Clinical Decision Support for Patient Cases with Asymptomatic Carotid Artery Stenosis Using AI Models and Electronic Medical Records
Abstract
:1. Introduction
2. Methods
2.1. Data Collection and Processing
2.2. Machine Learning Algorithms
2.3. Evaluation Metrics and Statistics Calculations
3. Results
3.1. Statistics of the Study Cohort
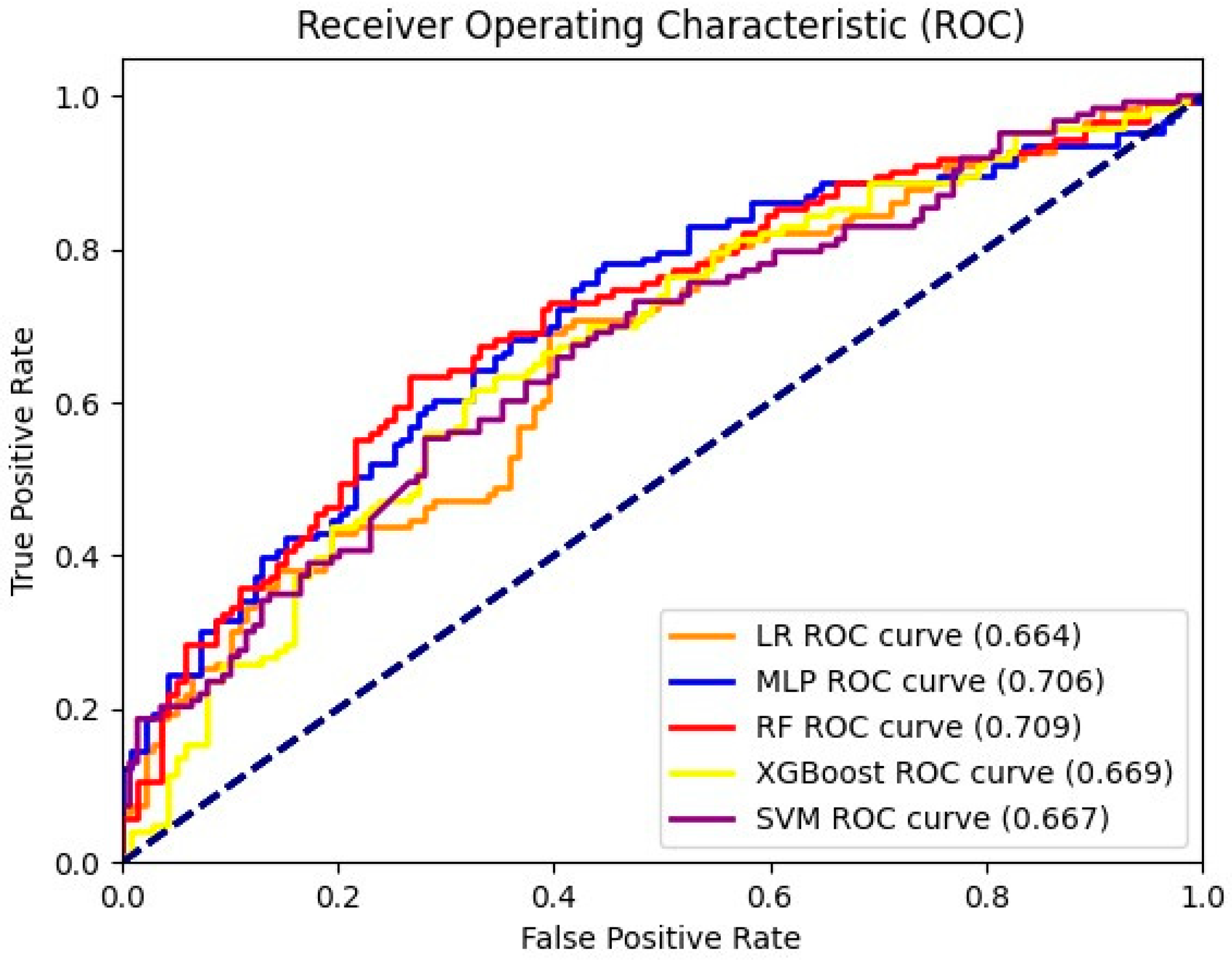
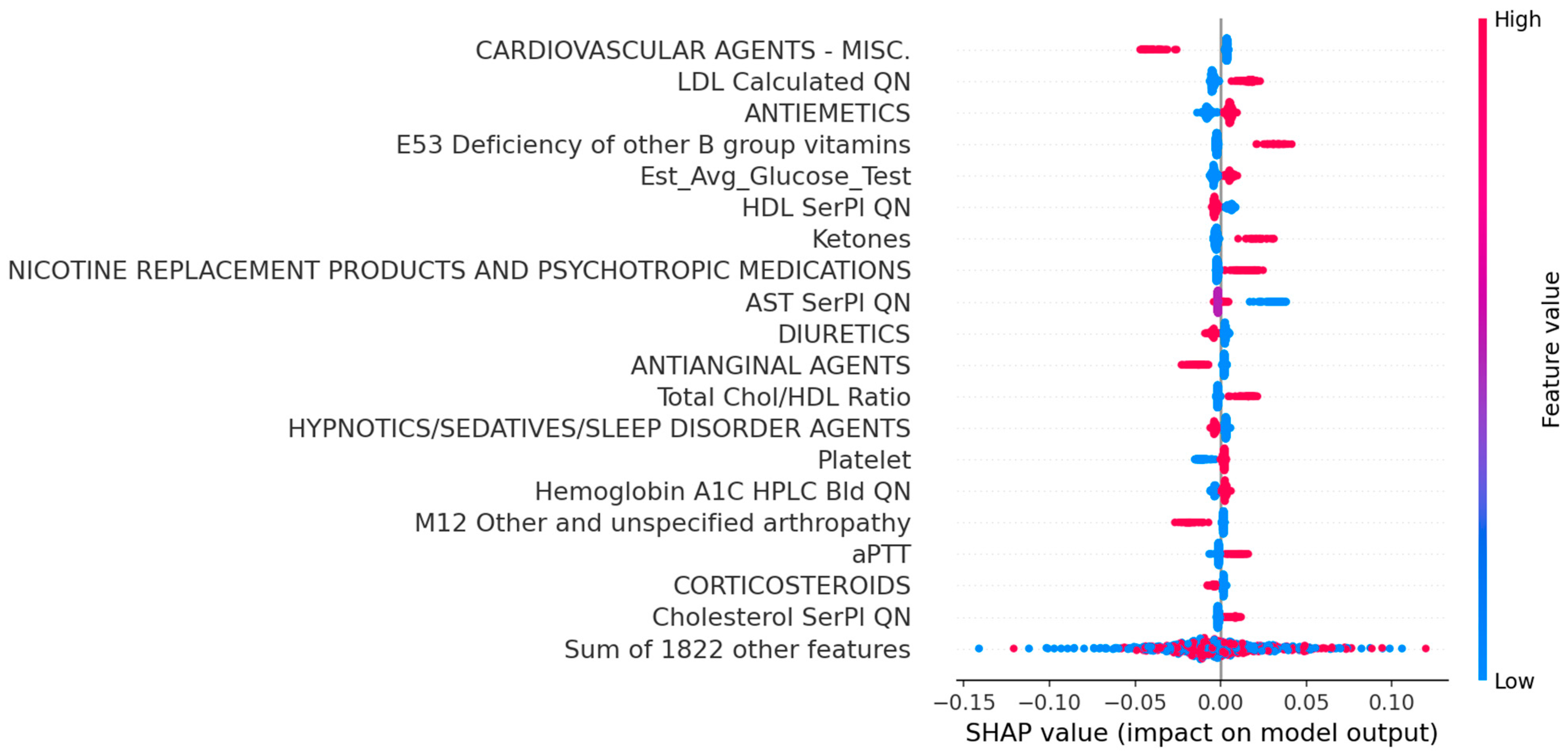
3.2. Performance Comparison and Interpretation
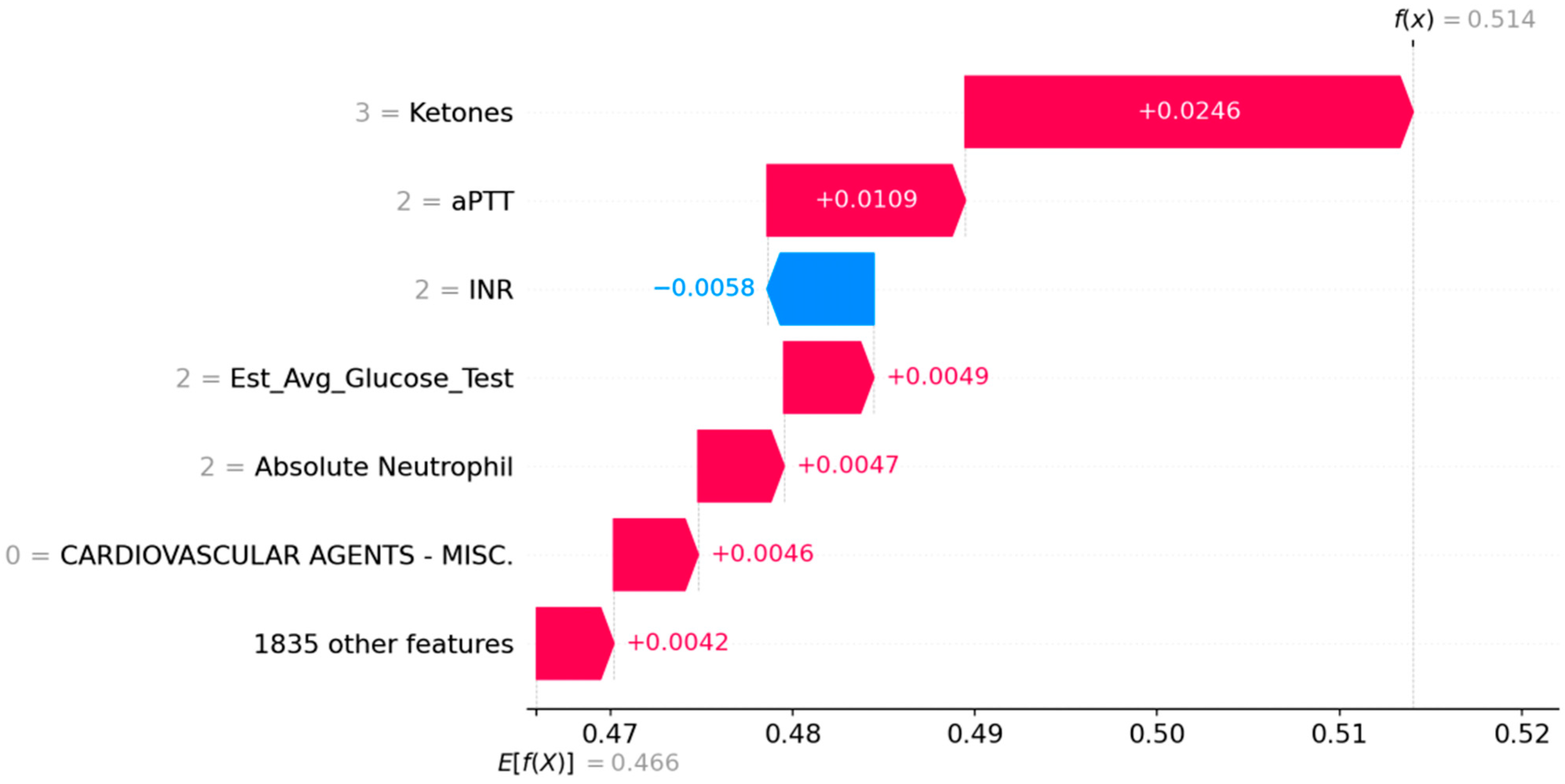
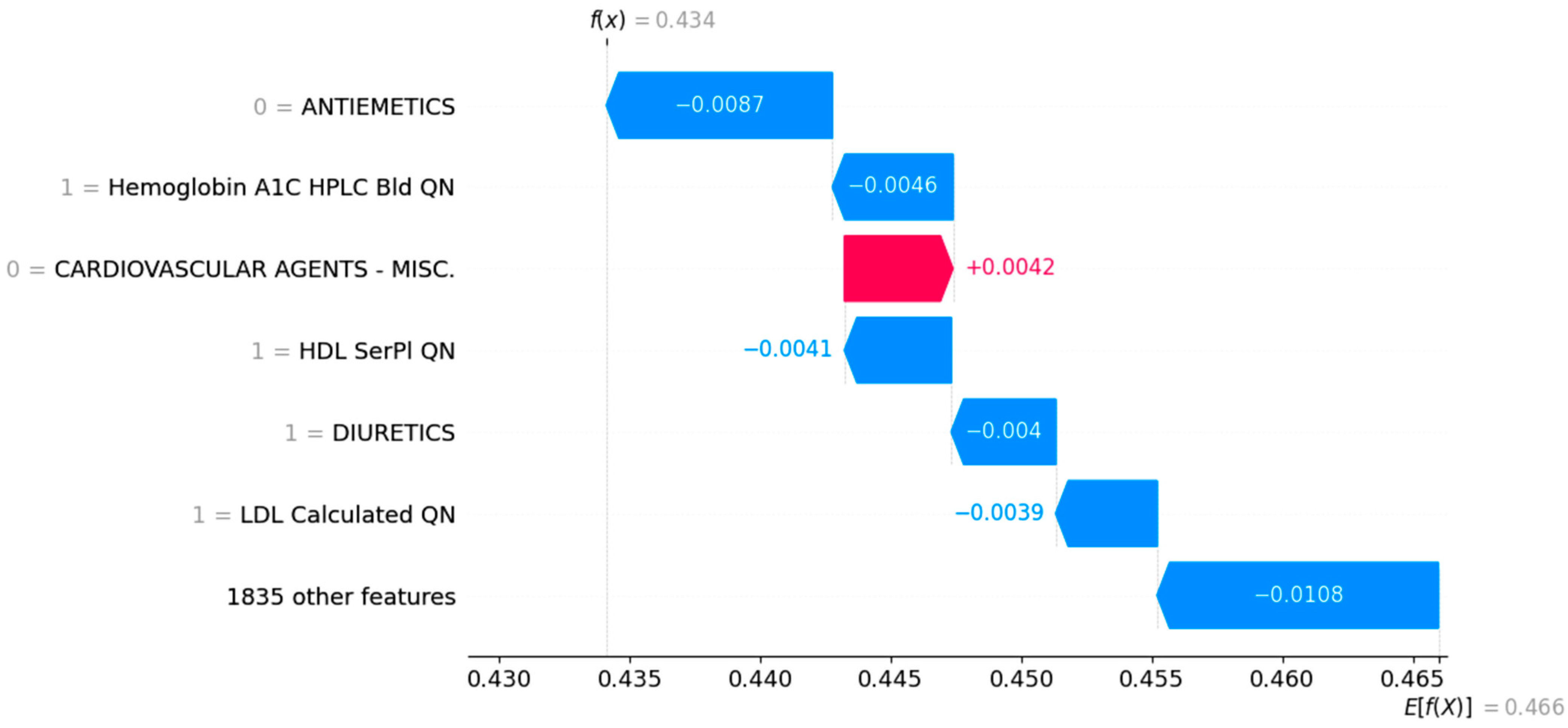
3.3. Case Study
4. Discussion
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AbuRahma, A. An analysis of the recommendations of the 2022 Society for Vascular Surgery clinical practice guidelines for patients with asymptomatic carotid stenosis. J. Vasc. Surg. 2024, 79, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Naylor, A.R. Why is the management of asymptomatic carotid disease so controversial? Surgeon 2015, 13, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Paraskevas, K.I.; Mikhailidis, D.P.; Veith, F.J. Are Symptomatic Patients Appropriate Candidates for Carotid Artery Stenting? No (at Least Not at Present). Vascular 2010, 18, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Ding, H.; Broyles, A.; Warden, S.J.; Moorthi, R.N.; Imel, E.A. Using machine learning to detect sarcopenia from electronic health records. Digit. Health 2023, 9. [Google Scholar] [CrossRef]
- Teoh, D. Towards stroke prediction using electronic health records. BMC Med. Inform. Decis. Mak. 2018, 18, 127. [Google Scholar] [CrossRef]
- Dev, S.; Wang, H.; Nwosu, C.S.; Jain, N.; Veeravalli, B.; John, D. A predictive analytics approach for stroke prediction using machine learning and neural networks. Health Anal. 2022, 2, 100032. [Google Scholar] [CrossRef]
- Zhao, Y.; Fu, S.; Bielinski, S.J.; Decker, P.A.; Chamberlain, A.M.; Roger, V.L.; Liu, H.; Larson, N.B. Natural Language Processing and Machine Learning for Identifying Incident Stroke from Electronic Health Records: Algorithm Development and Validation. J. Med. Internet Res. 2021, 23, e22951. [Google Scholar] [CrossRef]
- Reed, M.; Huang, J.; Graetz, I.; Brand, R.; Hsu, J.; Fireman, B.; Jaffe, M. Outpatient Electronic Health Records and the Clinical Care and Outcomes of Patients with Diabetes Mellitus. Ann. Intern. Med. 2012, 157, 482–489. [Google Scholar] [CrossRef]
- Norgeot, B.; Glicksberg, B.S.; Trupin, L.; Lituiev, D.; Gianfrancesco, M.; Oskotsky, B.; Schmajuk, G.; Yazdany, J.; Butte, A.J. Assessment of a Deep Learning Model Based on Electronic Health Record Data to Forecast Clinical Outcomes in Patients with Rheumatoid Arthritis. JAMA Netw. Open 2019, 2, e190606. [Google Scholar] [CrossRef]
- Goessens, B.M.; Visseren, F.L.; Kappelle, L.J.; Algra, A.; Van der Graaf, Y. Asymptomatic carotid artery stenosis and the risk of new vascular events in patients with manifest arterial disease: The SMART study. Stroke 2007, 38, 1470–1475. [Google Scholar] [CrossRef]
- McDonald, C.J.; Overhage, J.M.; Barnes, M.; Schadow, G.; Blevins, L.; Dexter, P.R.; Mamlin, B.; INPC Management Committee. The Indiana Network for Patient Care: A Working Local Health Information Infrastructure. Health Aff. 2005, 24, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.W.; Xu, J.; Bodenreider, O. The new International Classification of Diseases 11th edition: A comparative analysis with ICD-10 and ICD-10-CM. J. Am. Med. Inform. Assoc. 2020, 27, 738–746. [Google Scholar] [CrossRef]
- Wright, A.; Wright, A.P.; Aaron, S.; Sittig, D.F. Smashing the strict hierarchy: Three cases of clinical decision support malfunctions involving carvedilol. J. Am. Med. Inform. Assoc. 2018, 25, 1552–1555. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, S.M.; Lee, S.-I. A Unified Approach to Interpreting Model Predictions. Neural Inf. Process. Syst. 2017, 4768–4777. [Google Scholar] [CrossRef]
- Prendin, F.; Pavan, J.; Cappon, G.; Del Favero, S.; Sparacino, G.; Facchinetti, A. The importance of interpreting machine learning models for blood glucose prediction in diabetes: An analysis using SHAP. Sci. Rep. 2023, 13, 16865. [Google Scholar] [CrossRef]
- Gao, X.R.; Chiariglione, M.; Qin, K.; Nuytemans, K.; Scharre, D.W.; Li, Y.-J.; Martin, E.R. Explainable machine learning aggregates polygenic risk scores and electronic health records for Alzheimer’s disease prediction. Sci. Rep. 2023, 13, 450. [Google Scholar] [CrossRef]
- Liu, S.; Schlesinger, J.J.; McCoy, A.B.; Reese, T.J.; Steitz, B.; Russo, E.; Koh, B.; Wright, A. New onset delirium prediction using machine learning and long short-term memory (LSTM) in electronic health record. J. Am. Med. Inform. Assoc. 2022, 30, 120–131. [Google Scholar] [CrossRef]
- Walker, M.D.; Marler, J.R.; Goldstein, M.; Grady, P.A.; Toole, J.F.; Baker, W.H.; Castaldo, J.E.; Chambless, L.E.; Moore, W.S.; Robertson, J.T.; et al. Endarterectomy for Asymptomatic Carotid Artery Stenosis. JAMA 1995, 273, 1421–1428. [Google Scholar] [CrossRef]
- Halliday, A.; Harrison, M.; Hayter, E.; Kong, X.; Mansfield, A.; Marro, J.; Pan, H.; Peto, R.; Potter, J.; Rahimi, K.; et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): A multicentre randomised trial. Lancet 2010, 376, 1074–1084. [Google Scholar] [CrossRef]
- Marquardt, L.; Geraghty, O.C.; Mehta, Z.; Rothwell, P.M. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: A prospective, population-based study. Stroke 2010, 41, e11–e17. [Google Scholar] [CrossRef]
- Reiff, T.; Eckstein, H.-H.; Mansmann, U.; Jansen, O.; Fraedrich, G.; Mudra, H.; Böckler, D.; Böhm, M.; Debus, E.S.; Fiehler, J.; et al. Carotid endarterectomy or stenting or best medical treatment alone for moderate-to-severe asymptomatic carotid artery stenosis: 5-year results of a multicentre, randomised controlled trial. Lancet Neurol. 2022, 21, 877–888. [Google Scholar] [CrossRef]
- Van de Schoot, R.; Depaoli, S.; King, R.; Kramer, B.; Märtens, K.; Tadesse, M.G.; Vannucci, M.; Gelman, A.; Veen, D.; Willemsen, J. Bayesian statistics and modelling. Nat. Rev. Methods Primers 2021, 1, 1–26. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, S.; Zhang, J.; Wang, F.; He, W. Multimodal ultrasound parameters aided carotid plaque risk stratification in patients with asymptomatic carotid stenosis. Acta Radiol. 2021, 63, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Spagnolo-Allende, A.; Yang, D.; Qiao, Y.J. Epidemiology, pathophysiology, and imaging of atherosclerotic intracranial disease. Stroke 2024, 55, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Fabris, F.; Zanocchi, M.; Bo, M.; Fonte, G.; Poli, L.; Bergoglio, I.; Ferrario, E.; Pernigotti, L. Carotid plaque, aging, and risk factors. A study of 457 subjects. Stroke 1994, 25, 1133–1140. [Google Scholar] [CrossRef]
- North America Symptomatic Carotid Endarterectomy Trial Steering Committee. North America Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke 1991, 22, 711–720. [Google Scholar] [CrossRef]
- Thomas, K.H.; Dalili, M.N.; López-López, J.A.; Keeney, E.; Phillippo, D.; Munafò, M.R.; Stevenson, M.; Caldwell, D.M.; Welton, N.J. Smoking cessation medicines and e-cigarettes: A systematic review, network meta-analysis and cost-effectiveness analysis. Health Technol. Assess. 2021, 25, 1–224. [Google Scholar] [CrossRef]
- Welch, G.N.; Loscalzo, J. Homocysteine and Atherothrombosis. N. Engl. J. Med. 1998, 338, 1042–1050. [Google Scholar] [CrossRef]
- Agrawal, M.; Hegselmann, S.; Lang, H.; Kim, Y.; Sontag, D. Large language models are zero-shot clinical information extractors. arXiv 2022, arXiv:2205.12689. [Google Scholar] [CrossRef]
- Benary, M.; Wang, X.D.; Schmidt, M.; Soll, D.; Hilfenhaus, G.; Nassir, M.; Sigler, C.; Knödler, M.U.; Beule, D. Leveraging large language models for decision support in personalized oncology. AMA Netw. Open 2023, 6, e2343689. [Google Scholar] [CrossRef]
- Paraskevas, K.I.; Nicolaides, A.N.; Kakkos, S.K. Asymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) study: What have we learned from it? Ann. Transl. Med. 2020, 8, 1271. [Google Scholar] [CrossRef]

| Total | Asymptomatic | Symptomatic | p-Value | |
|---|---|---|---|---|
| Number of Patients | 872 | 464 | 408 | |
| Encounters per Patient, mean (STD) | 24.24 (21.00) | 25.20 (19.79) | 23.14 (22.21) | 0.151 |
| Demographics | ||||
| Age, mean (std) | 68.99 (10.51) | 69.89 (10.29) | 67.98 (10.69) | 0.007 |
| Gender | <0.05 | |||
| Male | 423 | 293 (63.1%) | 230 (56.4%) | |
| Female | 349 | 171 (36.9%) | 178 (43.6%) | |
| Race | 0.315 | |||
| White | 793 | 430 (92.7%) | 363 (90.0%) | |
| African American | 65 | 27 (5.8%) | 38 (9.3%) | |
| Asian | 7 | 3 (0.6%) | 4 (1.0%) | |
| Other or Unknown | 7 | 4 (0.9%) | 3 (0.7%) | |
| Medication | ||||
| Cardiovascular Agents | 87 | 71 (15.3%) | 16 (3.9%) | <0.01 |
| Antiemetics | 449 | 273 (58.8%) | 276 (67.6%) | <0.01 |
| Psychotherapeutic and Neurological Agents | 124 | 45 (9.7%) | 79 (19.4%) | <0.01 |
| Diuretics | 359 | 211 (45.5%) | 148 (36.3%) | <0.01 |
| Antianginal Agents | 97 | 62 (13.3%) | 35 (8.6%) | <0.05 |
| Hypnotics/Sedatives/Sleep Disorder Agents | 404 | 243 (52.4%) | 161 (39.5%) | <0.01 |
| Corticosteroids | 229 | 135 (29.1%) | 94 (23.0%) | 0.051 |
| Laboratory Test | ||||
| LDL Calculated QN (high) | 182 | 67 (14.4%) | 115 (28.2%) | <0.01 |
| Est Avg Glucose Test (high) | 348 | 151 (32.5%) | 197 (48.3%) | <0.01 |
| HDL SerPl QN (low) | 566 | 329 (70.9%) | 237 (58.1%) | <0.01 |
| Ketones (high) | 63 | 24 (5.2%) | 39 (9.6%) | <0.05 |
| AST SerPl QN | 111 | 49 (10.3%) | 63 (15.4%) | <0.05 |
| AST SerPl QN (low) | 59 | 21 (4.5%) | 38 (9.3%) | |
| AST SerPl QN (high) | 52 | 27 (5.8%) | 25 (6.1%) | |
| Total Chol/HDL Ratio (high) | 98 | 33 (7.1%) | 65 (15.9%) | <0.01 |
| Platelet | 129 | 78 (16.8%) | 51 (12.5%) | 0.100 |
| Platelet (low) | 112 | 70 (15.1%) | 42 (10.3%) | |
| Platelet (high) | 17 | 8 (1.7%) | 9 (2.2%) | |
| Hemoglobin A1C Bld QN | 505 | 240 | 265 | <0.01 |
| Hemoglobin A1C Bld QN (low) | 4 | 0 (0%) | 4 (0.1%) | |
| Hemoglobin A1C Bld QN (high) | 501 | 240 (51.7%) | 261 (64.0%) | |
| aPTT | 100 | 40 (8.6%) | 60 (14.7%) | <0.05 |
| aPTT (low) | 6 | 3 (0.6%) | 3 (0.7%) | |
| aPTT (high) | 94 | 37 (8.0%) | 57 (14.0%) | |
| Cholesterol SerPl QN (high) | 114 | 42 (9.1%) | 72 (17.6%) | <0.01 |
| Diagnosis | ||||
| E53 Deficiency of other B Group Vitamins | 88 | 26 (5.6%) | 52 (12.7%) | <0.01 |
| M12 Other and Unspecified Arthropathy | 62 | 41 (8.8%) | 21 (5.1%) | <0.05 |
| Models | Sensitivity | Specificity | ||
|---|---|---|---|---|
| Training | Test | Training | Test | |
| LR | 0.856 | 0.569 | 0.901 | 0.626 |
| SVM | 0.852 | 0.626 | 0.923 | 0.626 |
| MLP | 0.684 | 0.569 | 0.818 | 0.726 |
| RF | 0.533 | 0.317 | 0.978 | 0.906 |
| XGBoost | 0.715 | 0.455 | 0.870 | 0.776 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madison, M.; Luo, X.; Silvey, J.; Brenner, R.; Gannamaneni, K.; Sawchuk, A.P. Clinical Decision Support for Patient Cases with Asymptomatic Carotid Artery Stenosis Using AI Models and Electronic Medical Records. J. Cardiovasc. Dev. Dis. 2025, 12, 61. https://doi.org/10.3390/jcdd12020061
Madison M, Luo X, Silvey J, Brenner R, Gannamaneni K, Sawchuk AP. Clinical Decision Support for Patient Cases with Asymptomatic Carotid Artery Stenosis Using AI Models and Electronic Medical Records. Journal of Cardiovascular Development and Disease. 2025; 12(2):61. https://doi.org/10.3390/jcdd12020061
Chicago/Turabian StyleMadison, Mackenzie, Xiao Luo, Jackson Silvey, Robert Brenner, Kartik Gannamaneni, and Alan P. Sawchuk. 2025. "Clinical Decision Support for Patient Cases with Asymptomatic Carotid Artery Stenosis Using AI Models and Electronic Medical Records" Journal of Cardiovascular Development and Disease 12, no. 2: 61. https://doi.org/10.3390/jcdd12020061
APA StyleMadison, M., Luo, X., Silvey, J., Brenner, R., Gannamaneni, K., & Sawchuk, A. P. (2025). Clinical Decision Support for Patient Cases with Asymptomatic Carotid Artery Stenosis Using AI Models and Electronic Medical Records. Journal of Cardiovascular Development and Disease, 12(2), 61. https://doi.org/10.3390/jcdd12020061







