Subtraction CT Angiography for the Evaluation of Lower Extremity Artery Disease with Severe Arterial Calcification
Abstract
:1. Introduction
2. Materials and Methods
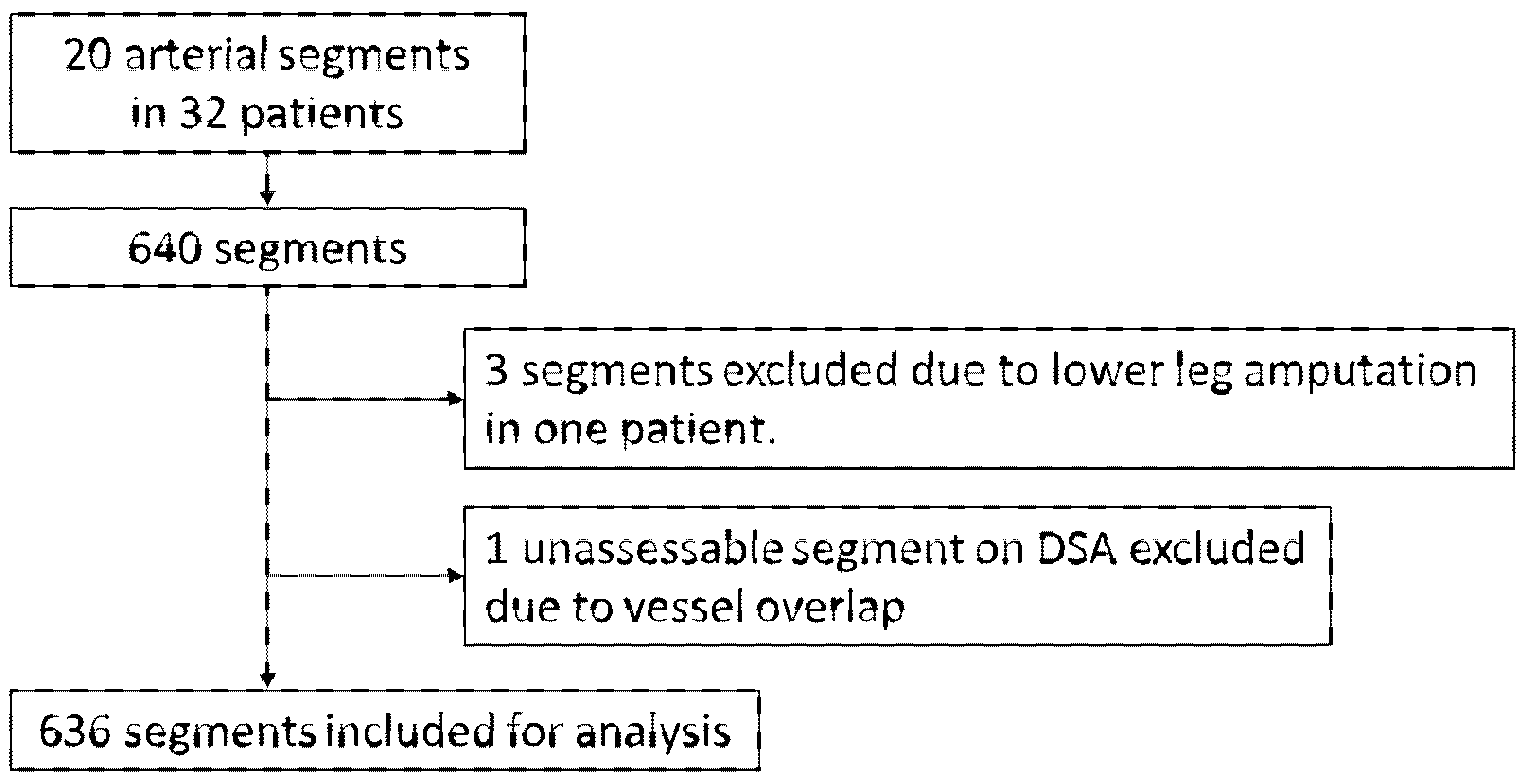
2.1. Study Design and Patient Population
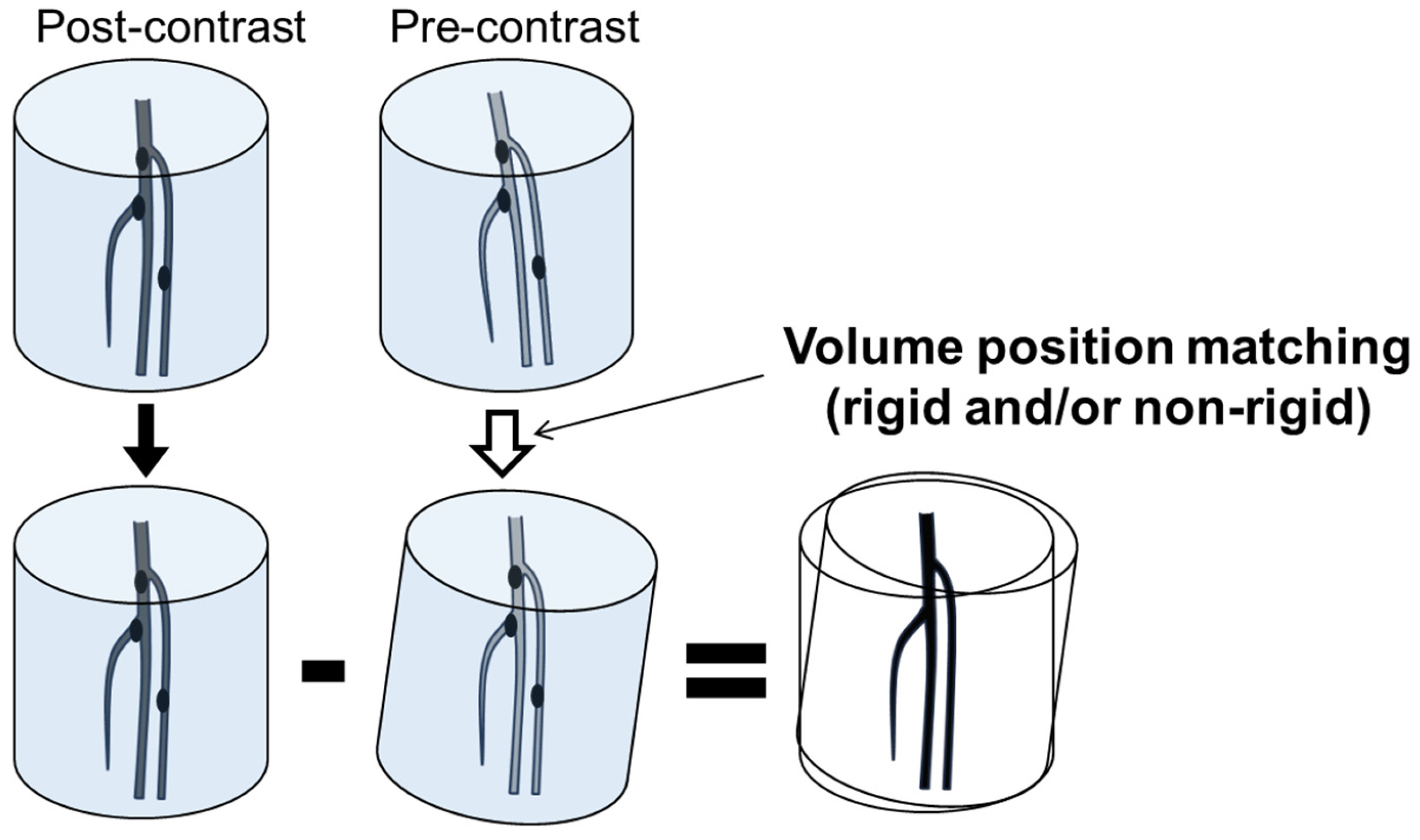
2.2. CT Angiography
2.3. Digital Subtraction Angiography
2.4. Image Analysis
2.5. Statistical Analysis
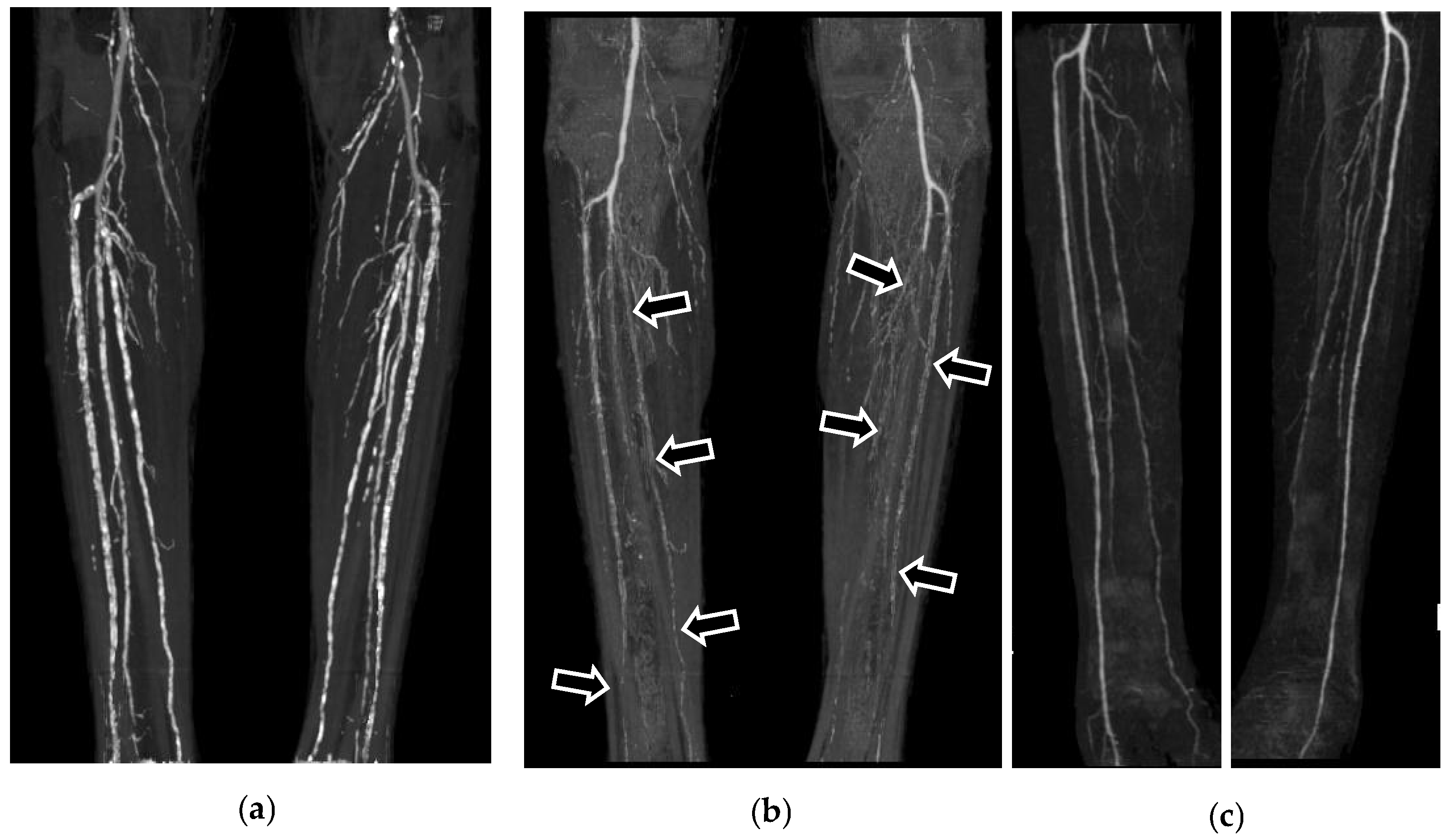
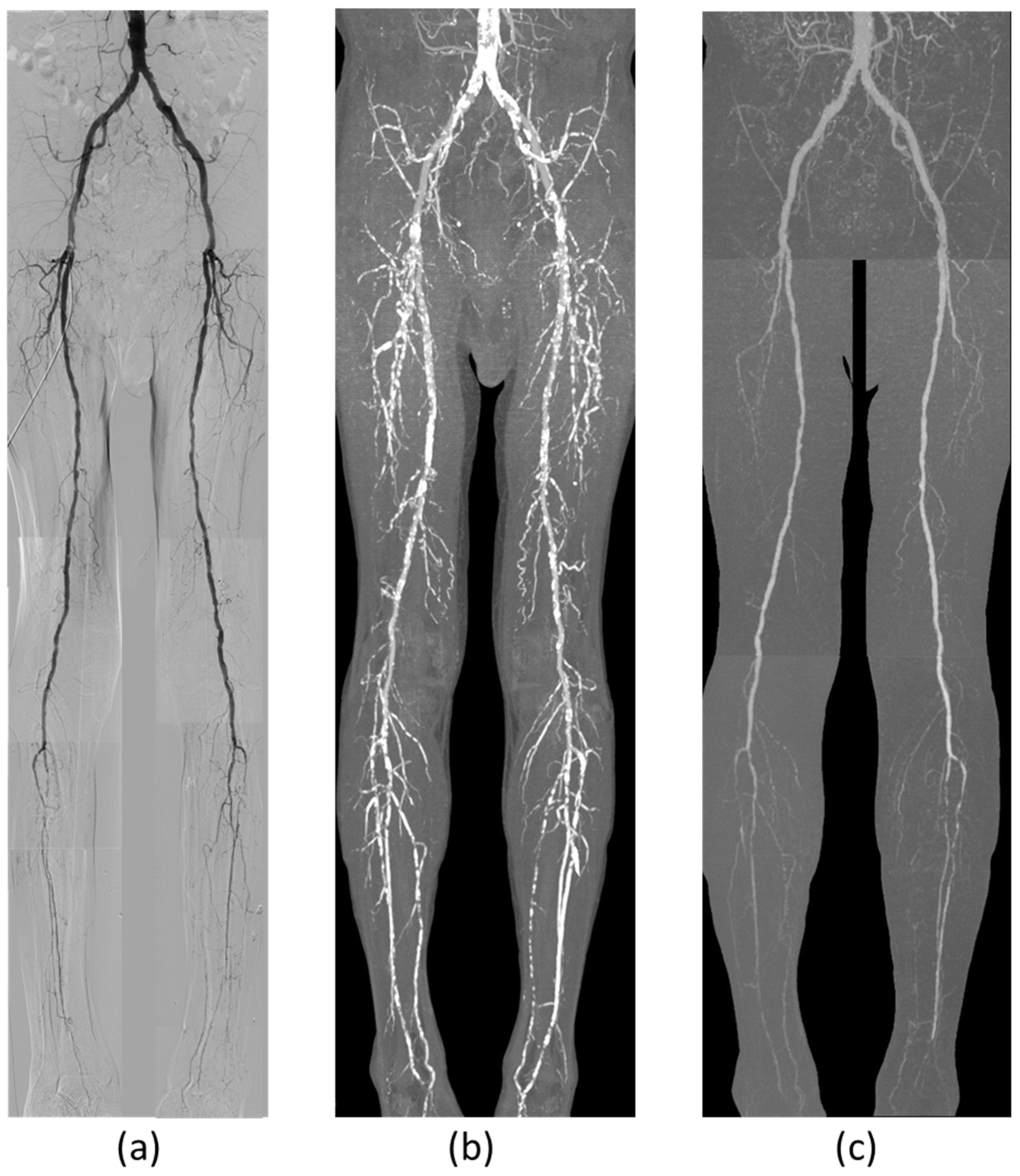
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTA | computed tomography angiography |
| LEAD | lower extremity artery disease |
| CLI | critical limb ischemia |
| DSA | digital subtraction angiography |
| MRA | magnetic resonance angiography |
| MIP | maximum intensity projection |
| ROC | receiver operating characteristic |
| AUC | area under the curve |
| SD | standard deviation |
| CI | confidence interval |
References
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Bjorck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: The European Stroke Organization (ESO) the Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef] [PubMed]
- Itoga, N.K.; Kim, T.; Sailer, A.M.; Fleischmann, D.; Mell, M.W. Lower extremity computed tomography angiography can help predict technical success of endovascular revascularization in the superficial femoral and popliteal artery. J. Vasc. Surg. 2017, 66, 835–843.e1. [Google Scholar] [CrossRef] [PubMed]
- Butt, T.; Lehti, L.; Apelqvist, J.; Gottsäter, A.; Acosta, S. Influence of diabetes on diagnostic performance of computed tomography angiography of the calf arteries in acute limb ischemia. Acta Radiol. 2021, 63, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.-J.; Won, J.-H.; Choi, H.-C.; Na, J.-B.; Kim, J.-E.; Park, M.-J.; Jo, S.-H.; Park, H.-O.; Lee, C.-E.; Kim, M.-J.; et al. Automatic Plaque Removal Using Dual-Energy Computed Tomography Angiography: Diagnostic Accuracy and Utility in Patients with Peripheral Artery Disease. Medicina 2022, 58, 1435. [Google Scholar] [CrossRef]
- Suzuki, M.; Tanaka, R.; Yoshioka, K.; Abiko, A.; Ehara, S. Subtraction CT angiography for the diagnosis of iliac arterial steno-occlusive disease. Jpn. J. Radiol. 2015, 34, 194–202. [Google Scholar] [CrossRef]
- Masuda, T.; Funama, Y.; Nakaura, T.; Sato, T.; Okimoto, T.; Masuda, S.; Yamashita, Y.; Yoshiura, T.; Noda, N.; Baba, Y.; et al. Diagnostic performance of computed tomography digital subtraction angiography of the lower extremities during haemodialysis in patients with suspected peripheral artery disease. Radiography 2021, 27, 888–896. [Google Scholar] [CrossRef]
- Imakita, S.; Onishi, Y.; Hashimoto, T.; Motosugi, S.; Kuribayashi, S.; Takamiya, M.; Hashimoto, N.; Yamaguchi, T.; Sawada, T. Subtraction ct angiography with controlled-orbit helical scanning for detection of intracranial aneurysms. AJNR Am. J. Neuroradiol. 1998, 19, 291–295. [Google Scholar]
- Nambu, K.; Suzuki, R.; Hirakawa, K. Cerebral blood flow: Measurement with xenon-enhanced dynamic helical CT. Radiology 1995, 195, 53–57. [Google Scholar] [CrossRef]
- Watanabe, Y.; Kashiwagi, N.; Yamada, N.; Higashi, M.; Fukuda, T.; Morikawa, S.; Onishi, Y.; Iihara, K.; Miyamoto, S.; Naito, H. Subtraction 3D CT Angiography with the Orbital Synchronized Helical Scan Technique for the Evaluation of Postoperative Cerebral Aneurysms Treated with Cobalt-Alloy Clips. Am. J. Neuroradiol. 2008, 29, 1071–1075. [Google Scholar] [CrossRef]
- Jurriaans, E.; Wells, I. Bolus chasing: A new technique in peripheral arteriography. Clin. Radiol. 1993, 48, 182–185. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2024. Available online: https://www.R-project.org/ (accessed on 20 January 2025).
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- McCullough, P.A. The Impact of Systemic Calcified Atherosclerosis in Patients With Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2008, 15, 335–337. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Agrawal, V.; Danielewicz, E.; Abela, G.S. Accelerated atherosclerotic calcification and Monckeberg’s sclerosis: A continuum of advanced vascular pathology in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1585–1598. [Google Scholar] [CrossRef]
- Amann, K. Media Calcification and Intima Calcification Are Distinct Entities in Chronic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1599–1605. [Google Scholar] [CrossRef]
- Blacher, J.; Guerin, A.P.; Pannier, B.; Marchais, S.J.; London, G.M. Arterial Calcifications, Arterial Stiffness, and Cardiovascular Risk in End-Stage Renal Disease. Hypertension 2001, 38, 938–942. [Google Scholar] [CrossRef]
- Heijenbrok-Kal, M.H.; Kock, M.C.J.M.; Hunink, M.G.M. Lower Extremity Arterial Disease: Multidetector CT Angiography—Meta-Analysis. Radiology 2007, 245, 433–439. [Google Scholar] [CrossRef]
- Hiatt, W.R. Medical Treatment of Peripheral Arterial Disease and Claudication. N. Engl. J. Med. 2001, 344, 1608–1621. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45 (Suppl. S), S5–S67. [Google Scholar] [CrossRef]
- Clorius, S.; Technau, K.; Watter, T.; Schwertfeger, E.; Fischer, K.-G.; Walz, G.; Gerke, P. Nephrogenic systemic fibrosis following exposure to gadolinium-containing contrast agent. Clin. Nephrol. 2007, 68, 249–252. [Google Scholar] [CrossRef]
- Thomaston, J.L.; Woldeyes, R.A.; Nakane, T.; Yamashita, A.; Tanaka, T.; Koiwai, K.; Brewster, A.S.; Barad, B.A.; Chen, Y.; Lemmin, T.; et al. XFEL structures of the influenza M2 proton channel: Room temperature water networks and insights into proton conduction. Proc. Natl. Acad. Sci. USA 2017, 114, 13357–13362. [Google Scholar] [CrossRef]
- Malikova, H.; Holesta, M. Gadolinium contrast agents—Are they really safe? J. Vasc. Access. 2017, 18, 1–7. [Google Scholar] [PubMed]
- Ota, H.; Takase, K.; Igarashi, K.; Chiba, Y.; Haga, K.; Saito, H.; Takahashi, S. MDCT Compared with Digital Subtraction Angiography for Assessment of Lower Extremity Arterial Occlusive Disease: Importance of Reviewing Cross-Sectional Images. Am. J. Roentgenol. 2004, 182, 201–209. [Google Scholar] [CrossRef]
- Kau, T.; Eicher, W.; Reiterer, C.; Niedermayer, M.; Rabitsch, E.; Senft, B.; Hausegger, K.A. Dual-energy CT angiography in peripheral arterial occlusive disease—Accuracy of maximum intensity projections in clinical routine and subgroup analysis. Eur. Radiol. 2011, 21, 1677–1686. [Google Scholar] [CrossRef]
- Klink, T.; Wilhelm, T.; Roth, C.; Heverhagen, J.T. Dual-Energy CTA in Patients with Symptomatic Peripheral Arterial Occlusive Disease: Study of Diagnostic Accuracy and Impeding Factors. Rofo-Fortschritte Geb. Rontgenstrahlen Bild. Verfahr. 2017, 189, 441–452. [Google Scholar] [CrossRef]
- Jakobs, T.F.; Wintersperger, B.J.; Becker, C.R. MDCT-imaging of peripheral arterial disease. Semin. Ultrasound CT MRI 2004, 25, 145–155. [Google Scholar] [CrossRef]

| Characteristic | Value |
|---|---|
| Age (y) | |
| Mean ± SD | 69.6 ± 10.8 |
| Range | 51–87 |
| Gender (n) | |
| Male | 28 (87.5%) |
| Female | 4 (12.5%) |
| Body | |
| Length (cm) | 160.8 ± 6.0 |
| Weight (kg) | 56.1 ± 10.4 |
| Body mass index | 21.7 ± 3.7 |
| Risk factors (n) | |
| Hypertension | 27 (84.4%) |
| Diabetes | 10 (31.3%) |
| Hypercholesterolemia | 13 (40.6%) |
| Smoking | 8 (25%) |
| Previous smoking history | 8 (25%) |
| Chronic renal failure | 10 (31.3%) |
| Hemodialysis | 6 (18.8%) |
| Fontaine classification (n) | |
| I | 1 (3.1%) |
| IIa | 8 (25%) |
| IIb | 17 (53.1%) |
| III | 2 (6.3%) |
| IV | 4 (12.5%) |
| Duration between CTA and DSA (days) | |
| Mean ± SD | 45.2 ± 22.0 |
| Range | 3–127 |
| Metrics | Overall (mGy⋅cm) | Without IR (mGy⋅cm) | With IR (mGy⋅cm) |
|---|---|---|---|
| Mean ± SD | 1707.5 ± 1050.4 | 2962.7 ± 117.8 | 977.7 ± 69.7 |
| Range | 589.0–3672.6 | 1456–3672 | 659–1826 |
| Characteristic | Conventional CTA | Subtraction CTA |
|---|---|---|
| Total number of segments | 636 | |
| Unassessable segments | 160 (25.2%) | 13 (2.0%) |
| Calcification | 152 (23.9%) | 6 (0.9%) |
| Metallic stent | 3 (0.5%) | 0 (0.0%) |
| Overlap of branches | 4 (0.6%) | 5 (0.8%) |
| Unclear margin | 1 (0.2%) | 2 (0.3%) |
| Region | Metrics | Conventional CTA | Subtraction CTA |
|---|---|---|---|
| Total | # of Missing Values | 160 (25.16%) | 13 (2.04%) |
| Accuracy | 0.657 | 0.885 | |
| Precision | 0.744 | 0.884 | |
| Recall | 0.675 | 0.936 | |
| F1 Score | 0.708 | 0.909 | |
| Correlation with DSA (R2) | 0.358 | 0.873 | |
| AUC (95% CI) * | 0.594 (0.546–0.642) | 0.913 (0.886–0.940) | |
| Iliac | # of Missing Values | 98 (51.31%) | 5 (2.62%) |
| Accuracy | 0.471 | 0.927 | |
| Precision | 0.741 | 0.971 | |
| Recall | 0.444 | 0.930 | |
| F1 Score | 0.555 | 0.950 | |
| AUC (95% CI) * | 0.343 (0.246–0.441) | 0.933 (0.876–0.990) | |
| Femoropopliteal | # of Missing Values | 52 (20.31%) | 6 (2.34%) |
| Accuracy | 0.707 | 0.891 | |
| Precision | 0.808 | 0.914 | |
| Recall | 0.768 | 0.934 | |
| F1 Score | 0.788 | 0.924 | |
| AUC (95% CI) * | 0.590 (0.500–0.680) | 0.902 (0.851–0.952) | |
| Below the Knee | # of Missing Values | 10 (5.29%) | 2 (1.06%) |
| Accuracy | 0.778 | 0.836 | |
| Precision | 0.633 | 0.689 | |
| Recall | 0.911 | 0.956 | |
| F1 Score | 0.747 | 0.808 | |
| AUC (95% CI) * | 0.830 (0.768–0.892) | 0.903 (0.857–0.950) | |
| CLI patients (6 cases, 111 segments) | |||
| Total | # of Missing Values | 49 (41.88%) | 8 (6.84%) |
| Accuracy | 0.513 | 0.889 | |
| Precision | 0.571 | 0.892 | |
| Recall | 0.438 | 0.906 | |
| F1 Score | 0.496 | 0.899 | |
| AUC (95% CI) * | 0.443 (0.339–0.547) | 0.854 (0.769–0.940) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, R.; Yoshioka, K. Subtraction CT Angiography for the Evaluation of Lower Extremity Artery Disease with Severe Arterial Calcification. J. Cardiovasc. Dev. Dis. 2025, 12, 131. https://doi.org/10.3390/jcdd12040131
Tanaka R, Yoshioka K. Subtraction CT Angiography for the Evaluation of Lower Extremity Artery Disease with Severe Arterial Calcification. Journal of Cardiovascular Development and Disease. 2025; 12(4):131. https://doi.org/10.3390/jcdd12040131
Chicago/Turabian StyleTanaka, Ryoichi, and Kunihiro Yoshioka. 2025. "Subtraction CT Angiography for the Evaluation of Lower Extremity Artery Disease with Severe Arterial Calcification" Journal of Cardiovascular Development and Disease 12, no. 4: 131. https://doi.org/10.3390/jcdd12040131
APA StyleTanaka, R., & Yoshioka, K. (2025). Subtraction CT Angiography for the Evaluation of Lower Extremity Artery Disease with Severe Arterial Calcification. Journal of Cardiovascular Development and Disease, 12(4), 131. https://doi.org/10.3390/jcdd12040131






