavi, N.; Pilgrim, T.; Praz, F.; et al. Optimal Fluoroscopic Projections of Coronary Ostia and Bifurcations Defined by Computed Tomographic Coronary Angiography. JACC Cardiovasc. Interv. 2020, 13, 2560–2570. [Google Scholar] [CrossRef]
- Voros, S.; Rinehart, S.; Qian, Z.; Joshi, P.; Vazquez, G.; Fischer, C.; Belur, P.; Hulten, E.; Villines, T.C. Coronary atherosclerosis imaging by coronary CT angiography: Current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovasc. Imaging 2011, 4, 537–548. [Google Scholar] [CrossRef]
- Fischer, C.; Hulten, E.; Belur, P.; Smith, R.; Voros, S.; Villines, T.C. Coronary CT angiography versus intravascular ultrasound for estimation of coronary stenosis and atherosclerotic plaque burden: A meta-analysis. J. Cardiovasc. Comput. Tomogr. 2013, 7, 256–266. [Google Scholar] [CrossRef]
- Ko, B.; Ohashi, H.; Mizukami, T.; Sakai, K.; Sonck, J.; Nørgaard, B.L.; Maeng, M.; Jensen, J.M.; Ihdayhid, A.; Tajima, A.; et al. Stent sizing by coronary CT angiography compared with optical coherence tomography. J. Cardiovasc. Comput. Tomogr. 2024, 18, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, B.; Vera-Urquiza, R.; Mejía-Rentería, H.; Gonzalo, N.; Escaned, J. Contemporary use of coronary computed tomography angiography in the planning of percutaneous coronary intervention. Int. J. Cardiovasc. Imaging 2020, 36, 2441–2459. [Google Scholar] [CrossRef]
- Van Mieghem, C.A.; Thury, A.; Meijboom, W.B.; Cademartiri, F.; Mollet, N.R.; Weustink, A.C.; Sianos, G.; de Jaegere, P.P.; Serruys, P.W.; de Feyter, P. Detection and characterization of coronary bifurcation lesions with 64-slice computed tomography coronary angiography. Eur. Heart J. 2007, 28, 1968–1976. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.M.; Song, Y.B.; Park, T.K.; Yang, J.H.; Hahn, J.Y.; Choi, S.H.; Gwon, H.C.; Lee, S.H.; Kim, S.M.; et al. Prediction of side branch occlusions in percutaneous coronary interventions by coronary computed tomography: The CT bifurcation score as a novel tool for predicting intraprocedural side branch occlusion. EuroIntervention 2019, 15, e788–e795. [Google Scholar] [CrossRef]
- Ide, S.; Sumitsuji, S.; Yamaguchi, O.; Sakata, Y. Cardiac computed tomography-derived myocardial mass at risk using the Voronoi-based segmentation algorithm: A histological validation study. J. Cardiovasc. Comput. Tomogr. 2017, 11, 179–182. [Google Scholar] [CrossRef]
- Ihdayhid, A.R.; Fairbairn, A.; Gulsin, G.S.; Tzimas, G.; Danehy, E.; Updegrove, A.; Jensen, J.M.; Taylor, C.A.; Bax, J.J.; Sellers, S.L.; et al. Cardiac computed tomography-derived coronary artery volume to myocardial mass. J. Cardiovasc. Comput. Tomogr. 2022, 16, 198–206. [Google Scholar] [CrossRef]
- Kim, H.Y.; Doh, J.H.; Lim, H.S.; Nam, C.W.; Shin, E.S.; Koo, B.K.; Lee, J.M.; Park, T.K.; Yang, J.H.; Song, Y.B.; et al. Identifcation of coronary artery side branch supplying myocardial mass that may beneft from revascularization. JACC Cardiovasc. Interv. 2017, 10, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Jeon, W.K.; Park, J.; Koo, B.K.; Suh, M.; Yang, S.; Kim, H.Y.; Lee, J.M.; Kim, K.J.; Choi, J.H.; Lim, H.S.; et al. Anatomical attributes of clinically relevant diagonal branches in patients with left anterior descending coronary artery bifurcation lesions. EuroIntervention 2020, 16, e715–e723. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Okura, H.; Okamura, A.; Iwai, S.; Keshi, A.; Kamon, D.; Isojima, T.; Ueda, T.; Soeda, T.; Saito, Y. Usefulness of longitudinal reconstructed optical coherence tomography images for predicting the need for the reverse wire technique during coronary bifurcation interventions. Catheter. Cardiovasc. Interv. 2019, 94, E54–E60. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A.; Angiolillo, D.J.; Tannenbaum, M.; Driesman, M.; Chu, A.; Patterson, J.; Kuehl, W.; Battaglia, J.; Dabbons, S.; Shamoon, F.; et al. Impact of stent deployment procedural factors on long-term effectiveness and safety of sirolimus-eluting stents (final results of the multicenter prospective STLLR trial). Am. J. Cardiol. 2008, 101, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Bavishi, C.; Sardar, P.; Chatterjee, S.; Khan, A.R.; Shah, A.; Ather, S.; Lemos, P.A.; Moreno, P.; Stone, G.W. Intravascular ultrasound-guided vs angiography-guided drug-eluting stent implantation in complex coronary lesions: Meta-analysis of randomized trials. Am. Heart J. 2017, 185, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Collet, C.; Sonck, J.; Leipsic, J.; Monizzi, G.; Buytaert, D.; Kitslaar, P.; Andreini, D.; De Bruyne, B. Implementing coronary computed tomography angiography in the catheterization laboratory. JACC Cardiovasc. Imaging 2021, 14, 1846–1855. [Google Scholar] [CrossRef] [PubMed]
- Maehara, A.; Matsumura, M.; Ali, Z.A.; Mintz, G.S.; Stone, G.W. IVUS-guided versus OCT-guided coronary stent implantation: A critical appraisal. J. Am. Coll. Cardiol. Imaging 2017, 10, 1487–1503. [Google Scholar] [CrossRef]
- Monizzi, G.; Sonck, J.; Nagumo, S.; Buytaert, D.; Van Hoe, L.; Grancini, L.; Bartorelli, A.L.; Vanhoenacker, P.; Simons, P.; Bladt, O.; et al. Quantification of calcium burden by coronary CT angiography compared to optical coherence tomography. Int. J. Cardiovasc. Imaging 2020, 36, 2393–2402. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.F.; Henry, T.D.; Mahmud, E.; Kirtane, A.J.; Brilakis, E.S.; Goyal, A.; Grines, C.L.; Lombardi, W.L.; Maran, A.; Rab, T.; et al. SCAI position statement on optimal percutaneous coronary interventional therapy for complex coronary artery disease. Catheter. Cardiovasc. Interv. 2020, 96, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Raff, G.L.; Abidov, A.; Achenbach, S.; Berman, D.S.; Boxt, L.M.; Budoff, M.J.; Cheng, V.; DeFrance, T.; Hellinger, J.C.; Karlsberg, R.P. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J. Cardiovasc. Comput. Tomogr. 2009, 3, 122–136. [Google Scholar] [CrossRef]
- Uetani, T.; Amano, T.; Kunimura, A.; Kumagai, S.; Ando, H.; Yokoi, K.; Yoshida, T.; Kato, B.; Kato, M.; Marui, N.; et al. The association between plaque characterization by CT angiography and post-procedural myocardial infarction in patients with elective stent implantation. JACC Cardiovasc. Imaging 2010, 3, 19–28. [Google Scholar] [CrossRef]
- Ali, Z.A.; Brinton, T.J.; Hill, J.M.; Maehara, A.; Matsumura, M.; Karimi Galougahi, K.; Illindala, U.; Götberg, M.; Whitbourn, R.; Van Mieghem, N.; et al. Optical coherence tomography characterization of coronary lithoplasty for treatment of calcified lesions: First description. J. Am. Coll. Cardiol. Imaging 2017, 10, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Ito, Y.; Yamawaki, M.; Araki, M.; Sakai, T.; Sakamoto, Y.; Mori, S.; Tsutsumi, M.; Nauchi, M.; Honda, Y.; et al. Optical frequency-domain imaging findings to predict good stent expansion after rotational atherectomy for severely calcified coronary lesions. Int. J. Cardiovasc. Imaging 2018, 34, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Chen, W.J.; Chen, Y.W.; Lai, C.H.; Su, C.S.; Chang, W.C.; Wang, C.Y.; Liang, K.W.; Liu, T.J.; Lee, W.L. Incidence and Mechanisms of Coronary Perforations during Rotational Atherectomy in Modern Practice. J. Interv. Cardiol. 2020, 2020, 1894389. [Google Scholar] [CrossRef]
- Bouisset, F.; Ohashi, H.; Andreini, D.; Collet, C. Role of coronary computed tomography angiography to optimise percutaneous coronary intervention outcomes. Heart 2023, 110, 1056–1062. [Google Scholar] [CrossRef]
- Schussler, J.M.; Dockery, W.D.; Johnson, K.B.; Rosenthal, R.L.; Stoler, R.C. Critical left main coronary artery stenosis diagnosed by computed tomographic coronary angiography. Bayl. Univ. Med. Cent. Proc. 2005, 18, 407. [Google Scholar] [CrossRef]
- Yeboah, J.; McClelland, R.L.; Polonsky, T.S.; Burke, G.L.; Sibley, C.T.; O’Leary, D.; Carr, J.J.; Goff, D.C.; Greenland, P.; Herrington, D.M. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediaterisk individuals. JAMA 2012, 308, 788–795. [Google Scholar] [CrossRef]
- Conte, E.; Mushtaq, S.; Pontone, G.; Li Piani, L.; Ravagnani, P.; Galli, S.; Collet, C.; Sonck, J.; Di Odoardo, L.; Guglielmo, M.; et al. Plaque quantification by coronary computed tomography angiography using intravascular ultrasound as a reference standard: A comparison between standard and last generation computed tomography scanners. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 191–201. [Google Scholar] [CrossRef]
- Watabe, H.; Sato, A.; Akiyama, D.; Kakefuda, Y.; Adachi, T.; Ojima, E.; Hoshi, T.; Murakoshi, N.; Ishizu, T.; Seo, Y.; et al. Impact of coronary plaque composition on cardiac troponin elevation after percutaneous coronary intervention in stable angina pectoris: A computed tomography analysis. J. Am. Coll. Cardiol. 2012, 59, 1881–1888. [Google Scholar] [CrossRef]
- Kashiwagi, M.; Tanaka, A.; Kitabata, H.; Tsujioka, H.; Kataiwa, H.; Komukai, K.; Tanimoto, T.; Takemoto, K.; Takarada, S.; Kubo, T.; et al. Feasibility of noninvasive assessment of thin-cap fibroatheroma by multidetector computed tomography. JACC Cardiovasc. Imaging 2009, 2, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Maurovich-Horvat, P.; Hoffmann, U.; Vorpahl, M.; Nakano, M.; Virmani, R.; Alkadhi, H. The napkin-ring sign: CT signature of high-risk coronary plaques? JACC Cardiovasc. Imaging 2010, 3, 440–444. [Google Scholar] [CrossRef]
- van Velzen, J.E.; de Graaf, F.R.; de Graaf, M.A.; Schuijf, J.D.; Kroft, L.J.; de Roos, A.; Reiber, J.H.; Bax, J.J.; Jukema, J.W.; Boersma, E.; et al. Comprehensive assessment of spotty calcifications on computed tomography angiography: Comparison to plaque characteristics on intravascular ultrasound with radiofrequency backscatter analysis. J. Nucl. Cardiol. 2011, 18, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.C.; Kwiecinski, J.; Doris, M.; McElhinney, P.; D’Souza, M.S.; Cadet, S.; Adamson, P.D.; Moss, A.J.; Alam, S.; Hunter, A.; et al. Low-Attenuation Noncalcified Plaque on Coronary Computed Tomography Angiography Predicts Myocardial Infarction: Results from the Multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART). Circulation 2020, 141, 1452–1462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gaur, S.; Øvrehus, K.A.; Dey, D.; Leipsic, J.; Bøtker, H.E.; Jensen, J.M.; Narula, J.; Ahmadi, A.; Achenbach, S.; Ko, B.S.; et al. Coronary plaque quantification and fractional flow reserve by coronary computed tomography angiography identify ischaemia-causing lesions. Eur. Heart J. 2016, 37, 1220–1227. [Google Scholar] [CrossRef]
- Nakazawa, G.; Tanabe, K.; Onuma, Y.; Yachi, S.; Aoki, J.; Yamamoto, H.; Higashikuni, Y.; Yagishita, A.; Nakajima, H.; Hara, K. Efficacy of culprit plaque assessment by 64-slice multidetector computed tomography to predict transient no-reflow phenomenon during percutaneous coronary intervention. Am. Heart J. 2008, 155, 1150–1157. [Google Scholar] [CrossRef]
- Budoff, M.J.; Muhlestein, J.B.; Bhatt, D.L.; Le Pa, V.T.; May, H.T.; Shaikh, K.; Shekar, C.; Kinninger, A.; Lakshmanan, S.; Roy, S.K.; et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: Final results of the EVAPORATE trial. Eur. Heart J. 2020, 41, 3925–3932. [Google Scholar] [CrossRef]
- Curzen, N.; Rana, O.; Nicholas, Z.; Golledge, P.; Zaman, A.; Oldroyd, K.; Hanratty, C.; Banning, A.; Wheatcroft, S.; Hobson, A.; et al. Does routine pressure wire assessment influence management strategy at coronary angiography for diagnosis of chest pain?: The RIPCORD study. Circ. Cardiovasc. Interv. 2014, 7, 248–255. [Google Scholar] [CrossRef]
- Tonino, P.A.; Fearon, W.F.; De Bruyne, B.; Oldroyd, K.G.; Leesar, M.A.; Ver Lee, P.N.; Maccarthy, P.A.; Van’t Veer, M.; Pijls, N.H. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography. J. Am. Coll. Cardiol. 2010, 55, 2816–2821. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Fonte, T.A.; Min, J.K. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: Scientific basis. J. Am. Coll. Cardiol. 2013, 61, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Khav, N.; Ihdayhid, A.R.; Ko, B. CT-derived fractional flow reserve (CT-FFR) in the evaluation of coronary artery disease. Heart Lung Circ. 2020, 29, 1621–1632. [Google Scholar] [CrossRef]
- Tzimas, G.; Gulsin, G.S.; Takagi, H.; Mileva, N.; Sonck, J.; Muller, O.; Leipsic, J.A.; Collet, C. Coronary CT Angiography to Guide Percutaneous Coronary Intervention. Radiol. Cardiothorac. Imaging 2022, 4, e210171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takagi, H.; Leipsic, J.A.; McNamara, N.; Martin, I.; Fairbairn, T.A.; Akasaka, T.; Nørgaard, B.L.; Berman, D.S.; Chinnaiyan, K.; Hurwitz-Koweek, L.M.; et al. Trans-lesional fractional flow reserve gradient as derived from coronary CT improves patient management: ADVANCE registry. J. Cardiovasc. Comput. Tomogr. 2022, 16, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Collet, C.; Sonck, J.; Vandeloo, B.; Mizukami, T.; Roosens, B.; Lochy, S.; Argacha, J.F.; Schoors, D.; Colaiori, I.; Di Gioia, G.; et al. Measurement of hyperemic pullback pressure gradients to characterize patterns of coronary atherosclerosis. J. Am. Coll. Cardiol. 2019, 74, 1772–1784. [Google Scholar] [CrossRef] [PubMed]
- Fournier, S.; Ciccarelli, G.; Toth, G.G.; Milkas, A.; Xaplanteris, P.; Tonino, P.A.L.; Fearon, W.F.; Pijls, N.H.J.; Barbato, E.; De Bruyne, B. Association of Improvement in Fractional Flow Reserve with Outcomes, Including Symptomatic Relief, After Percutaneous Coronary Intervention. JAMA Cardiol. 2019, 4, 370–374. [Google Scholar] [CrossRef]
- Lee, J.M.; Hwang, D.; Choi, K.H.; Rhee, T.M.; Park, J.; Kim, H.Y.; Jung, H.W.; Hwang, J.W.; Lee, H.J.; Jang, H.J.; et al. Prognostic Implications of Relative Increase and Final Fractional Flow Reserve in Patients with Stent Implantation. JACC Cardiovasc. Interv. 2018, 11, 2099–2109. [Google Scholar] [CrossRef]
- Al-Lamee, R.; Howard, J.P.; Shun-Shin, M.J.; Thompson, D.; Dehbi, H.M.; Sen, S.; Nijjer, S.; Petraco, R.; Davies, J.; Keeble, T.; et al. Fractional Flow Reserve and Instantaneous Wave-Free Ratio as Predictors of the Placebo-Controlled Response to Percutaneous Coronary Intervention in Stable Single-Vessel Coronary Artery Disease. Circulation 2018, 138, 1780–1792. [Google Scholar] [CrossRef] [PubMed]
- Dourado, L.O.C.; Bittencourt, M.S.; Pereira, A.C.; Poppi, N.T.; Dallan, L.A.O.; Krieger, J.E.; Cesar, L.A.M.; Gowdak, L.H.W. Coronary Artery Bypass Surgery in Diffuse Advanced Coronary Artery Disease: 1-Year Clinical and Angiographic Results. Thorac. Cardiovasc. Surg. 2018, 66, 477–482. [Google Scholar]
- Sonck, J.; Nagumo, S.; Norgaard, B.L.; Otake, H.; Ko, B.; Zhang, J.; Mizukami, T.; Maeng, M.; Andreini, D.; Takahashi, Y.; et al. (Eds.) Clinical Validation of a virtual planner of coronary interventions based on coronary CT angiography and blood flow simulations. In Proceedings of the EuroPCR, Virtual, 18–20 May 2021. [Google Scholar]
- Hokimoto, S.; Kaikita, K.; Yasuda, S.; Tsujita, K.; Ishihara, M.; Matoba, T.; Matsuzawa, Y.; Mitsutake, Y.; Mitani, Y.; Murohara, T.; et al. JCS/CVIT/JCC 2023 guideline focused update on diagnosis and treatment of vasospastic angina (coronary spastic angina) and coronary microvascular dysfunction. Circ. J. 2023, 87, 879–936. [Google Scholar] [CrossRef]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.H.E.M.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European society of cardiology working group on coronary pathophysiology & microcirculation endorsed by coronary vasomotor disorders international study group. Eur. Heart J. 2020, 41, 3504–3520. [Google Scholar]
- Toth, G.; Hamilos, M.; Pyxaras, S.; Mangiacapra, F.; Nelis, O.; De Vroey, F.; Di Serafino, L.; Muller, O.; Van Mieghem, C.; Wyffels, E.; et al. Evolving concepts of angiogram: Fractional flow reserve discordances in 4000 coronary stenoses. Eur. Heart J. 2014, 35, 2831–2838. [Google Scholar] [CrossRef]
- Serruys, P.W.; Kageyama, S.; Garg, S.; Onuma, Y. In the beginning there was angina pectoris, at the end there was still angina pectoris. JACC Cardiovasc. Interv. 2022, 15, 2519–2522. [Google Scholar] [CrossRef]
- Taylor, C.A.; Gaur, S.; Leipsic, J.; Achenbach, S.; Berman, D.S.; Jensen, J.M.; Dey, D.; Bøtker, H.E.; Kim, H.J.; Khem, S.; et al. Effect of the ratio of coronary arterial lumen volume to left ventricle myocardial mass derived from coronary CT angiography on fractional flow reserve. J. Cardiovasc. Comput. Tomogr. 2017, 11, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, S.K.; Kühl, J.T.; Fuchs, A.; Norsk, J.B.; Køber, L.V.; Nordestgaard, B.G.; Kofoed, K.F. Volume and dimensions of angiographically normal coronary arteries assessed by multidetector computed tomography. J. Cardiovasc. Comput. Tomogr. 2017, 11, 295–301. [Google Scholar] [CrossRef]
- Mahnken, A.H.; Mühlenbruch, G.; Günther, R.W.; Wildberger, J.E. Cardiac CT: Coronary arteries and beyond. Eur. Radiol. 2007, 17, 994–1008. [Google Scholar] [CrossRef]
- Krombach, G.A.; Niendorf, T.; Günther, R.W.; Mahnken, A.H. Characterization of myocardial viability using MR and CT imaging. Eur. Radiol. 2007, 17, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Klocke, F.J.; Baird, M.G.; Lorell, B.H.; Bateman, T.M.; Messer, J.V.; Berman, D.S.; O’Gara, P.T.; Carabello, B.A.; Russell, R.O., Jr.; Cerqueira, M.D.; et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging—Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). J. Am. Coll. Cardiol. 2003, 42, 1318–1333. [Google Scholar] [CrossRef] [PubMed]
- Safley, D.M.; Koshy, S.; Grantham, J.A.; Bybee, K.A.; House, J.A.; Kennedy, K.F.; Rutherford, B.D. Changes in myocardial ischemic burden following percutaneous coronary intervention of chronic total occlusions. Catheter. Cardiovasc. Interv. 2011, 78, 337–343. [Google Scholar] [CrossRef]
- Patel, A.R.; Bamberg, F.; Branch, K.; Carrascosa, P.; Chen, M.; Cury, R.C.; Ghoshhajra, B.; Ko, B.; Nieman, K.; Pugliese, F.; et al. Society of cardiovascular computed tomography expert consensus document on myocardial computed tomography perfusion imaging. J. Cardiovasc. Comput. Tomogr. 2020, 14, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Coenen, A.; Rossi, A.; Lubbers, M.M.; Kurata, A.; Kono, A.K.; Chelu, R.G.; Segreto, S.; Dijkshoorn, M.L.; Wragg, A.; van Geuns, R.M.; et al. Integrating CT myocardial perfusion and CTFFR in the work-up of coronary artery disease. JACC Cardiovasc. Imaging 2017, 10, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Sadamatsu, K.; Nagaoka, K.; Koga, Y.; Kagiyama, K.; Muramatsu, K.; Hironaga, K.; Tashiro, H.; Ueno, T.; Fukumoto, Y. The functional severity assessment of coronary stenosis using coronary computed tomography angiography-based myocardial mass at risk and minimal lumen diameter. Cardiovasc. Ther. 2020, 2020, 6716130. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Lu, Z.; Shen, C.; Yan, J.; Wang, Y.; Lu, B.; Zhang, J. The best predictor of ischemic coronary stenosis: Subtended myocardial volume, machine learning–based FFRCT, or high-risk plaque features? Eur. Radiol. 2019, 29, 3647–3657. [Google Scholar] [CrossRef]
- Mushtaq, S.; Conte, E.; Pontone, G. State-of-the-art-myocardial perfusion stress testing: Static CT perfusion. J. Cardiovasc. Comput. Tomogr. 2020, 14, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Berbarie, R.F.; Dockery, W.D.; Johnson, K.B.; Rosenthal, R.L.; Stoler, R.C.; Schussler, J.M. Use of multislice computed tomographic coronary angiography for the diagnosis of anomalous coronary arteries. Am. J. Cardiol. 2006, 98, 402–406. [Google Scholar] [CrossRef]
- Yamanaka, O.; Hobbs, R.E. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc. Diagn. 1990, 21, 28–40. [Google Scholar] [CrossRef]
- Li, N.; Zhao, P.; Wu, D.; Liang, C. Coronary artery fistulas detected with coronary CT angiography: A pictorial review of 73 cases. Br. J. Radiol. 2020, 93, 20190523. [Google Scholar] [CrossRef] [PubMed]
- Schussler, J.M. Cardiovascular CT: Interventional cardiology applications. In Cardiac CT Imaging; Budoff, M.J., Shinbane, J.S., Eds.; Springer: Cham, Switzerland, 2016; pp. 487–505. [Google Scholar]
- Jones, D.A.; Beirne, A.M.; Kelham, M.; Rathod, K.S.; Andiapen, M.; Wynne, L.; Godec, T.; Forooghi, N.; Ramaseshan, R.; Moon, J.C.; et al. Computed tomography cardiac angiography before invasive coronary angiography in patients with previous bypass surgery: The BYPASS-CTCA trial. Circulation 2023, 148, 1371–1380. [Google Scholar] [CrossRef]
- Machado, M.F.; Felix, N.; Melo, P.H.C.; Gauza, M.M.; Calomeni, P.; Generoso, G.; Khatri, S.; Altmayer, S.; Blankstein, R.; Bittencourt, M.S.; et al. Coronary CT angiography versus invasive coronary angiography in patients with coronary artery disease: A randomized controlled trial (CORE320 study). Eur. Heart J. 2015, 36, 1464–1472. [Google Scholar] [CrossRef]
- Galassi, A.R.; Tomasello, S.D.; Reifart, N.; Werner, G.S.; Sianos, G.; Bonnier, H.; Sievert, H.; Ehladad, S.; Bufe, A.; Shofer, J.; et al. In-hospital outcomes of percutaneous coronary intervention in patients with chronic total occlusion: Insights from the ERCTO (European Registry of Chronic Total Occlusion) registry. EuroIntervention 2011, 7, 472–479. [Google Scholar] [CrossRef]
- Morino, Y.; Abe, M.; Morimoto, T.; Kimura, T.; Hayashi, Y.; Muramatsu, T.; Ochiai, M.; Noguchi, Y.; Kato, K.; Shibata, Y.; et al. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes. JACC Cardiovasc. Interv. 2011, 4, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Karmpaliotis, D.; Michael, T.T.; Brilakis, E.S.; Papayannis, A.C.; Tran, D.L.; Kirkland, B.L.; Lembo, N.; Kalynych, A.; Carlson, H.; Banerjee, S.; et al. Retrograde coronary chronic total occlusion revascularization: Procedural and in-hospital outcomes from a multicenter registry in the United States. JACC Cardiovasc. Interv. 2012, 5, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Tsuchikane, E.; Katoh, O.; Muramatsu, T.; Muto, M.; Kishi, K.; Hamazaki, Y.; Oikawa, Y.; Kawasaki, T.; Okamura, A. Outcomes of percutaneous coronary interventions for chronic total occlusion performed by highly experienced Japanese specialists: The first report from the Japanese CTO-PCI Expert Registry. JACC Cardiovasc. Interv. 2017, 10, 2144–2154. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, N.V.; Werner, G.S.; Deftereos, S.; Di Mario, C.; Galassi, A.R.; Buettner, J.H.; Avran, A.; Reifart, N.; Goktekin, O.; Garbo, R.; et al. Temporal trends in chronic total occlusion interventions in Europe: 17626 procedures from the European Registry of Chronic total occlusion. Circ. Cardiovasc. Interv. 2018, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Simsek, B.; Jaffer, F.A.; Kostantinis, S.; Karacsonyi, J.; Koike, H.; Doshi, D.; Alaswad, K.; Gorgulu, S.; Goktekin, O.; Khatri, J.; et al. Preprocedural coronary computed tomography angiography in chronic total occlusion percutaneous coronary intervention: Insights from the PROGRESS-CTO registry. Int. J. Cardiol. 2022, 367, 20–25. [Google Scholar] [CrossRef]
- Hong, S.J.; Kim, B.K.; Cho, I.; Kim, H.Y.; Rha, S.W.; Lee, S.H.; Park, S.M.; Kim, Y.H.; Chang, H.J.; Ahn, C.M.; et al. CT-CTO Investigators. Effect of Coronary CTA on Chronic Total Occlusion Percutaneous Coronary Intervention: A Randomized Trial. JACC Cardiovasc. Imaging 2021, 10, 1993–2004. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, K.; Pyrpyris, N.; Theofilis, P.; Mantzouranis, E.; Beneki, E.; Kostakis, P.; Koutsopoulos, G.; Aznaouridis, K.; Aggeli, K.; Tsioufis, K. Computed Tomography Angiography Identified High-Risk Coronary Plaques: From Diagnosis to Prognosis and Future Management. Diagnostics 2024, 14, 1671. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zyśk, A.; Wolny, R.; Kruk, M.; Kwieciński, J.; Dębski, A.; Barbero, U.; Kępka, C.; Demkow, M.; Witkowski, A.; Opolski, M.P. Computed Tomography Angiography-Derived Scores for Prediction of Chronic Total Occlusion Percutaneous Coronary Intervention Using the Hybrid Algorithm. J. Cardiovasc. Dev. Dis. 2023, 11, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schlattmann, P.; Wieske, V.; Bressem, K.K.; Götz, T.; Schuetz, G.M.; Andreini, D.; Pontone, G.; Alkadhi, H.; Hausleiter, J.; Zimmermann, E.; et al. The effectiveness of coronary computed tomography angiography and functional testing for the diagnosis of obstructive coronary artery disease: Results from the individual patient data Collaborative Meta-Analysis of Cardiac CT (COME-CCT). Insights Imaging 2024, 15, 208. [Google Scholar] [CrossRef] [PubMed]
- Haase, R.; Schlattmann, P.; Gueret, P.; Andreini, D.; Pontone, G.; Alkadhi, H.; Hausleiter, J.; Garcia, M.J.; Leschka, S.; Meijboom, W.B.; et al. COME-CCT Consortium. Diagnosis of obstructive coronary artery disease using computed tomography angiography in patients with stable chest pain depending on clinical probability and in clinically important subgroups: Meta-analysis of individual patient data. BMJ 2019, 365, l1945. [Google Scholar] [CrossRef]
- Griffin, W.F.; Choi, A.D.; Riess, J.S.; Marques, H.; Chang, H.J.; Choi, J.H.; Doh, J.H.; Her, A.Y.; Koo, B.K.; Nam, C.W.; et al. AI Evaluation of Stenosis on Coronary CTA, Comparison with Quantitative Coronary Angiography and Fractional Flow Reserve: A CREDENCE Trial Substudy. JACC Cardiovasc. Imaging 2023, 16, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.D.; Marques, H.; Kumar, V.; Griffin, W.F.; Rahban, H.; Karlsberg, R.P.; Zeman, R.K.; Katz, R.J.; Earls, J.P. CT Evaluation by Artificial Intelligence for Atherosclerosis, Stenosis and Vascular Morphology (CLARIFY): A Multi-center, international study. J. Cardiovasc. Comput. Tomogr. 2021, 15, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Nurmohamed, N.S.; Min, J.K.; Anthopolos, R.; Reynolds, H.R.; Earls, J.P.; Crabtree, T.; Mancini, G.B.J.; Leipsic, J.; Budoff, M.J.; Hague, C.J.; et al. Atherosclerosis quantification and cardiovascular risk: The ISCHEMIA trial. Eur. Heart J. 2024, 45, 3735–3747. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ihdayhid, A.R.; Tzimas, G.; Peterson, K.; Ng, N.; Mirza, S.; Maehara, A.; Safian, R.D. Diagnostic Performance of AI-enabled Plaque Quantification from Coronary CT Angiography Compared with Intravascular Ultrasound. Radiol. Cardiothorac. Imaging 2024, 6, e230312. [Google Scholar] [CrossRef] [PubMed]
- Dundas, J.; Leipsic, J.A.; Sellers, S.; Blanke, P.; Miranda, P.; Ng, N.; Mullen, S.; Meier, D.; Akodad, M.; Sathananthan, J.; et al. Artificial Intelligence-based Coronary Stenosis Quantification at Coronary CT Angiography versus Quantitative Coronary Angiography. Radiol. Cardiothorac. Imaging 2023, 5, e230124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dundas, J.; Leipsic, J.; Fairbairn, T.; Ng, N.; Sussman, V.; Guez, I.; Rosenblatt, R.; Hurwitz Koweek, L.M.; Douglas, P.S.; Rabbat, M.; et al. Interaction of AI-Enabled Quantitative Coronary Plaque Volumes on Coronary CT Angiography, FFRCT, and Clinical Outcomes: A Retrospective Analysis of the ADVANCE Registry. Circ. Cardiovasc. Imaging 2024, 17, e016143. [Google Scholar] [CrossRef] [PubMed]
- Tzimas, G.; Gulsin, G.S.; Everett, R.J.; Akodad, M.; Meier, D.; Sewnarain, K.; Ally, Z.; Alnamasy, R.; Ng, N.; Mullen, S.; et al. Age- and Sex-Specific Nomographic CT Quantitative Plaque Data From a Large International Cohort. JACC Cardiovasc. Imaging 2024, 17, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Tortora, M.; Gemini, L.; D’Iglio, I.; Ugga, L.; Spadarella, G.; Cuocolo, R. Spectral Photon-Counting Computed Tomography: A Review on Technical Principles and Clinical Applications. J. Imaging 2022, 8, 112. [Google Scholar] [CrossRef]
- Cademartiri, F.; Meloni, A.; Pistoia, L.; Degiorgi, G.; Clemente, A.; Gori, C.D.; Positano, V.; Celi, S.; Berti, S.; Emdin, M.; et al. Dual-Source Photon-Counting Computed Tomography—Part I: Clinical Overview of Cardiac CT and Coronary CT Angiography Applications. J. Clin. Med. 2023, 12, 3627. [Google Scholar] [CrossRef]
- Van der Bie, J.; van Straten, M.; Booij, R.; Bos, D.; Dijkshoorn, M.L.; Hirsch, A.; Sharma, S.P.; Oei, E.H.G.; Budde, R.P.J. Photon-counting CT: Review of initial clinical results. Eur. J. Radiol. 2023, 163, 110829. [Google Scholar] [CrossRef]
- Rajagopal, J.R.; Farhadi, F.; Richards, T.; Nikpanah, M.; Sahbaee, P.; Shanbhag, S.M.; Bandettini, W.P.; Saboury, B.; Malayeri, A.A.; Pritchard, W.F.; et al. Evaluation of Coronary Plaques and Stents with Conventional and Photon-counting CT: Benefits of High-Resolution Photon-counting CT. Radiol. Cardiothorac. Imaging 2021, 3, e210102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oncel, D.; Oncel, G.; Tastan, A.; Tamci, B. Evaluation of coronary stent patency and in-stent restenosis with dual-source CT coronary angiography without heart rate control. AJR Am. J. Roentgenol. 2008, 191, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Mergen, V.; Eberhard, M.; Manka, R.; Euler, A.; Alkadhi, H. First in-human quantitative plaque characterization with ultra-high resolution coronary photon-counting CT angiography. Front. Cardiovasc. Med. 2022, 9, 981012. [Google Scholar] [CrossRef] [PubMed]
- Baturin, P.; Alivov, Y.; Molloi, S. Spectral CT imaging of vulnerable plaque with two independent biomarkers. Phys. Med. Biol. 2012, 57, 4117–4138. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beneki, E.; Dimitriadis, K.; Pyrpyris, N.; Antonopoulos, A.; Aznaouridis, K.; Antiochos, P.; Fragoulis, C.; Lu, H.; Meier, D.; Tsioufis, K.; et al. Computed Tomography Angiography in the Catheterization Laboratory: A Guide Towards Optimizing Coronary Interventions. J. Cardiovasc. Dev. Dis. 2025, 12, 28. https://doi.org/10.3390/jcdd12010028
Beneki E, Dimitriadis K, Pyrpyris N, Antonopoulos A, Aznaouridis K, Antiochos P, Fragoulis C, Lu H, Meier D, Tsioufis K, et al. Computed Tomography Angiography in the Catheterization Laboratory: A Guide Towards Optimizing Coronary Interventions. Journal of Cardiovascular Development and Disease. 2025; 12(1):28. https://doi.org/10.3390/jcdd12010028
Chicago/Turabian StyleBeneki, Eirini, Kyriakos Dimitriadis, Nikolaos Pyrpyris, Alexios Antonopoulos, Konstantinos Aznaouridis, Panagiotis Antiochos, Christos Fragoulis, Henri Lu, David Meier, Konstantinos Tsioufis, and et al. 2025. "Computed Tomography Angiography in the Catheterization Laboratory: A Guide Towards Optimizing Coronary Interventions" Journal of Cardiovascular Development and Disease 12, no. 1: 28. https://doi.org/10.3390/jcdd12010028
APA StyleBeneki, E., Dimitriadis, K., Pyrpyris, N., Antonopoulos, A., Aznaouridis, K., Antiochos, P., Fragoulis, C., Lu, H., Meier, D., Tsioufis, K., Fournier, S., Aggeli, C., & Tzimas, G. (2025). Computed Tomography Angiography in the Catheterization Laboratory: A Guide Towards Optimizing Coronary Interventions. Journal of Cardiovascular Development and Disease, 12(1), 28. https://doi.org/10.3390/jcdd12010028









