A Fluoroscopy-Free Ablation Workflow for Persistent Atrial Fibrillation Using a Pentaspline Pulse Field Ablation Catheter Guided by Left-Sided Intracardiac Echo Imaging and Electroanatomic Mapping: A Case Series
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Procedure Description and Workflow
- -
- Via the access in left femoral vein, the ICE catheter was advanced to the RA and the decapolar catheter was advanced to the coronary sinus. Baseline ICE images were obtained from the RA and then RV to assess the anatomy of the interatrial septum, LV function, and the presence of preprocedural pericardial effusion. Then, the ICE probe was advanced to the SVC to visualize the transseptal wire and sheath.
- -
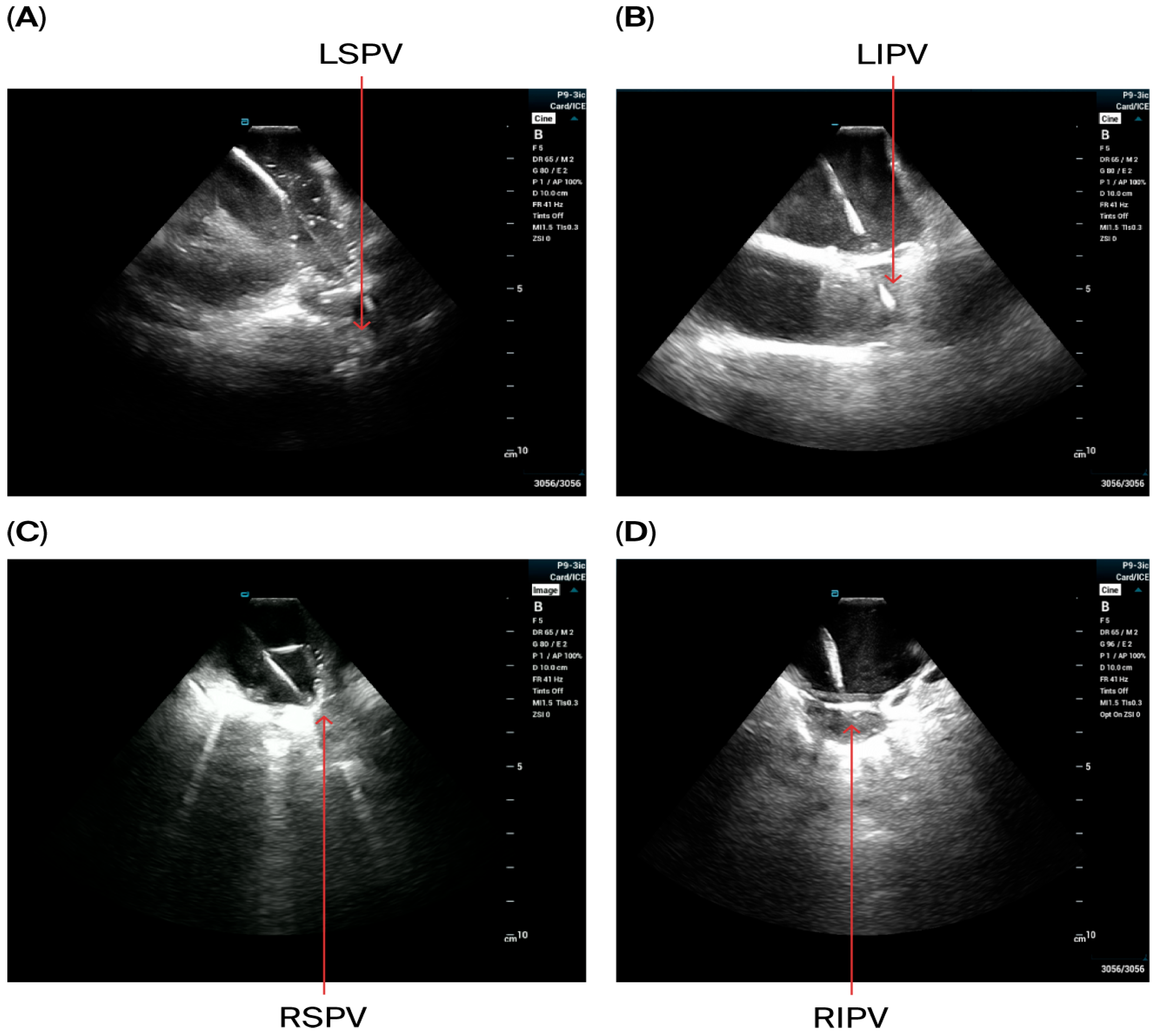
- Via the access in the right femoral vein, the VersaCross Access Solution wire (Boston Scientific, Marlborough, MA, USA) and sheath were advanced to the SVC under ICE monitoring. A single transseptal approach was performed and after crossing the septum VersaCross wire was placed in the left superior pulmonary vein, which was confirmed in ICE views from RA and from RV if needed.
- -
- The VersaCross sheath was exchanged for the Faradrive sheath and dilatator. After predilatation of transseptal puncture, the Faradrive sheath was retracted, and the ICE catheter was advanced along the VersaCross wire to the left atrium (Figure 1A–D). Then, the Faradrive sheath was moved back to the LA, and the dilator and Versacross wire were removed, with the ICE catheter and PFA sheath placed in the LA via a single transseptal puncture.
- -
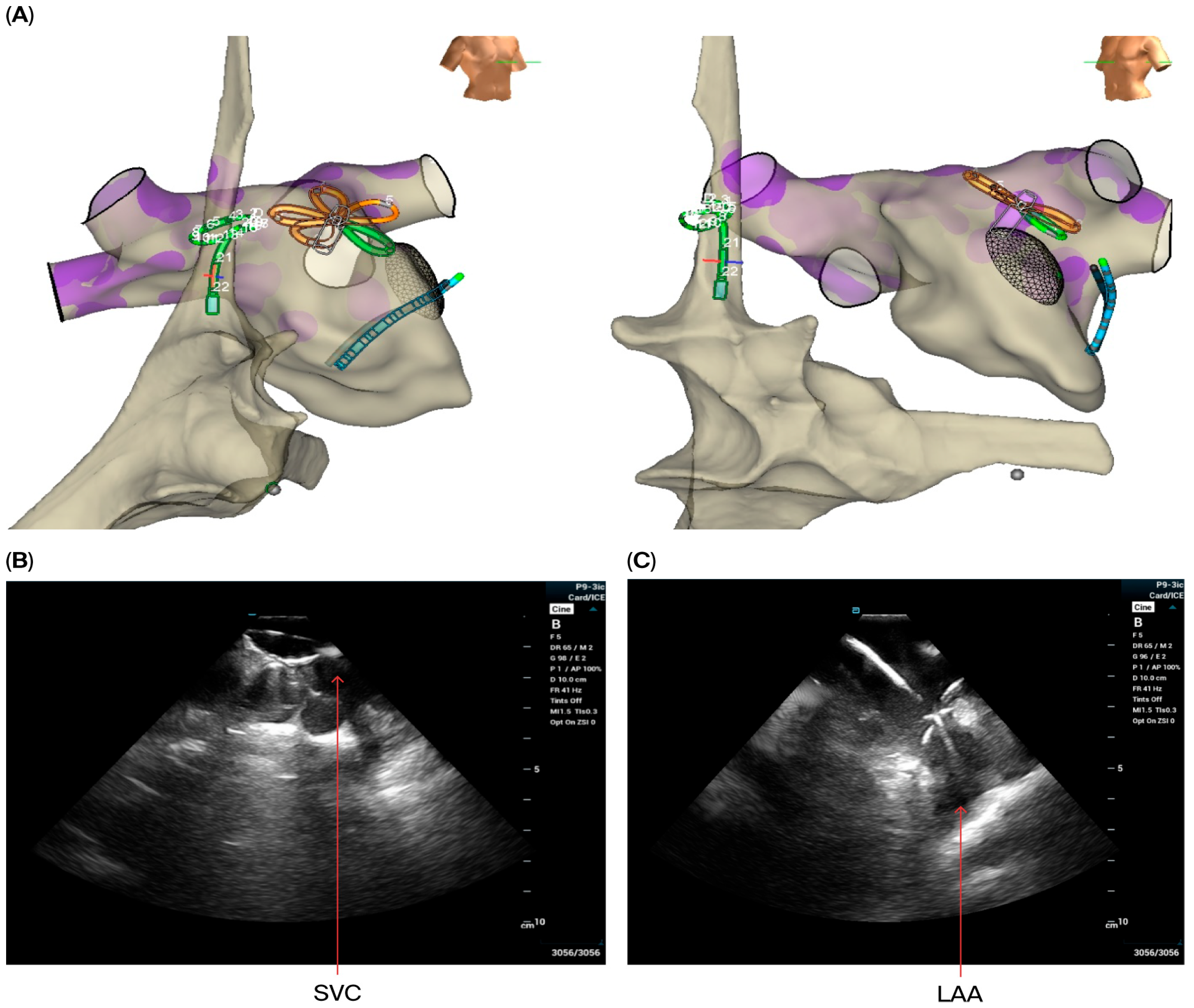
- ICE images of the LA, including the LAA, were obtained from all patients to exclude LAA thrombus (Figure 1E).

- -
- A circular mapping catheter (CMC) or high-density catheter (with repeated ablation) was advanced to the LA via the Faradrive sheath. Electroanatomic mapping of the LA, including detailed voltage assessment was performed. Then, over the J-tip wire, the Farawave pentaspline catheter was advanced to the LA via the Faradrive sheath. The 0.035-inch J-tip guidewire was attached to the EnSite mapping system’s pin box via a DuoMode extension cable (Boston Scientific, Marlborough, MA, USA) for additional visualization in LA and pulmonary veins. ICE catheter imaging was used to ensure appropriate catheter positioning and catheter–tissue contact with each PV and LAPW PFA lesion delivered. Prior to ablation, 0.2 mg of intravenous glycopyrrolate was administered to all patients to avoid vagal reactions during PVI.
- -
- PV isolation was performed with the PFA catheter placed at the PV ostium, with 4 applications in the basket configuration and 4 in the flower configuration for a total of 8 applications per PV. Appropriate rotation of the catheter following 2 applications in each position was assured with LA ICE and EAM visualization. Two additional applications in the flower configuration were delivered at the operator’s discretion in the left and right carina, towards the LA ridge and septal aspect of the right pulmonary veins based on the anatomy and presence of atrial potentials.
- -
- LAPW isolation was achieved with overlapping lesions placed in the flower configuration, with 2 application per site. With the J-tip wire in the left PV serving as a ‘hook’, rotating the catheter clockwise in the flower configuration allowed ablation of the posterior wall near the left veins; similarly, with the J-tip wire in the right pulmonary veins, rotating the catheter counterclockwise allowed appropriate contact for the posterior wall isolation near right veins. Remaining mid-posterior wall was ablated with overlying lesions in the flower configuration. ICE was used with each ablation application to ensure optimal contact.
- -
- Cardioversion was performed if the patient remained in atrial fibrillation post-ablation. PV isolation was confirmed by the presence of entrance and exit blocks in all PVs, while posterior wall isolation was determined by the complete abolishment of posterior wall potentials from the LA roof to just below the lower regions of the inferior PVs.
- -
- A high-dose isoproterenol challenge at a rate of 20 mg/min was performed in all patients unless the dose was not tolerated. If the presence of spontaneous or induced non-PV triggers or atrial arrhythmias was detected, further PFA was performed.
- -
- For superior vena cava (SVC) isolation, the ICE catheter was retracted to the top right of the atrium to guide the ablation procedure. Prior to and after ablating the SVC, the CMC was placed at the junction of the SVC and right atrium to map the phrenic nerve and to detect potential phrenic nerve injury. Then, the Pentaspline catheter was placed at the junction of right atrium and SVC (the lower edge of the right pulmonary artery level in ICE imaging). A total of 4 applications in the basket configuration were delivered (2 followed by 2 other applications after rotating the catheter 30 degree), and heart rate was monitored during the procedure.
- -
- To ablate induced perimitral flutter, an anterior mitral line was created using PFA lesions delivered in the flower configuration, with 3 applications per site from the mitral annulus to the right superior pulmonary veins. Intravenous nitroglycerin was given prior to PFA, with dosing based on a previously published protocol [14]. The ST segment was monitored during and post-ablation.
- -
- For ablation of induced focal atrial tachycardias, high-density catheter was used for activation mapping, and PFA was delivered in 3 applications in the flower configuration in each targeted site.
2.3. Follow-Up
3. Results
4. Discussion
Study Limitations
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PFA | pulse field ablation |
| AF | atrial fibrillation |
| ICE | intracardiac echo |
| EAM | electroanatomic mapping |
| PVI | pulmonary vein isolation |
| LAPW | left atrial posterior wall ablation |
| RA | right atrium |
| LA | left atrium |
References
- Zheng, N.N.; Fu, Y.B.; Xue, F.; Xu, M.Z.; Ling, L.; Jiang, T.B. Which ablation strategy is the most effective for treating persistent atrial fibrillation? A systematic review and bayesian network meta-analysis of randomized controlled trials. Heart Rhythm 2025, 22, e60–e73. [Google Scholar] [CrossRef] [PubMed]
- Ekanem, E.; Reddy, V.Y.; Schmidt, B.; Reichlin, T.; Neven, K.; Metzner, A.; Hansen, J.; Blaauw, Y.; Maury, P.; Arentz, T.; et al. Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF). Europace 2022, 24, 1256–1266. [Google Scholar] [CrossRef] [PubMed]
- Turagam, M.K.; Neuzil, P.; Schmidt, B.; Reichlin, T.; Neven, K.; Metzner, A.; Hansen, J.; Blaauw, Y.; Maury, P.; Arentz, T.; et al. Safety and effectiveness of pulsed field ablation to treat atrial fibrillation: One-year outcomes from the MANIFEST-PF registry. Circulation 2023, 148, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Duytschaever, M.; De Potter, T.; Grimaldi, M.; Anic, A.; Vijgen, J.; Neuzil, P.; Van Herendael, H.; Verma, A.; Skanes, A.; Scherr, D.; et al. Paroxysmal atrial fibrillation ablation using a novel variable loop biphasic pulsed field ablation catheter integrated with a 3-dimensional mapping system: 1-year outcomes of the multicenter inspIRE study. Circ. Arrhythmia Electrophysiol. 2023, 16, e011780. [Google Scholar] [CrossRef] [PubMed]
- Turagam, M.; Neuzil, P.; Petru, J.; Funasako, M.; Koruth, J.; Skoda, J.; Kralovec, S.; Reddy, V.Y. AF ablation using a novel “single-shot” map-and-ablate spherical array pulsed field ablation catheter: 1-Year outcomes of the first-in-human PULSE-EU trial. Heart Rhythm 2024, 21, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Bordignon, S.; Neven, K.; Reichlin, T.; Blaauw, Y.; Hansen, J.; Adelino, R.; Ouss, A.; Füting, A.; Roten, L.; et al. EUropean real-world outcomes with Pulsed field ablation in patients with symptomatic atrial fibrillation: Lessons from the multi-centre EU-PORIA registry. Europace 2023, 25, euad185. [Google Scholar] [CrossRef]
- Ahn, J.; Shin, D.G.; Han, S.J.; Lim, H.E. Safety and efficacy of intracardiac echocardiography-guided zero-fluoroscopic cryoballoon ablation for atrial fibrillation: A prospective randomized controlled trial. Europace 2023, 25, euad086. [Google Scholar] [CrossRef]
- Debreceni, D.; Janosi, K.; Bocz, B.; Turcsan, M.; Lukacs, R.; Simor, T.; Antolic, B.; Vamos, M.; Komocsi, A.; Kupo, P. Zero fluoroscopy catheter ablation for atrial fibrillation: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2023, 10, 1178783. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.R.; Holmqvist, F.; Parish, A.; Green, C.L.; Piccini, J.P.; Bahnson, T.D. Safety of continuous left atrial phased-array intracardiac echocardiography during left atrial ablation for atrial fibrillation. Heart Rhythm O2 2022, 3, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Mattison, L.; Verma, A.; Tarakji, K.G.; Reichlin, T.; Hindricks, G.; Sack, K.L.; Önal, B.; Schmidt, M.M.; Miklavčič, D.; Sigg, D.C. Effect of contact force on pulsed field ablation lesions in porcine cardiac tissue. J. Cardiovasc. Electrophysiol. 2023, 34, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Rauber, M.; Manninger, M.; Eberl, A.S.; Scherr, D. Zero-fluoroscopy ablation with multielectrode pulse field ablation system: Case series. Pacing Clin. Electrophysiol. 2024, 47, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Hirata, S.; Nagashima, K.; Watanabe, R.; Wakamatsu, Y.; Hirata, M.; Kurokawa, S.; Otsuka, N.; Sawada, M.; Okumura, Y. Workflow of the zero-fluoro pulsed field ablation. J. Arrhythmia 2024, 40, 1529–1532. [Google Scholar] [CrossRef]
- Mohanty, S.; Casella, M.; Doty, B.; Schiavone, M.; Gabrah, K.; Valeri, Y.; Torlapati, P.G.; Fazia, V.M.L.; Gianni, C.; Al-Ahmad, A.; et al. Ensuring catheter-tissue contact with intracardiac echocardiography during pulsed-field ablation improves procedure outcome in patients with atrial fibrillation. Heart Rhythm 2025, 22, E875–E881. [Google Scholar] [CrossRef]
- Malyshev, Y.; Neuzil, P.; Petru, J.; Funasako, M.; Hala, P.; Kopriva, K.; Schneider, C.; Achyutha, A.; Vanderper, A.; Musikantow, D.; et al. Nitroglycerin to Ameliorate Coronary Artery Spasm During Focal Pulsed-Field Ablation for Atrial Fibrillation. JACC Clin. Electrophysiol. 2024, 10, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Lakkireddy, D.; Katapadi, A.; Garg, J.; Herink, E.; Klotz, M.; Ganta, J.; Kabra, A.; Kabra, R.; Pothineni, N.V.; Darden, D.; et al. NEMESIS-PFA Investigating Collateral Tissue Injury Associated with Pulsed Field Ablation. JACC Clin. Electrophysiol. 2025, 11, 1746–1756. [Google Scholar]
- Reddy, V.Y.; Gerstenfeld, E.P.; Schmidt, B.; Nair, D.; Natale, A.; Saliba, W.; Verma, A.; Sommer, P.; Metzner, A.; Turagam, M.; et al. Pulsed Field Ablation for Persistent Atrial Fibrillation: 1-Year Results of ADVANTAGE AF. JACC 2025, 85, 1664–1678. [Google Scholar]
- Russo, A.D.; Tondo, C.; Schillaci, V.; Casella, M.; Iacopino, S.; Bianchi, S.; Fassini, G.; Rossillo, A.; Compagnucci, P.; Schiavone, M. Intracardiac echocardiography-guided pulsed-field ablation for successful ablation of atrial fibrillation: A propensity-matched analysis from a large nationwide multicenter experience. J. Interv. Card. Electrophysiol. 2024, 67, 1257–1266. [Google Scholar] [CrossRef]
- Pierucci, N.; Fazia, V.M.L.; Mohanty, S.; Schiavone, M.; Doty, B.; Gabrah, K.; Rocca, D.G.D.; Burkhardt, D.; Al-Ahmad, A.; Di Biase, L.; et al. Results of ICE-Guided Isolation of the Superior Vena Cava with Pulsed Field Ablation. JACC Clin. Electrophysiol. 2025, 11, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Harmouch, W.; Cuellar, S.; Narayanan, A.; Taii, H.A.; Sabayon, M.D. An Antiquated Concept in the Novel Era of Ablation: Zero-fluoroscopy Pulsed Field Ablation for Treatment of Atrial Fibrillation. J. Innov. Card. Rhythm. Manage. 2025, 16, 6268–6270. [Google Scholar] [CrossRef] [PubMed]

| Group size | 30 patients |
| Sex distribution | 12 females 18 males |
| Mean age (range) | 70.3 (41–81 years old) |
| Average time since diagnosis of atrial fibrillation (range) | 3.5 years (3 months–12 years) |
| Prior use of antiarrhythmics (%) | 23 patients (77%) |
| Patients who had prior AF ablation (%) | 8 patients (26%) |
| Number of procedures completed (%) | 30 (100%) |
| Successful isolation of the PVI + posterior wall (%) | 30 (100%) |
| Other ablation sites (%) | 4 patients (13%)
|
| Average procedural time (skin-to-skin) | 91 min (57–147 min) |
Average number of PFA applications
| 54 applications 58 applications 42 applications |
| Major complications | None |
| 30-day readmissions (%) | 1 patient (3.3%) |
| Early AF recurrences within 30 days | 5 patients (16.6%) |
| AAD discontinued at 2 months | 28 patients (93.3%) |
| Free from AF at 3 months (%) | 27 patients (90%) |
| Absence of clinical recurrence at 6 months (%) | 25 patients (83.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohmand-Borkowski, A. A Fluoroscopy-Free Ablation Workflow for Persistent Atrial Fibrillation Using a Pentaspline Pulse Field Ablation Catheter Guided by Left-Sided Intracardiac Echo Imaging and Electroanatomic Mapping: A Case Series. J. Cardiovasc. Dev. Dis. 2025, 12, 412. https://doi.org/10.3390/jcdd12100412
Mohmand-Borkowski A. A Fluoroscopy-Free Ablation Workflow for Persistent Atrial Fibrillation Using a Pentaspline Pulse Field Ablation Catheter Guided by Left-Sided Intracardiac Echo Imaging and Electroanatomic Mapping: A Case Series. Journal of Cardiovascular Development and Disease. 2025; 12(10):412. https://doi.org/10.3390/jcdd12100412
Chicago/Turabian StyleMohmand-Borkowski, Adam. 2025. "A Fluoroscopy-Free Ablation Workflow for Persistent Atrial Fibrillation Using a Pentaspline Pulse Field Ablation Catheter Guided by Left-Sided Intracardiac Echo Imaging and Electroanatomic Mapping: A Case Series" Journal of Cardiovascular Development and Disease 12, no. 10: 412. https://doi.org/10.3390/jcdd12100412
APA StyleMohmand-Borkowski, A. (2025). A Fluoroscopy-Free Ablation Workflow for Persistent Atrial Fibrillation Using a Pentaspline Pulse Field Ablation Catheter Guided by Left-Sided Intracardiac Echo Imaging and Electroanatomic Mapping: A Case Series. Journal of Cardiovascular Development and Disease, 12(10), 412. https://doi.org/10.3390/jcdd12100412





