Incidence and Risk Factors for Developing Type 2 Diabetes Mellitus After Acute Myocardial Infarction—A Long-Term Follow-Up
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Outcomes
2.2. Data Collection and Definitions
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Follow-Up and Outcome
3.3. The Cumulative Incidence of NODM by the Baseline Characteristics
3.4. The Risk of Developing NODM Based on the Investigated Parameters—Multivariable Analysis
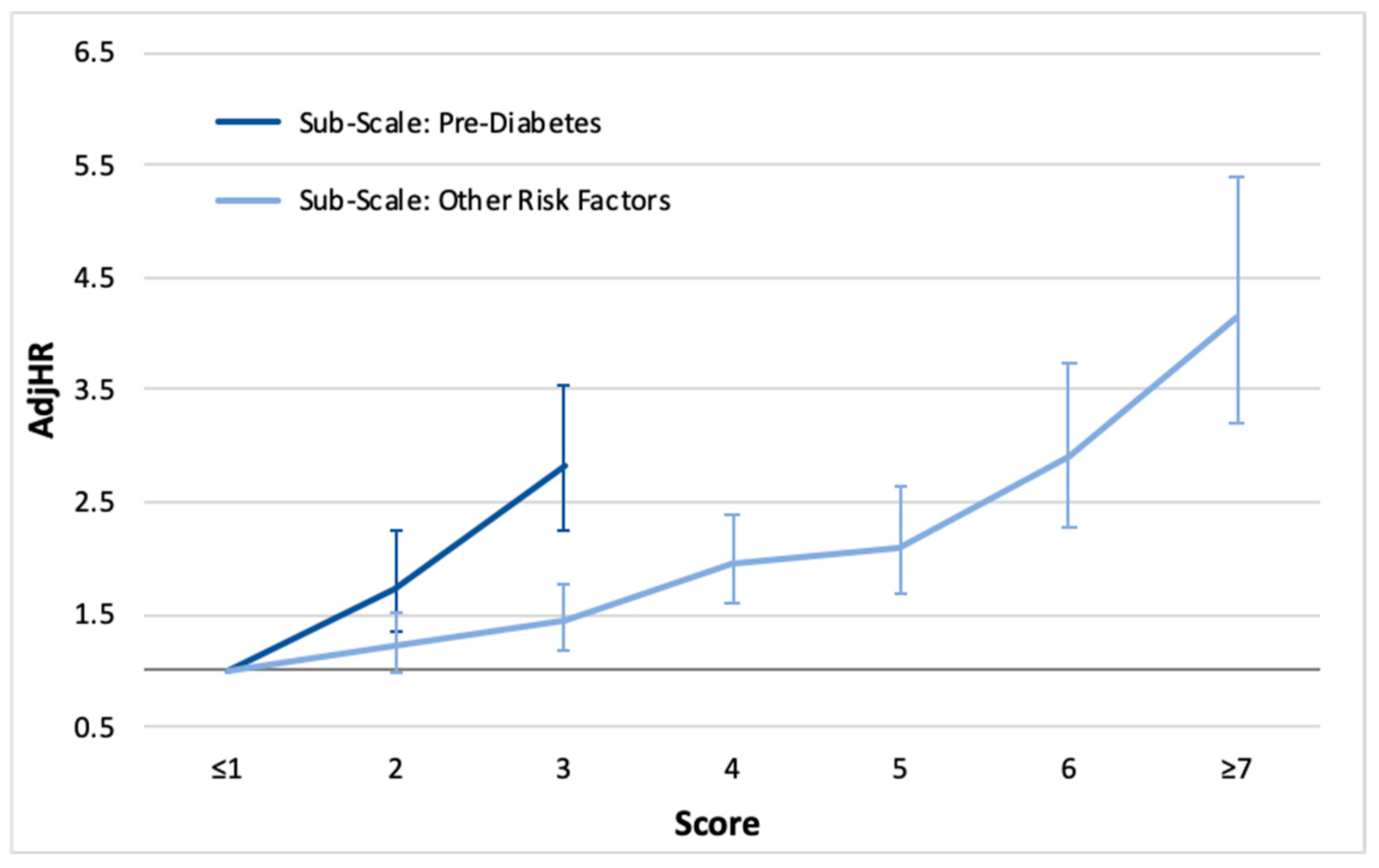
3.5. Risk Scoring
4. Discussion
4.1. HbA1C Is the Strongest Predictor
4.2. “No Results” Phenomenon
4.3. The Cause and Effect of Risk Factors on NODM
4.4. Total Score
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Gaglia, J.L.; Hilliard, M.E.; Isaacs, D.; et al. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes: Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.; Rosengren, A.; Young, K.; Jennings, E. Mortality and morbidity trends after the first year in survivors of acute myocardial infarction: A systematic review. BioMed Cent. Cardiovasc. Disord. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Abu Tailakh, M.; Friger, M.; Zahger, D.; Sidi, A.; Mazor-Dray, E.; Novack, V. Prospective study of the impact of diabetes mellitus newly diagnosed by glycated hemoglobin on outcomes in patients undergoing percutaneous coronary intervention. Eur. J. Intern. Med. 2017, 37, 69–74. [Google Scholar] [CrossRef]
- Aguilar, D.; Solomon, S.D.; Køber, L. Newly diagnosed and previously known diabetes mellitus and 1-year outcomes of acute myocardial infarction: The VALsartan in Acute myocardial iNfarcTion (VALIANT) trial. Circulation 2004, 110, 1572–1578. [Google Scholar] [CrossRef]
- Aggarwal, B.; Shah, G.; Randhawa, M.S.; Lincoff, A.M.; Ellis, S.G.; Menon, V. Patients with newly diagnosed diabetes have comparable long term mortality with known diabetics after ST segment elevation myocardial infarction. Circulation 2014, 130 (Suppl. S2), A17910. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Marfisi, R.; Levantesi, G.; Silletta, M.G.; Tavazzi, L.; Tognoni, G.; Valagussa, F.; Marchioli, R. Incidence of new-onset diabetes and impaired fasting glucose in patients with recent myocardial infarction and the effect of clinical and lifestyle risk factors. Lancet 2007, 370, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Choi, Y.S.; Kim, P.J.; Lee, J.M.; Baek, K.-H.; Kim, H.Y.; Yoo, K.D.; Song, K.-H.; Chung, W.S.; Seung, K.B.; et al. Acute myocardial infarction is a risk factor for new onset diabetes in patients with coronary artery disease. PLoS ONE 2015, 10, e0136354. [Google Scholar] [CrossRef]
- Gyldenkerne, C.; Kahlert, J.; Thrane, P.G.; Olesen, K.K.W.; Mortensen, M.B.; Sørensen, H.T.; Thomsen, R.W.; Maeng, M. 2-Fold More Cardiovascular Disease Events Decades Before Type 2 Diabetes Diagnosis: A Nationwide Registry Study. J. Am. Coll. Cardiol. 2024, 84, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Prevalence of Both Diagnosed and Undiagnosed Diabetes. 2024. Available online: https://www.cdc.gov/diabetes/php/data-research/ (accessed on 1 November 2022).
- Appelman, Y.; van Rijn, B.B.; Ten Haaf, M.E.; Boersma, E.; Peters, S.A.E. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis 2015, 241, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Kazlauskienė, L.; Butnorienė, J.; Norkus, A. Metabolic syndrome related to cardiovascular events in a 10-year prospective study. Diabetol. Metab. Syndr. 2015, 7, 102. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.; Go, A.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; Ferranti, S.D.; Després, J.-P.; Fullerton, H.J.; et al. Executive summary: Heart disease and stroke statistics—2016 update: A report from the American Heart Association. Circulation 2016, 133, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.M.; Shalaby, M.A.; El-Shiekh, R.A.; El-Banna, H.A.; Emam, S.R.; Bakr, A.F. Metabolic syndrome: Risk factors, diagnosis, pathogenesis, and management with natural approaches. Food Chem. Adv. 2023, 3, 10035. [Google Scholar] [CrossRef]
- Frantz, S.; Hundertmark, M.J.; Schulz-Menger, J.; Bengel, F.M.; Bauersachs, J. Left ventricular remodeling post-myocardial infarction: Pathophysiology, imaging, and novel therapies. Eur. Heart J. 2022, 43, 2549–2561. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Leancă, S.A.; Crișu, D.; Petriș, A.O.; Afrăsânie, I.; Genes, A.; Costache, A.D.; Tesloianu, D.N.; Costache, I.I. Left ventricular remodeling after myocardial infarction: From physiopathology to treatment. Life 2022, 12, 1111. [Google Scholar] [CrossRef]
- Ibánez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar]
- Choi, J.Y.; Choi, C.U.; Hwang, S.Y.; Choi, B.G.; Jang, W.Y.; Kim, D.Y.; Kim, W.; Park, E.J.; Lee, S.; Na, J.O.; et al. Effect of Pitavastatin compared with atorvastatin and Rosuvastatin on new-onset diabetes mellitus in patients with acute myocardial infarction. Am. J. Cardiol. 2018, 122, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Boyer, E.W.; Pozner, C.N.; Geib, A.-J.; Thomsen, T.; Mick, N.; Thomas, S. Assessment of hyperglycemia after calcium channel blocker overdoses involving diltiazem or verapamil. Crit. Care Med. 2007, 35, 2071–2075. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Y.; Liu, Y.; Xu, W.; Wang, Q. Comparative risk of new-onset diabetes mellitus for antihypertensive drugs: A network meta-analysis. J. Clin. Hypertens. 2017, 19, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Ganda, O.P. Statin-induced diabetes: Incidence, mechanisms, and implications. F1000Research 2016, 5, F1000. [Google Scholar] [CrossRef] [PubMed]
- Porath, A.; Arbelle, J.E.; Fund, N.; Cohen, A.; Mosseri, M. Statin therapy: Diabetes mellitus risk and cardiovascular benefit in primary prevention. Isr. Med. Assoc. J. 2018, 20, 480–485. [Google Scholar] [PubMed]
- Rizos, C.V.; Elisaf, M.S. Antihypertensive drugs and glucose metabolism. World J. Cardiol. 2014, 6, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Rosei, E.A.; Rizzoni, D. Metabolic profile of nebivolol, a beta-adrenoceptor antagonist with unique characteristics. Drugs 2007, 67, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Roebuck, A.; Furze, G.; Thompson, D.R. Health-related quality of life after myocardial infarction: An interview study. J. Adv. Nurs. 2001, 34, 787–794. [Google Scholar] [CrossRef]
- Gaalema, D.E.; Elliott, R.J.; Morford, Z.H.; Higgins, S.T.; Ades, P.A. Effect of socioeconomic status on propensity to change risk behaviors following myocardial infarction: Implications for healthy lifestyle medicine. Prog. Cardiovasc. Dis. 2017, 60, 159–168. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart disease and stroke statistics—2023 update: A report from the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar]
- Feng, H.-P.; Chien, W.C.; Cheng, W.T.; Chung, C.H.; Cheng, S.-M.; Tzeng, W.-C. Risk of anxiety and depressive disorders in patients with myocardial infarction: A nationwide population-based cohort study. Medicine 2016, 95, e4464. [Google Scholar] [CrossRef] [PubMed]
- Crane, P.B.; Abel, W.M.; McCoy, T.P. Fatigue and physical activity after myocardial infarction. Biol. Res. Nurs. 2015, 17, 276–284. [Google Scholar] [CrossRef]
- Horne, C.E.; Johnson, S.; Crane, P.B. Comparing comorbidity measures and fatigue post myocardial infarction. Appl. Nurs. Res. 2019, 45, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Minges, K.E.; Strait, K.M.; Owen, N.; Dunstan, D.W.; Camhi, S.M.; Lichtman, J.; Geda, M.; Dreyer, R.P.; Bueno, H.; Beltrame, J.F.; et al. Gender differences in physical activity following acute myocardial infarction in adults: A prospective, observational study. Eur. J. Prev. Cardiol. 2017, 24, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Thompson, D.R.; Ski, C.F.; Liu, M. Health-related quality of life and its associated factors in Chinese myocardial infarction patients. Eur. J. Prev. Cardiol. 2014, 21, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Ernstsen, L.; Rangul, V.; Nauman, J.; Nes, B.M.; Dalen, H.; Krokstad, S.; Lavie, C.J.; Blair, S.N.; Wisløff, U. Protective effect of regular physical activity on depression after myocardial infarction: The HUNT study. Am. J. Med. 2016, 129, 82–88. [Google Scholar] [CrossRef] [PubMed]
- White, S.; Bissell, P.; Anderson, C. A qualitative study of cardiac rehabilitation patients’ perspectives on making dietary changes. J. Hum. Nutr. Diet. 2011, 24, 122–127. [Google Scholar] [CrossRef]
- Ma, Y.; Li, W.; Olendzki, B.C.; Pagoto, S.L.; Merriam, P.A.; Chiriboga, D.E.; Griffith, J.A.; Bodenlos, J.; Wang, Y.; Ockene, I.S. Dietary quality 1 year after diagnosis of coronary heart disease. J. Am. Diet. Assoc. 2008, 108, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Plakht, Y.; Gilutz, H.; Shiyovich, A. Temporal trends in acute myocardial infarction: What about survival of hospital survivors? Disparities between STEMI & NSTEMI remain. Soroka Acute Myocardial Infarction II (SAMI-II) project. Int. J. Cardiol. 2016, 203, 1073–1081. [Google Scholar] [PubMed]
- Plakht, Y.; Shiyovich, A.; Weitzman, S.; Fraser, D.; Zahger, D.; Gilutz, H. A new risk score predicting 1- and 5-year mortality following acute myocardial infarction Soroka Acute Myocardial Infarction (SAMI) Project. Int. J. Cardiol. 2012, 154, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Plakht, Y.; Abu Tailakh, M.; Barabi, T.; Shiyovich, A. Ethnic disparities in emergency department utilization patterns in southern Israel: A population-based study. Intern. Emerg. Med. 2012, 7, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Plakht, Y.; Gilutz, H.; Arbelle, J.E.; Greenberg, D.; Shiyovich, A. Sex and Ethnic Disparities in Health-Related Outcomes Following Acute Myocardial Infarction in Israel. Isr. Med. Assoc. J. 2020, 22, 303–309. [Google Scholar]
- Plakht, Y.; Gilutz, H.; Shiyovich, A. Ethnical disparities in temporal trends of acute myocardial infarction (AMI) throughout a decade in Israel. Soroka acute myocardial infarction (SAMI-II) project. Int. J. Cardiol. 2016, 214, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Plakht, Y.; Shiyovich, A.; Weitzman, S.; Fraser, D.; Zahger, D.; Gilutz, H. Soroka acute myocardial infarction (SAMI) score predicting 10-year mortality following acute myocardial infarction. Int. J. Cardiol. 2012, 167, 3068–3070. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control. ICD-9-CM Diagnosis and Procedure Codes. 2014. Available online: https://www.cms.gov/Medicare/Coding/ICD9ProviderDiagnosticCodes/codes (accessed on 1 January 2024).
- Berberich, A.J.; Hegele, R.A. A Modern Approach to Dyslipidemia. Endocr. Rev. 2021, 43, 611–653. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control. Defining Adult Overweight and Obesity. 2020. Available online: https://www.lb7.uscourts.gov/documents/19-927URL1defining.pdf (accessed on 24 May 2023).
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Available online: https://www.who.int/publications/i/item/WHO-NMH-NHD-MNM-11.1 (accessed on 1 January 2024).
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Elyse Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Ligthart, S.; van Herpt, T.T.W.; Leening, M.J.G.; Kavousi, M.; Hofman, A.; Stricker, B.H.C.; van Hoek, M.; Sijbrands, E.J.G.; Franco, O.H.; Dehghan, A. Lifetime risk of developing impaired glucose metabolism and eventual progression from prediabetes to type 2 diabetes: A prospective cohort study. Lancet Diabetes Endocrinol. 2016, 4, 44–51. [Google Scholar] [CrossRef]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef]
- Cavagnolli, G.; Pimentel, A.L.; Freitas, P.A.; Gross, J.L.; Camargo, J.L. Effect of ethnicity on HbA1c levels in individuals without diabetes: Systematic review and meta-analysis. PLoS ONE 2017, 12, e0171315. [Google Scholar] [CrossRef]
- Mainous, A.G.; Rooks, B.J.; Wright, R.U.; Sumfest, J.M.; Carek, P.J. Diabetes Prevention in a U.S. Healthcare System: A Portrait of Missed Opportunities. Am. J. Prev. Med. 2022, 62, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Azadnajafabad, S.; Ahmadi, N.; Rezaei, N.; Rashidi, M.-M.; Moghaddam, S.S.; Mohammadi, E.; Abbasi-Kangevari, M.; Naderian, M.; Ghasemi, E.; Farzi, Y.; et al. Evaluation of the diabetes care cascade and compliance with WHO global coverage targets in Iran based on STEPS survey 2021. Sci. Rep. 2023, 13, 13528. [Google Scholar] [CrossRef]
- Gilutz, H.; Steinberg, S.; Zahger, D.; Grotto, I. Secondary prevention after myocardial infarction: It takes two (physician and patient) to tango. Int. J. Cardiol. 2014, 172, e259–e260. [Google Scholar] [CrossRef] [PubMed]
- Tamir, O.; Peleg, R.; Dreiher, J.; Abu-Hammad, T.; Abu Rabia, Y.; Abu Rashid, M.; Eisenberg, A.; Sibersky, D.; Kazanovich, A.; Khalil, E.; et al. Cardiovascular risk factors in the Bedouin population: Management and compliance. Isr. Med. Assoc. J. 2007, 9, 652–655. [Google Scholar] [PubMed]
- Benderly, M.; Chetrit, A.; Murad, H.; Abu-Saad, K.; Gillon-Keren, M.; Rogowski, O.; Sela, B.-A.; Kanety, H.; Harats, D.; Atamna, A.; et al. Cardiovascular health among two ethnic groups living in the same region: A population-based study. Int. J. Cardiol. 2017, 228, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Guh, D.P.; Zhang, W.; Bansback, N.; Amarsi, Z.; Birmingham, C.L.; Anis, A.H. The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health 2009, 9, 1–20. [Google Scholar] [CrossRef]
- Ridker, P.M. Targeting inflammatory pathways for the treatment of cardiovascular disease. Eur. Heart J. 2014, 35, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, hypertension, and cardiovascular disease: Clinical insights and vascular mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef]
- Jialal, I.; Bajaj, M. Therapy and clinical trials: Management of diabetic dyslipidemia. Curr. Opin. Lipidol. 2009, 20, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Schiffrin, E.L. Vascular endothelin in hypertension. Vasc. Pharmacol. 2005, 43, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Lambelet, M.; Terra, L.F.; Fukaya, M.; Meyerovich, K.; Labriola, L.; Cardozo, A.K.; Allagnat, F. Dysfunctional autophagy following exposure to pro-inflammatory cytokines contributes to pancreatic β-cell apoptosis. Cell Death Dis. 2018, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Smoking and Cardiovascular Disease. 2024. Available online: https://www.cdc.gov/tobacco/patient-care/care-settings/pdfs/cdc-osh-hcp-cardio-factsheet-508.pdf (accessed on 1 November 2024).
- Signorelli, S.S.; Anzaldi, M.; Fiore, V. Inflammation and peripheral arterial disease (PAD). Curr. Pharm. Des. 2012, 18, 4350–4357. [Google Scholar] [CrossRef]
- Shammas, N.W. Epidemiology, classification, and modifiable risk factors of peripheral arterial disease. Vasc. Health Risk Manag. 2007, 3, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sadoshima, J. Cardiomyopathy in obesity, insulin resistance and diabetes. J. Physiol. 2020, 598, 2977–2993. [Google Scholar] [CrossRef]
- Velez, M.; Kohli, S.; Sabbah, H.N. Animal models of insulin resistance and heart failure. Heart Fail. Rev. 2014, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bastien, M.; Poirier, P.; Lemieux, I.; Després, J.P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog. Cardiovasc. Dis. 2014, 56, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Polonski, L.; Gasior, M.; Gierlotka, M.; Osadnik, T.; Kalarus, Z.; Trusz-Gluza, M.; Zembala, M.; Wilczek, K.; Lekston, A.; Zdrojewski, T.; et al. A comparison of ST elevation versus non-ST elevation myocardial infarction outcomes in a large registry database: Are non-ST myocardial infarctions associated with worse long-term prognoses? Int. J. Cardiol. 2011, 152, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Aronson, D.; Rayfield, E.J.; Chesebro, J.H. Mechanisms determining course and outcome of diabetic patients who have had acute myocardial infarction. Ann. Intern. Med. 1997, 126, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Zaveri, M.P.; Perry, J.C.; Schuetz, T.M.; Memon, M.D.; Faiz, S.; Cancarevic, I. Diabetic Cardiomyopathy as a Clinical Entity: Is It a Myth? Cureus 2020, 12, e11100. [Google Scholar] [CrossRef]
- Rizza, V.; Tondi, L.; Patti, A.M.; Cecchi, D.; Lombardi, M.; Perone, F.; Ambrosetti, M.; Rizzo, M.; Cianflone, D.; Maranta, F. Diabetic cardiomyopathy: Pathophysiology, imaging assessment and therapeutical strategies. Int. J. Cardiol. Cardiovasc. Risk Prev. 2024, 23, 200338. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Ilic, S.; Cuspidi, C.; Ivanovic, B.; Bukarica, L.; Kostic, N.; Marjanovic, T.; Kocijancic, V.; Celic, V. Left and right atrial phasic function and deformation in untreated patients with prediabetes and type 2 diabetes mellitus. Int. J. Cardiovasc. Imaging 2015, 31, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.S.; Brownlee, M. Molecular and Cellular Mechanisms of Cardiovascular Disorders in Diabetes. Circ. Res. 2016, 118, 1808–1829. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xu, R.; Wang, J.R.; Xu, H.Y.; Fu, H.; Xie, L.J.; Yang, M.X.; Zhang, L.; Wen, L.-Y.; Liu, H.; et al. Association of myocardial fibrosis detected by late gadolinium-enhanced MRI with clinical outcomes in patients with diabetes: A systematic review and meta-analysis. BMJ Open 2022, 12, e055374. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Category | n (%) |
|---|---|---|
| Demographics | ||
| Age, years | <50 | 1017 (19.8) |
| 50–60 | 1220 (23.7) | |
| ≥60 | 2910 (56.5) | |
| Sex | Male | 3818 (74.2) |
| Ethnicity | Minorities (Arabs) | 829 (16.1) |
| Cardiac diseases | ||
| Cardiomegaly | 367 (7.1) | |
| Atrial fibrillation/flutter | 749 (14.6) | |
| CHF | 546 (10.6) | |
| Chronic pulmonary heart disease | 307 (6.0) | |
| History of MI | 580 (11.3) | |
| AV block | 165 (3.2) | |
| Cardiovascular risk factors | ||
| Renal diseases | 244 (4.7) | |
| Smoking | 2434 (47.3) | |
| PVD | 415 (8.1) | |
| Hypertension | 2384 (46.3) | |
| BMI, kg/m2 | No results | 3704 (72.0) |
| <30 | 1068 (74.1) | |
| ≥30 | 375 (25.9) | |
| Results of laboratory tests | ||
| HbA1C baseline, % | No results | 4119 (80.0) |
| <5.7 | 528 (10.3) | |
| 5.7–6.0 | 279 (5.4) | |
| ≥6.0 | 221 (4.3) | |
| LDL, mg/dL | No results | 560 (10.9) |
| <100 | 2031 (44.2) | |
| ≥100 | 2556 (55.7) | |
| Total cholesterol, mg/dL | No results | 361 (7.0) |
| <200 | 3523 (73.6) | |
| 200–240 | 903 (18.8) | |
| ≥240 | 360 (7.5) | |
| HDL, mg/dL | No results | 886 (17.2) |
| <40 | 2054 (48.2) | |
| 40–60 | 1899 (44.5) | |
| ≥60 | 308 (7.2) | |
| Triglycerides, mg/dL | No results | 370 (7.2) |
| <150 | 3008 (63.0) | |
| 150–200 | 991 (20.7) | |
| 200–500 | 745 (15.5) | |
| ≥500 | 33 (0.7) | |
| Other disorders | ||
| COPD | 334 (6.5) | |
| Neurological disorders | 557 (10.8) | |
| Malignancy | 139 (2.7) | |
| Anemia | 1881 (36.5) | |
| Clinical characteristics of AMI | ||
| Type of AMI | STEMI | 2788 (54.2) |
| Results of echocardiography (n = 4415, 85.8%) | ||
| Severe LV dysfunction | 343 (7.8) | |
| LV hypertrophy | 170 (3.9) | |
| Mitral regurgitation | 190 (4.3) | |
| Tricuspid regurgitation | 124 (2.8) | |
| Pulmonary hypertension | 222 (5.0) | |
| Results of angiography (n = 3971, 77.2%) | ||
| Measure of CAD | No or non-significant | 176 (4.4) |
| One vessel | 1222 (30.8) | |
| Two vessels | 1157 (29.1) | |
| Three vessels/LM | 1416 (35.7) | |
| No results | 1176 (29.6) | |
| Type of treatment | ||
| Type of treatment | Noninvasive | 1093 (21.2) |
| PCI | 3440 (66.8) | |
| CABG | 614 (11.9) | |
| Parameter | Category | Cumulative Incidence | p-Value |
|---|---|---|---|
| Demographics | |||
| Age, years | <50 | 0.599 | 0.04 |
| 50–60 | 0.538 | ||
| ≥60 | 0.451 | ||
| Sex | Female | 0.477 | 0.083 |
| Male | 0.547 | ||
| Ethnicity | Jews | 0.536 | 0.001 |
| Minorities (Arabs) | 0.569 | ||
| Cardiac diseases | |||
| Cardiomegaly | No | 0.541 | 0.003 |
| Yes | 0.503 | ||
| Atrial fibrillation/flutter | No | 0.533 | 0.029 |
| Yes | 0.63 | ||
| CHF | No | 0.544 | 0.022 |
| Yes | 0.55 | ||
| Chronic pulmonary heart disease | No | 0.541 | 0.127 |
| Yes | 0.438 | ||
| History of MI | No | 0.547 | 0.01 |
| Yes | 0.549 | ||
| AV block | No | 0.538 | 0.091 |
| Yes | 0.636 | ||
| Cardiovascular risk factors | |||
| Renal diseases | No | 0.542 | 0.322 |
| Yes | 0.416 | ||
| Smoking | No | 0.45 | 0.072 |
| Yes | 0.597 | ||
| PVD | No | 0.533 | <0.001 |
| Yes | 0.654 | ||
| Hypertension | No | 0.52 | <0.001 |
| Yes | 0.555 | ||
| BMI, kg/m2 | No results | 0.535 | <0.001 |
| <30 | 0.449 | ||
| ≥30 | 0.678 | ||
| Results of laboratory tests | |||
| HbA1C baseline, % | No results | 0.54 | <0.001 |
| <5.7 | 0.416 | ||
| 5.7–6.0 | 0.441 | ||
| ≥6 | 0.701 | ||
| LDL, mg/dL | No results | 0.839 | <0.001 |
| <100 | 0.551 | ||
| ≥100 | 0.525 | ||
| Total cholesterol, mg/dL | No results | 0.852 | 0.017 |
| <200 | 0.499 | ||
| 200–240 | 0.589 | ||
| ≥240 | 0.619 | ||
| HDL, mg/dL | No results | 0.605 | 0.028 |
| <40 | 0.552 | ||
| 40–60 | 0.514 | ||
| ≥60 | 0.631 | ||
| Triglycerides, mg/dL | No results | 0.853 | <0.001 |
| <150 | 0.43 | ||
| 150–200 | 0.682 | ||
| 200–500 | 0.654 | ||
| ≥500 | 0.845 | ||
| Other disorders | |||
| COPD | No | 0.543 | 0.009 |
| Yes | 0.483 | ||
| Neurological disorders | No | 0.541 | 0.197 |
| Yes | 0.486 | ||
| Malignancy | No | 0.542 | 0.814 |
| Yes | 0.336 | ||
| Anemia | No | 0.556 | 0.341 |
| Yes | 0.53 | ||
| Yes | 0.59 | ||
| Clinical characteristics of AMI | |||
| Type of AMI | NSTEMI | 0.557 | <0.001 |
| STEMI | 0.519 | ||
| Results of echocardiography | |||
| Severe LV dysfunction | No | 0.539 | 0.447 |
| Yes | 0.432 | ||
| LV hypertrophy | No | 0.535 | 0.022 |
| Yes | 0.595 | ||
| Mitral regurgitation | No | 0.534 | 0.006 |
| Yes | 0.667 | ||
| Tricuspid regurgitation | No | 0.537 | 0.115 |
| Yes | 0.447 | ||
| Pulmonary hypertension | No | 0.534 | 0.001 |
| Yes | 0.552 | ||
| Results of angiography | |||
| Measure of CAD | No or non-significant | 0.498 | 0.462 |
| One vessel | 0.554 | ||
| Two vessels | 0.445 | ||
| Three vessels/LM | 0.553 | ||
| No results | 0.605 | 0.001 | |
| Type of treatment | |||
| Type of treatment | Noninvasive | 0.621 | 0.002 |
| PCI | 0.544 | ||
| CABG | 0.401 | ||
| Parameter | Category | B (SE) | AdjHR | (95% CI) | p-Value |
|---|---|---|---|---|---|
| Age, years | <50 | 1 (ref.) | |||
| 50–60 | 0.191 (0.091) | 1.21 | (1.012; 1.448) | 0.037 | |
| ≥60 | 0.031 (0.096) | 1.031 | (0.854; 1.244) | 0.751 | |
| Ethnicity | Arabs vs. Jews | 0.250 (0.083) | 1.284 | (1.091; 1.511) | 0.003 |
| Cardiomegaly | Yes vs. No | 0.319 (0.124) | 1.373 | (1.078; 1.756) | 0.01 |
| History of MI | Yes vs. No | 0.221 (0.098) | 1.248 | (1.029; 1.513) | 0.024 |
| AV block | Yes vs. No | 0.479 (0.169) | 1.614 | (1.160; 2.246) | 0.005 |
| HbA1C baseline, % | No results | 0.065 (0.139) | 1.068 | (0.814; 1.401) | 0.637 |
| <5.7 | 1 (ref.) | ||||
| 5.7–6.0 | 0.654 (0.191) | 1.924 | (1.324; 2.795) | <0.001 | |
| ≥6.0 | 1.208 (0.180) | 3.346 | (2.353; 4.760) | <0.001 | |
| LDL, mg/dL | No results | 0.526 (0.126) | 1.692 | (1.321; 2.167) | <0.001 |
| <100 | 1 (ref.) | ||||
| ≥100 | 0.235 (0.072) | 1.264 | (1.098; 1.455) | 0.001 | |
| Hypertension | Yes vs. No | 0.310 (0.070) | 1.364 | (1.188; 1.565) | <0.001 |
| BMI, kg/m2 | No results | 0.120 (0.087) | 1.128 | (0.951; 1.337) | 0.167 |
| <30 | 1 (ref.) | <0.001 | |||
| ≥30 | 0.470 (0.128) | 1.599 | (1.245; 2.055) | <0.001 | |
| Smoking | Yes vs. No | 0.295 (0.073) | 1.343 | (1.164; 1.550) | <0.001 |
| PVD | Yes vs. No | 0.337 (0.117) | 1.401 | (1.114; 1.761) | 0.004 |
| Type of AMI | NSTEMI vs. STEMI | 0.210 (0.069) | 1.233 | (1.076; 1.413) | 0.003 |
| Mitral regurgitation | No results | 0.329 (0.123) | 1.389 | (1.092; 1.768) | 0.007 |
| No | 1 (ref.) | ||||
| Yes | 0.483 (0.165) | 1.622 | (1.173; 2.242) | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakubov, T.; Abu Tailakh, M.; Shiyovich, A.; Gilutz, H.; Plakht, Y. Incidence and Risk Factors for Developing Type 2 Diabetes Mellitus After Acute Myocardial Infarction—A Long-Term Follow-Up. J. Cardiovasc. Dev. Dis. 2025, 12, 89. https://doi.org/10.3390/jcdd12030089
Yakubov T, Abu Tailakh M, Shiyovich A, Gilutz H, Plakht Y. Incidence and Risk Factors for Developing Type 2 Diabetes Mellitus After Acute Myocardial Infarction—A Long-Term Follow-Up. Journal of Cardiovascular Development and Disease. 2025; 12(3):89. https://doi.org/10.3390/jcdd12030089
Chicago/Turabian StyleYakubov, Tamara, Muhammad Abu Tailakh, Arthur Shiyovich, Harel Gilutz, and Ygal Plakht. 2025. "Incidence and Risk Factors for Developing Type 2 Diabetes Mellitus After Acute Myocardial Infarction—A Long-Term Follow-Up" Journal of Cardiovascular Development and Disease 12, no. 3: 89. https://doi.org/10.3390/jcdd12030089
APA StyleYakubov, T., Abu Tailakh, M., Shiyovich, A., Gilutz, H., & Plakht, Y. (2025). Incidence and Risk Factors for Developing Type 2 Diabetes Mellitus After Acute Myocardial Infarction—A Long-Term Follow-Up. Journal of Cardiovascular Development and Disease, 12(3), 89. https://doi.org/10.3390/jcdd12030089







