Beyond the Lumen: Molecular Imaging to Unmask Vulnerable Coronary Plaques
Abstract
:1. Introduction
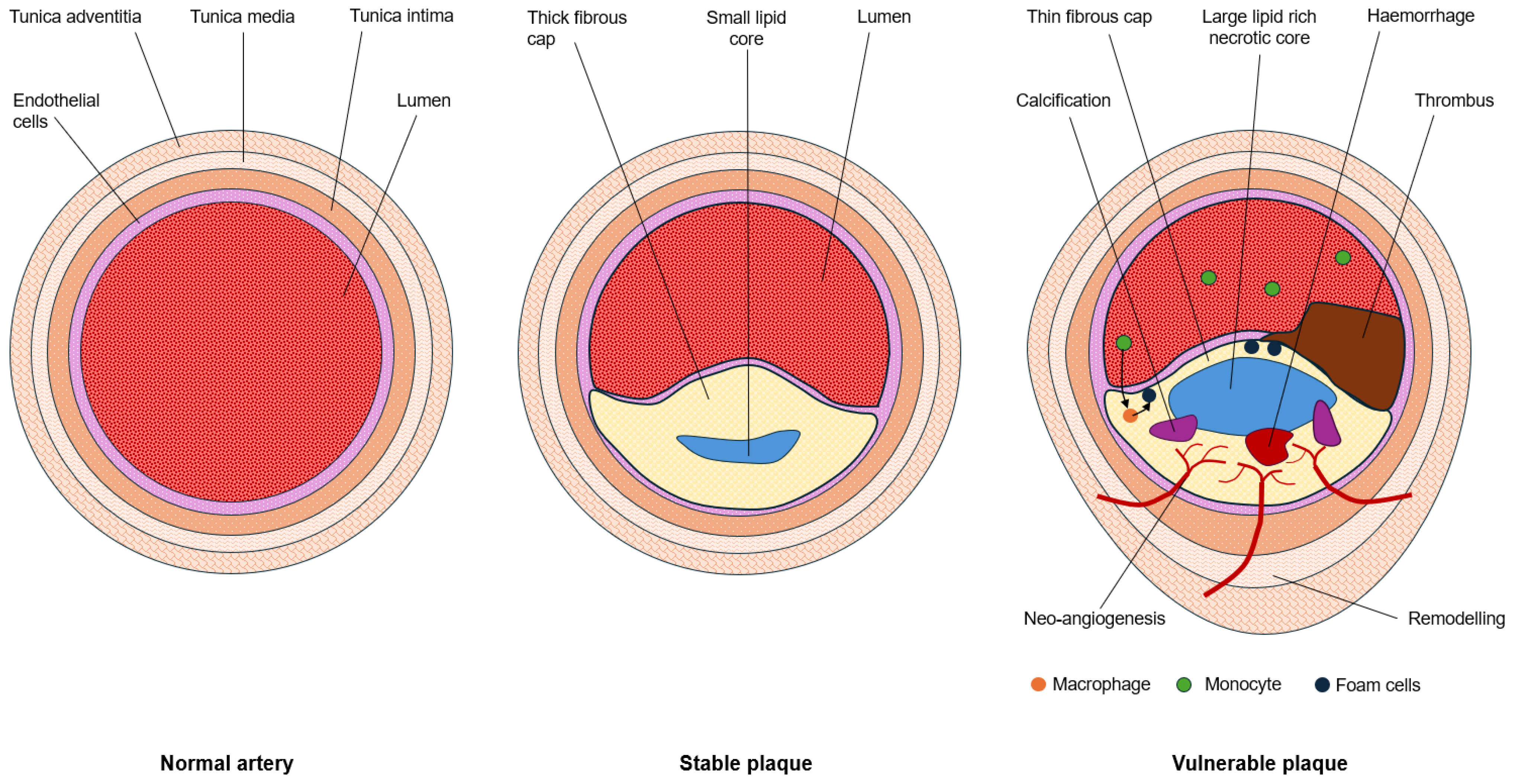
1.1. Hallmarks of Plaque Vulnerability
1.2. Imaging Plaque Morphology
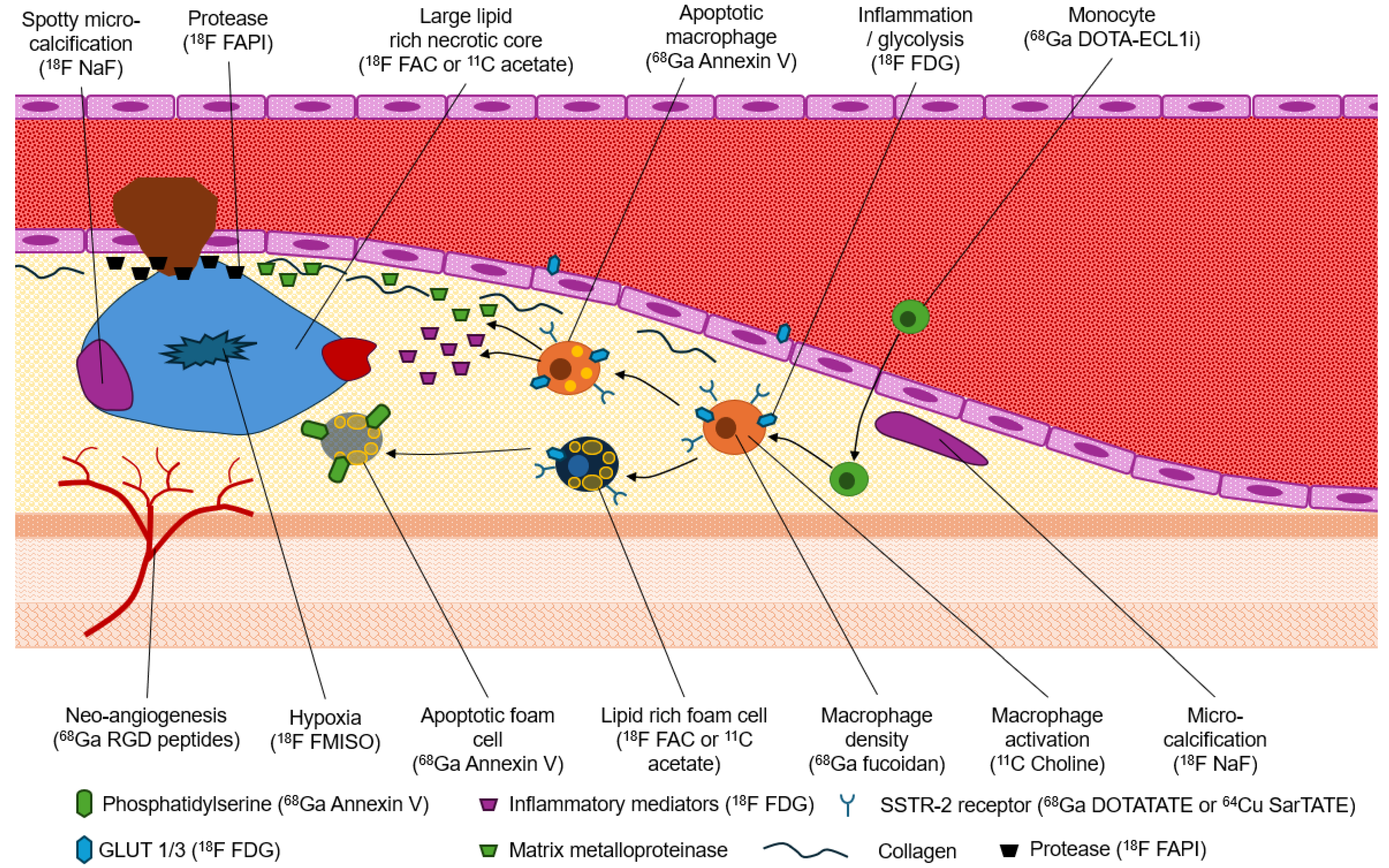
1.3. Molecular Imaging of the Hallmarks of Plaque Vulnerability
1.4. Inflammation
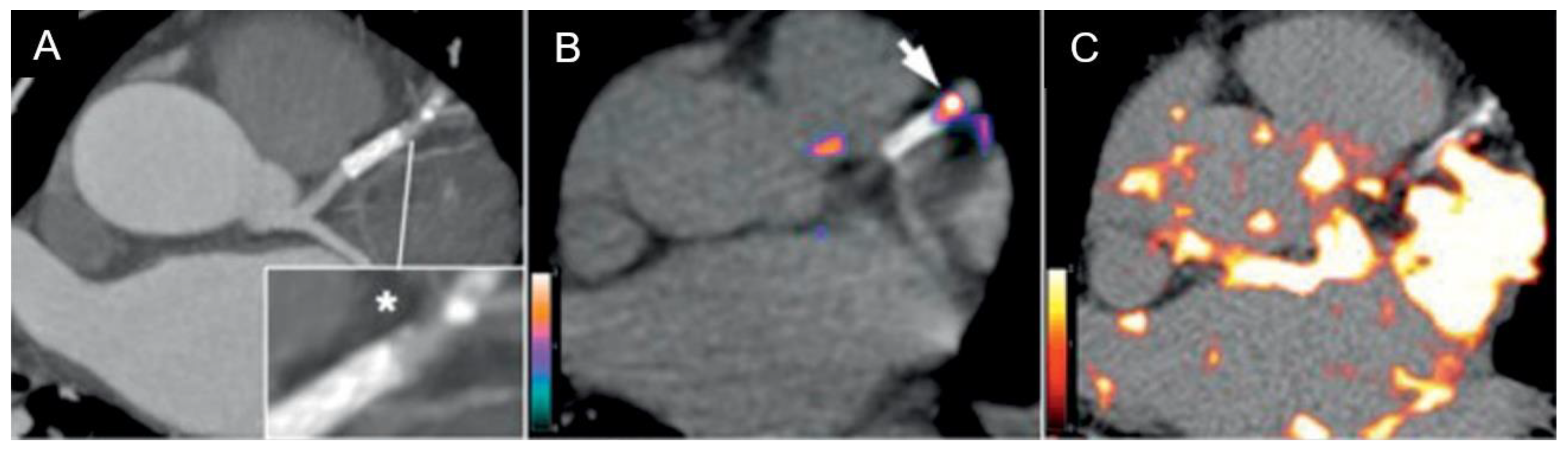
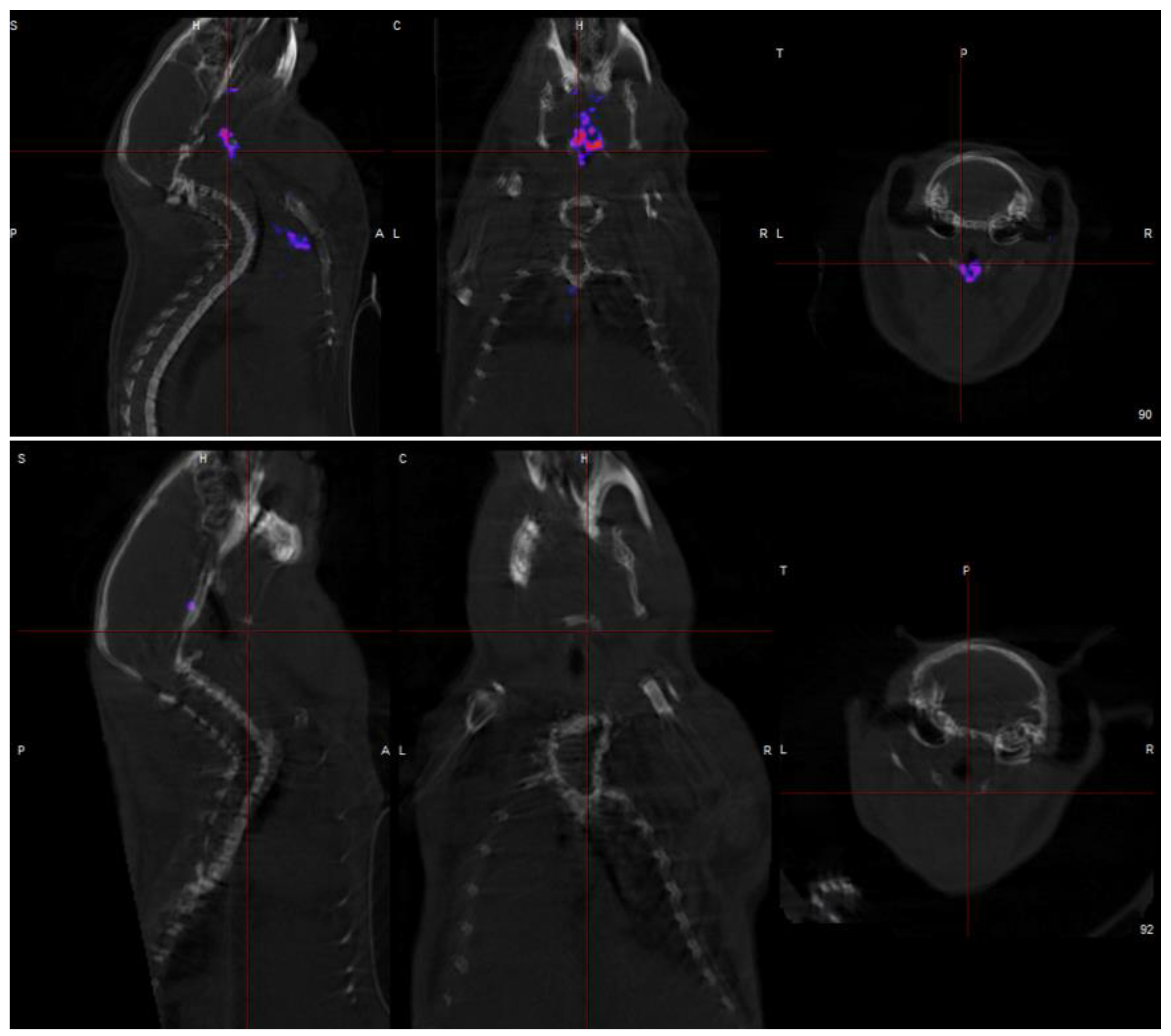
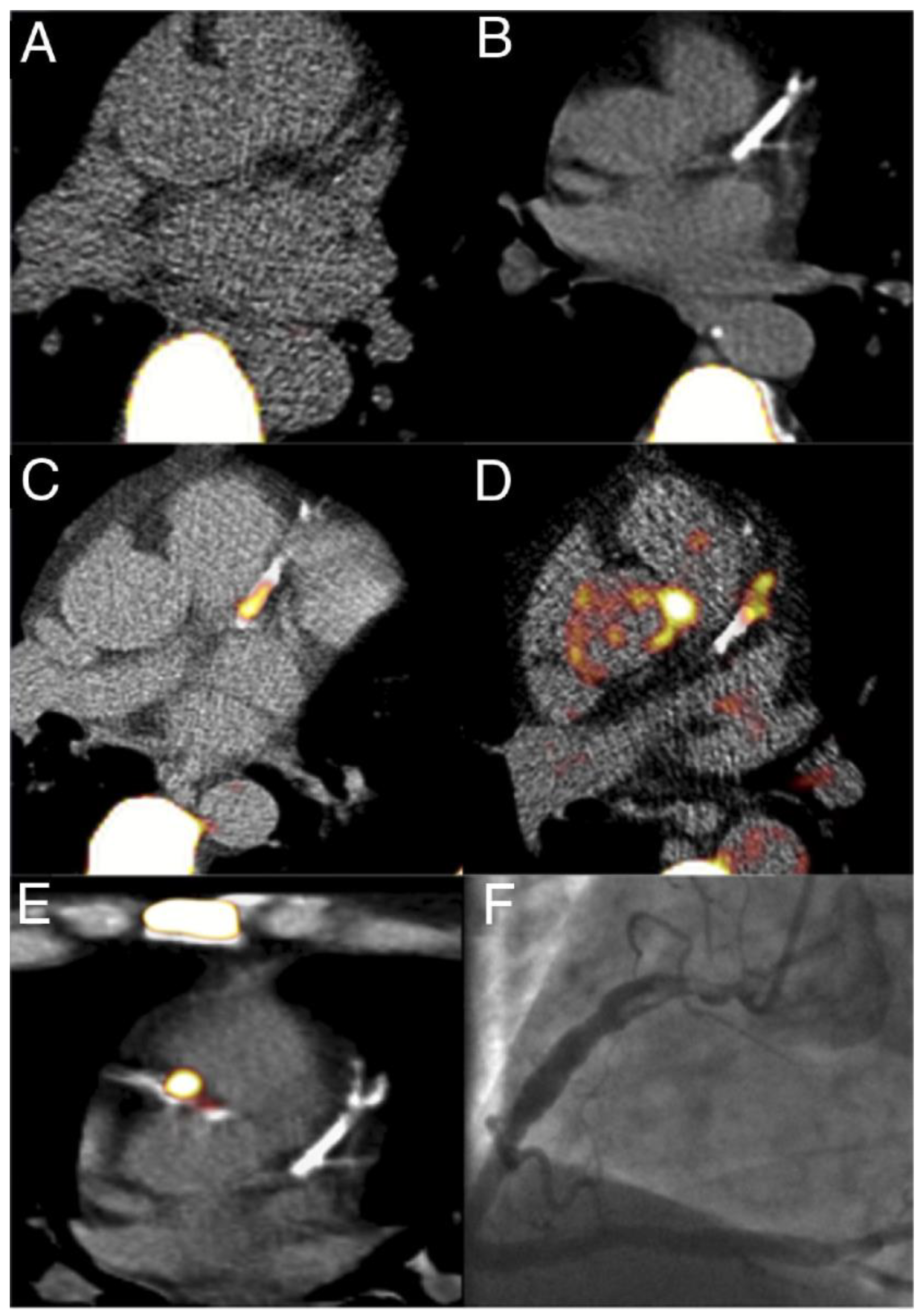
1.5. Micro-Calcifications
2. Systematic Review of 18F NaF PET in Vulnerable Plaque
2.1. Method
- Coronary artery
- Positron emission
- Sodium fluoride
- Coronary artery
- Positron emission
- Vulnerable plaque
2.2. Results
3. Discussion
- Coronary plaque specificity is demonstrated to be exceptional for detecting micro-calcification in coronary atherosclerotic plaques by 18F NaF, a hallmark of vulnerable lesions. This specificity arises from the ability of 18F NaF to reflect the exchange of hydroxyl groups in hydroxyapatite crystals, which is a crucial step in the calcification process of coronary plaques.
- Coronary imaging challenges due to their small size, constant motion, and proximity to the metabolically active myocardium is overcome by 18F NaF to provide:
- ◦
- Low background activity due to rapid clearance of 18F NaF from the circulation, resulting in minimal background activity in the myocardium even after just 1 h. This allows for accurate quantification of coronary plaque uptake without complex target-to-background ratio measurements.
- ◦
- Superior coronary visualization because the low myocardial uptake of 18F NaF enables clear visualization of coronary plaques.
- ◦
- Efficient imaging protocols with 18F NaF require no patient preparation and short imaging protocols.
- Early detection of vulnerable coronary plaques because 18F NaF accumulation in micro-calcification before visibility on CT, allowing earlier intervention. Furthermore, 18F NaF uptake can differentiate between active and indolent calcification in coronary arteries, providing crucial information on plaque activity and potential instability.
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.; Xie, Z.; Long, H.; Ren, K.; Hou, L.; Wang, Y.; Xu, X.; Lei, W.; Yang, Z.; Ahmed, S.; et al. Current advances in the imaging of atherosclerotic vulnerable plaque using nanoparticles. Mater. Today Bio 2022, 14, 100236. [Google Scholar] [CrossRef] [PubMed]
- Daghem, M.; Bing, R.; Fayad, Z.A.; Dweck, M.R. Noninvasive Imaging to Assess Atherosclerotic Plaque Composition and Disease Activity: Coronary and Carotid Applications. JACC Cardiovasc. Imaging 2020, 13, 1055–1068. [Google Scholar] [CrossRef] [PubMed]
- Benenati, S.; De Maria, G.L.; Kotronias, R.; Porto, I.; Banning, A.P. Why percutaneous revascularisation might not reduce the risk of myocardial infarction and mortality in patients with stable CAD? Open Hear. 2023, 10, e002343. [Google Scholar] [CrossRef] [PubMed]
- Perera, D.; Clayton, T.; O’kane, P.D.; Greenwood, J.P.; Weerackody, R.; Ryan, M.; Morgan, H.P.; Dodd, M.; Evans, R.; Canter, R.; et al. Percutaneous Revascularization for Ischemic Left Ventricular Dysfunction. N. Engl. J. Med. 2022, 387, 1351–1360. [Google Scholar] [CrossRef]
- Fox, K.A.A.; Metra, M.; Morais, J.; Atar, D. The myth of stable coronary artery disease. Nat. Rev. Cardiol. 2020, 17, 9–21. [Google Scholar] [CrossRef]
- Fayad, Z.A.; Fuster, V. Clinical Imaging of the High-Risk or Vulnerable Atherosclerotic Plaque. Circ. Res. 2001, 89, 305–316. [Google Scholar] [CrossRef]
- Bartlett, B.; Ludewick, H.P.; Lee, S.; Verma, S.; Francis, R.J.; Dwivedi, G. Imaging Inflammation in Patients and Animals: Focus on PET Imaging the Vulnerable Plaque. Cells 2021, 10, 2573. [Google Scholar] [CrossRef]
- SCOT-HEART Investigators; Newby, D.E.; Adamson, P.D.; Berry, C.; Boon, N.A.; Dweck, M.R.; Flather, M.; Forbes, J.; Hunter, A.; Lewis, S.; et al. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N. Engl. J. Med. 2018, 379, 924–933. [Google Scholar] [CrossRef]
- Budoff, M.J.; Shaw, L.J.; Liu, S.T.; Weinstein, S.R.; Tseng, P.H.; Flores, F.R.; Callister, T.Q.; Raggi, P.; Berman, D.S. Long-Term Prognosis Associated with Coronary Calcification: Observations From a Registry of 25,253 Patients. J. Am. Coll. Cardiol. 2007, 49, 1860–1870. [Google Scholar] [CrossRef]
- Hou, Z.-H.; Lu, B.; Gao, Y.; Jiang, S.-L.; Wang, Y.; Li, W.; Budoff, M.J. Prognostic Value of Coronary CT Angiography and Calcium Score for Major Adverse Cardiac Events in Outpatients. JACC: Cardiovasc. Imaging 2012, 5, 990–999. [Google Scholar] [CrossRef]
- Chang, H.J.; Lin, F.Y.; Lee, S.E.; Andreini, D.; Bax, J.; Cademartiri, F.; Chinnaiyan, K.; Chow, B.J.W.; Conte, E.; Cury, R.C.; et al. Coronary Atherosclerotic Precursors of Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2018, 71, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Kondakov, A.; Berdalin, A.; Beregov, M.; Lelyuk, V. Emerging Nuclear Medicine Imaging of Atherosclerotic Plaque Formation. J. Imaging 2022, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.J.; Hong, R.; Teo, L.L.Y.; Tan, R.-S.; Koh, A.S. Atherosclerotic cardiovascular disease in aging and the role of advanced cardiovascular imaging. npj Cardiovasc. Heal. 2024, 1, 1–8. [Google Scholar] [CrossRef]
- Nakahara, T.; Strauss, H.W.; Narula, J.; Jinzaki, M. Vulnerable Plaque Imaging. Semin. Nucl. Med. 2023, 53, 230–240. [Google Scholar] [CrossRef]
- Nayor, M.; Brown, K.J.; Vasan, R.S. The Molecular Basis of Predicting Atherosclerotic Cardiovascular Disease Risk. Circ. Res. 2021, 128, 287–303. [Google Scholar] [CrossRef]
- Ćorović, A.; Wall, C.; Mason, J.C.; Rudd, J.H.F.; Tarkin, J.M. Novel Positron Emission Tomography Tracers for Imaging Vascular Inflammation. Curr. Cardiol. Rep. 2020, 22, 1–11. [Google Scholar] [CrossRef]
- Tarkin, J.M.; Joshi, F.R.; Evans, N.R.; Chowdhury, M.M.; Figg, N.L.; Shah, A.V.; Starks, L.T.; Martin-Garrido, A.; Manavaki, R.; Yu, E.; et al. Detection of Atherosclerotic Inflammation by 68Ga-DOTATATE PET Compared to [18F]FDG PET Imaging. J. Am. Coll. Cardiol. 2017, 69, 1774–1791. [Google Scholar] [CrossRef]
- Dweck, M.R.; Chow, M.W.; Joshi, N.V.; Williams, M.C.; Jones, C.; Fletcher, A.M.; Richardson, H.; White, A.; McKillop, G.; van Beek, E.J.; et al. Coronary arterial 18F-sodium fluoride uptake: A novel marker of plaque biology. J. Am. Coll. Cardiol. 2012, 59, 1539–1548. [Google Scholar] [CrossRef]
- Moss, A.; Daghem, M.; Tzolos, E.; Meah, M.N.; Wang, K.-L.; Bularga, A.; Adamson, P.D.; Kwiecinski, J.; Fletcher, A.; Dawson, D.; et al. Coronary Atherosclerotic Plaque Activity and Future Coronary Events. JAMA Cardiol. 2023, 8, 755–764. [Google Scholar] [CrossRef]
- Moss, A.J.; Doris, M.K.; Andrews, J.P.M.; Bing, R.; Daghem, M.; van Beek, E.J.R.; Forsyth, L.; Shah, A.S.V.; Williams, M.C.; Sellers, S.; et al. Molecular Coronary Plaque Imaging Using 18F-Fluoride. Circ. Cardiovasc. Imaging 2019, 12, e008574. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-L.; Balmforth, C.; Meah, M.N.; Daghem, M.; Moss, A.J.; Tzolos, E.; Kwiecinski, J.; Molek-Dziadosz, P.; Craig, N.; Bularga, A.; et al. Coronary Atherosclerotic Plaque Activity and Risk of Myocardial Infarction. J. Am. Coll. Cardiol. 2024, 83, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J.; Tzolos, E.; Adamson, P.D.; Cadet, S.; Moss, A.J.; Joshi, N.; Williams, M.C.; van Beek, E.J.R.; Dey, D.; Berman, D.S.; et al. Coronary 18F-Sodium Fluoride Uptake Predicts Outcomes in Patients With Coronary Artery Disease. J. Am. Coll. Cardiol. 2020, 75, 3061–3074. [Google Scholar] [CrossRef]
- Kitagawa, T.; Yamamoto, H.; Nakamoto, Y.; Sasaki, K.; Toshimitsu, S.; Tatsugami, F.; Awai, K.; Hirokawa, Y.; Kihara, Y. Predictive Value of 18 F-Sodium Fluoride Positron Emission Tomography in Detecting High-Risk Coronary Artery Disease in Combination With Computed Tomography. J. Am. Hear. Assoc. 2018, 7, e010224. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, T.; Sasaki, K.; Fujii, Y.; Ikegami, Y.; Tatsugami, F.; Awai, K.; Hirokawa, Y.; Nakano, Y. 18F-sodium fluoride positron emission tomography following coronary computed tomography angiography in predicting long-term coronary events: A 5-year follow-up study. J. Nucl. Cardiol. 2023, 30, 2365–2378. [Google Scholar] [CrossRef]
- Sorci, O.; Batzdorf, A.S.; Mayer, M.; Rhodes, S.; Peng, M.; Jankelovits, A.R.; Hornyak, J.N.; Gerke, O.; Høilund-Carlsen, P.F.; Alavi, A.; et al. 18F-sodium fluoride PET/CT provides prognostic clarity compared to calcium and Framingham risk scoring when addressing whole-heart arterial calcification. Eur. J. Nucl. Med. 2019, 47, 1678–1687. [Google Scholar] [CrossRef]
- Gao, M.; Wen, W.; Li, H.; Zheng, Y.; Yun, M.; Meng, J.; Wang, S.; Wang, B.; Hu, B.; Mou, T.; et al. Coronary sodium [18F]fluoride activity predicts outcomes post-CABG: A comparative evaluation with conventional metrics. Eur. J. Nucl. Med. 2024, 51, 3235–3251. [Google Scholar] [CrossRef]
- Joshi, N.V.; Vesey, A.T.; Williams, M.C.; Shah, A.S.V.; A Calvert, P.; Craighead, F.H.M.; Yeoh, S.E.; Wallace, W.; Salter, D.; Fletcher, A.M.; et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: A prospective clinical trial. Lancet 2014, 383, 705–713. [Google Scholar] [CrossRef]
- Daghem, M.; Adamson, P.D.; Wang, K.L.; Doris, M.; Bing, R.; van Beek, E.J.R.; Forsyth, L.; Williams, M.C.; Tzolos, E.; Dey, D.; et al. Temporal Changes in Coronary 18F-Fluoride Plaque Uptake in Patients with Coronary Atherosclerosis. J. Nucl. Med. 2023, 64, 1478–1486. [Google Scholar] [CrossRef]
- Lee, J.M.; Bang, J.-I.; Koo, B.-K.; Hwang, D.; Park, J.; Zhang, J.; Yaliang, T.; Suh, M.; Paeng, J.C.; Shiono, Y.; et al. Clinical Relevance of 18 F-Sodium Fluoride Positron-Emission Tomography in Noninvasive Identification of High-Risk Plaque in Patients With Coronary Artery Disease. Circ. Cardiovasc. Imaging. 2017, 10. [Google Scholar] [CrossRef]
- Doris, M.K.; Meah, M.N.; Moss, A.J.; Andrews, J.P.; Bing, R.; Gillen, R.; Weir, N.; Syed, M.; Daghem, M.; Shah, A.; et al. Coronary 18 F-Fluoride Uptake and Progression of Coronary Artery Calcification. Circ. Cardiovasc. Imaging 2020, 13, e011438. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, T.; Yamamoto, H.; Toshimitsu, S.; Sasaki, K.; Senoo, A.; Kubo, Y.; Tatsugami, F.; Awai, K.; Hirokawa, Y.; Kihara, Y. 18F-sodium fluoride positron emission tomography for molecular imaging of coronary atherosclerosis based on computed tomography analysis. Atherosclerosis 2017, 263, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Tzolos, E.; Kwiecinski, J.; Lassen, M.L.; Cadet, S.; Adamson, P.D.; Moss, A.J.; Joshi, N.; Williams, M.C.; van Beek, E.J.R.; Dey, D.; et al. Observer repeatability and interscan reproducibility of 18F-sodium fluoride coronary microcalcification activity. J. Nucl. Cardiol. 2020, 29, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Rudd, J.H.; Myers, K.S.; Bansilal, S.; Machac, J.; Rafique, A.; Farkouh, M.; Fuster, V.; Fayad, Z.A. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: Implications for atherosclerosis therapy trials. J. Am. Coll. Cardiol. 2007, 50, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Rogers, I.S.; Nasir, K.; Figueroa, A.L.; Cury, R.C.; Hoffmann, U.; Vermylen, D.A.; Brady, T.J.; Tawakol, A. Feasibility of FDG imaging of the coronary arteries: Comparison between acute coronary syndrome and stable angina. JACC Cardiovasc. Imaging 2010, 3, 388–397. [Google Scholar] [CrossRef]



| Imaging Approach | Morphological Target | Invasiveness |
|---|---|---|
| CATH | Degree of stenosis, location of stenosis, number of stenoses | Invasive |
| CTCA | Degree of stenosis, location of stenosis, number of stenoses | Noninvasive |
| CAC | Atherosclerotic burden, stratification of cardiac event rate, and survival | Noninvasive |
| cMRI | Structural and functional changes associated with stenosis | Noninvasive |
| Echocardiography | Functional changes associated with stenosis | Noninvasive |
| Myocardial perfusion SPECT and PET | Hemodynamic significance of stenosis | Noninvasive |
| Intravascular ultrasound | High-resolution assessment of plaque morphology | Invasive |
| Optical coherence tomography | High-resolution assessment of plaque morphology | Invasive |
| Near-infrared spectroscopy | High-resolution assessment of plaque morphology | Invasive |
| 99mTc, 111In, 123I, 18F and 68Ga labelled LDL | Thickening of intima (fatty streak) | Noninvasive |
| 18F-4V | Monocyte adhesion to VCAM-1 | Noninvasive |
| 68Ga fucoidan | Monocyte adhesion to P-selectin | Noninvasive |
| 99mTc or 111In leukocytes | Monocyte migration | Noninvasive |
| Imaging Approach | Radiopharmaceutical/Probe | Molecular Target | Evidence (Coronary Arteries) |
|---|---|---|---|
| Monocyte migration | 111In or 99mTc WBCs 68Ga DOTA-ECL1i | Migrating leukocytes | Limited |
| Luminal thrombus | 111In-platelets 99mTc apcitide | Thrombus Glycoproteins activated by fibrinogen | Limited |
| Macrophage activity or density | 18F FDG 11C Acetate or 18F FAC 68Ga DOTATATE or 64Cu SarTATE 68Ga fucoidan 11C choline or 18F fluorocholine | Glycolysis, GLUT-1 and 3 expression Lipids in macrophages SSRT2 Macrophage density and P-selectin adhesion Macrophage activation | Moderate Limited |
| Receptor expression | 68Ga DOTATATE or 64CuSarTATE 68Ga Pentixafor Radiolabelled IL-2 | SSRT2 Chemokine IL-2 | Moderate for SSRT2 Mostly carotid |
| Neo-angiogenesis | 68Ga-NOTA-PRGD2 18F galacto-RGD 18F alphatide II 18F flotegatide 18F fluciclatide | Integrin αvβ3 expression | Mostly carotid |
| Glycolysis | 18F FDG | Inflammation | Moderate with limitations in coronaries |
| Calcification | 18F NaF | Hydroxyapatite | Strong for micro-calcification |
| Permeability (protease) | 11C, 18F, 123I, 68Ga and 99mTc labelled MMPs or MMP inhibitors 18F or 68Ga FAPI | MMP FAP | Emerging |
| Lipid concentration | 11C Acetate or 18F FAC 99mTc, 111In, 123I, 68Ga, 18F, 89Zr LDL | Fatty streaks, macrophages, foam cells, lipid-rich core | Limited |
| Hypoxia | 18F FMISO | Limited | |
| Apoptosis | 99mTc Annexin 68Ga-Annexin V | Apoptotic macrophages and foam cells | Limited |
| Inflammation | 18F FDG 18F-4V 18F Florbetaben | Endothelial activation due to inflammation Inflammation in endothelial cells and macrophages | Limited |
| Adhesion molecules | 18F-4V | VCAM-1 | Limited |
| Interleukin-2 expression | 99mTc-HYNIC-IL-2 | Activated T-lymphocytes | Mostly carotid |
| Patient Number | Study Type | Outcomes | Citation |
|---|---|---|---|
| 704 | Prospective | 18F NaF accumulation predicts all-cause mortality (HR = 2.43) and cardiac death or non-fatal MI (HR = 1.82), | [19] |
| 691 | Prospective | Increased CMA of 18F NaF has 2.1-fold increase in risk of MI over no uptake. Untreated had nearly fourfold increased risk of MI than those treated in patients with 18F NaF uptake. | [22] |
| 293 | Prospective | 18F NaF coronary accumulation predicts MI and, specifically, a CMA greater than 1.56 has HR of 7.1 for future MI. | [23] |
| 32 | Prospective | 18F NaF accumulation had HR of 8.2 for cardiac event in next 2 years. | [24] |
| 40 | Prospective | 18F NaF accumulation with maximum TBRmax greater than 1.29 had a HR of 5.4 for major cardiac event in the next 5-years. | [25] |
| 136 | Retrospective | 18F NaF uptake differentiated healthy controls from atherosclerotic patients, but CT-CAC could not. | [26] |
| 101 | Prospective | TBRmax > 3.0 had a 3.7-fold increase in perioperative MI while > 3.6 was associated with a 5.5-fold increased risk of major cardiovascular or cerebrovascular events. | [27] |
| 119 | Prospective | 18F NaF activity was higher in patients with atherosclerosis (p = 0.003), higher rates of previous cardiac events (p = 0.016), angina (p = 0.023), and correlated with CT-CAC (r = 0.652). | [18] |
| 37 | Prospective | 18F NaF TBRmax is higher (1.66) in culprit lesions than non-culprit lesions (1.24; p < 0.0001). By comparison, 18F FDG showed a ratio of 1.71 and 1.58 (p = 0.34) for culprit and non-culprit lesions, respectively. IVUS with features of high-risk plaques had a mean 18F NaF ratio of 1.90. | [28] |
| 101 | Prospective | Baseline 18F NaF CMA > 1.56 predicted progression of CT-CAC scores (p = 0.001). | [29] |
| 51 | Prospective | 18F NaF positive lesions had high plaque burden than IVUS and OCT. 14/15 IVUS and OCT identified high-risk lesions had high 18F NaF uptake. | [30] |
| 183 | Prospective | CT-CAC scores increased at 12 months in those with baseline increased accumulation of 18F NaF. | [31] |
| 32 | Retrospective | 18F NaF accumulation in coronary arteries correlates with a history of MI and CT-CAC. | [32] |
| 30 | Prospective | 18F NaF uptake in culprit lesions is reproducible. | [20] |
| 19 | Prospective | 100% intraobserver, interobserver, and interscan agreement for the presence (CMA > 0) or absence (CMA = 0) of coronary 18F NaF uptake. | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Currie, G.; Kiat, H. Beyond the Lumen: Molecular Imaging to Unmask Vulnerable Coronary Plaques. J. Cardiovasc. Dev. Dis. 2025, 12, 51. https://doi.org/10.3390/jcdd12020051
Currie G, Kiat H. Beyond the Lumen: Molecular Imaging to Unmask Vulnerable Coronary Plaques. Journal of Cardiovascular Development and Disease. 2025; 12(2):51. https://doi.org/10.3390/jcdd12020051
Chicago/Turabian StyleCurrie, Geoffrey, and Hosen Kiat. 2025. "Beyond the Lumen: Molecular Imaging to Unmask Vulnerable Coronary Plaques" Journal of Cardiovascular Development and Disease 12, no. 2: 51. https://doi.org/10.3390/jcdd12020051
APA StyleCurrie, G., & Kiat, H. (2025). Beyond the Lumen: Molecular Imaging to Unmask Vulnerable Coronary Plaques. Journal of Cardiovascular Development and Disease, 12(2), 51. https://doi.org/10.3390/jcdd12020051








