Echocardiographic Predictors of Postoperative Atrial Fibrillation After Cardiac Surgery: Assessing Atrial Mechanics for Risk Stratification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Preoperative Echocardiographic Atrial Function Assessment
2.3. Operative Procedure Characteristics
2.4. Statistical Analysis
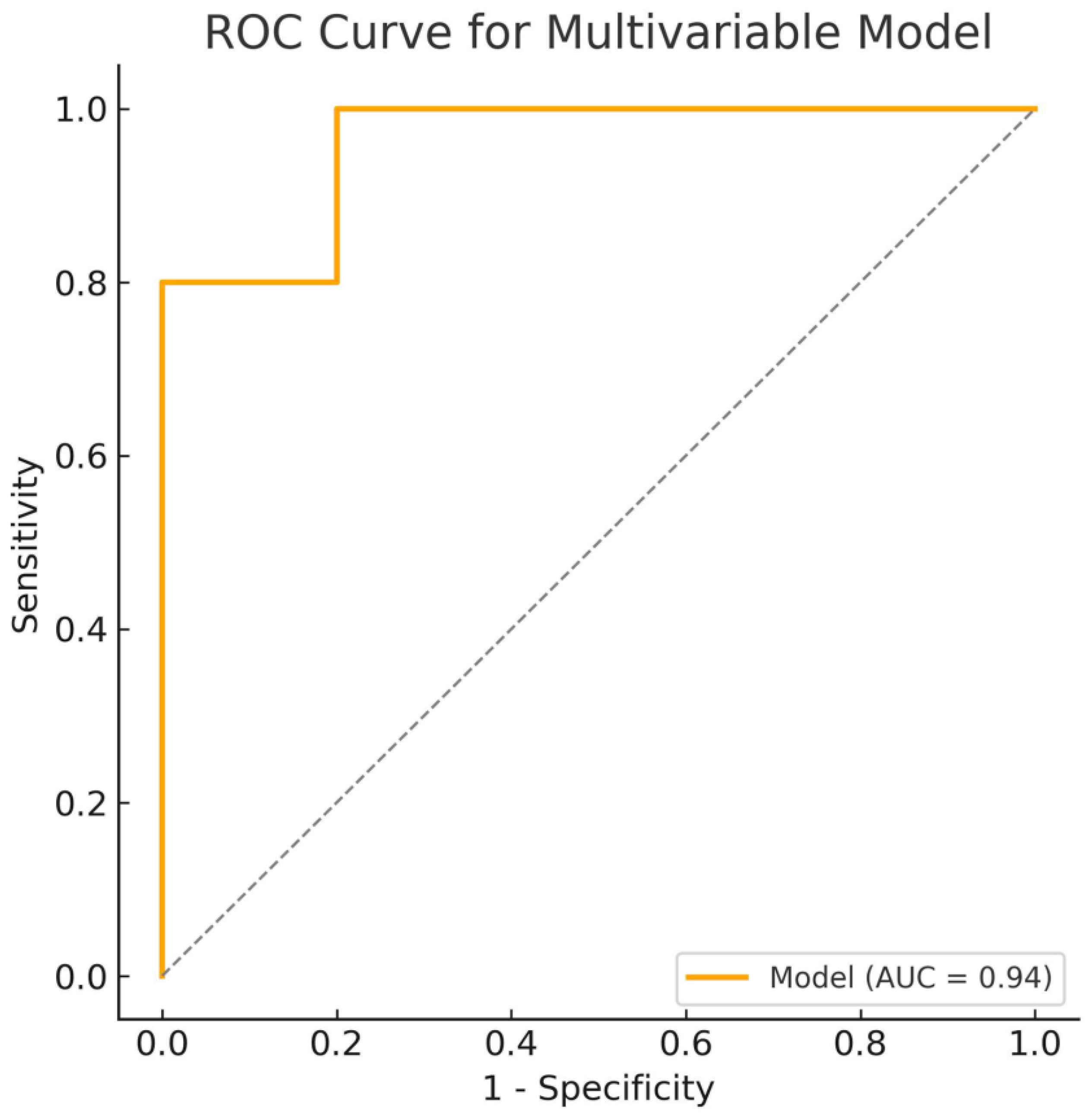
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [PubMed]
- Maisel, W.H.; Rawn, J.D.; Stevenson, W.G. Atrial fibrillation after cardiac surgery. Ann. Intern. Med. 2001, 135, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Ahlsson, A.; Fengsrud, E.; Bodin, L.; Englund, A. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur. J. Cardiothorac. Surg. 2010, 37, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Kosmidou, I.; Chen, S.; Kappetein, A.P.; Serruys, P.W.; Gersh, B.J.; Puskas, J.D.; Kandzari, D.E.; Taggart, D.P.; Morice, M.C.; Buszman, P.E.; et al. New-onset atrial fibrillation after PCI or CABG for left main disease: The EXCEL trial. J. Am. Col. Cardiol. 2018, 71, 739–748. [Google Scholar] [CrossRef]
- Aranki, S.F.; Shaw, D.P.; Adams, D.H.; Rizzo, R.J.; Couper, G.S.; VanderVliet, M.; Collins, J.J., Jr.; Cohn, L.H.; Burstin, H.R. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation 1996, 94, 390–397. [Google Scholar] [CrossRef]
- Mohamed Sabry, A.S.; El-Kader Mansour, H.A.; Abo El-Azm, T.H.; Sayed Akef, M.E.; Mostafa, S.A. Clinical and echocardiographic predictors of atrial fibrillation after coronary artery bypass grafting. J. Atr. Fibrillation 2020, 13, 2320. [Google Scholar]
- Pastore, M.C.; Degiovanni, A.; Grisafi, L.; Renda, G.; Sozzani, M.; Giordano, A.; Salvatici, C.; Lorenz, V.; Pierfelice, F.; Cappelli, C.; et al. Left atrial strain to predict postoperative atrial fibrillation in patients undergoing coronary artery bypass grafting. Circ. Cardiovasc. Imaging 2024, 17, e015969. [Google Scholar] [CrossRef]
- Inoue, K.; Kawakami, H.; Akazawa, Y.; Higashi, H.; Higaki, T.; Yamaguchi, O. Echocardiographic assessment of atrial function: From basic mechanics to specific cardiac diseases. J. Cardiovasc. Dev. Dis. 2022, 9, 68. [Google Scholar] [CrossRef]
- Jain, P.; Patel, V.; Patel, Y.; Rasool, J.; Gandhi, S.K.; Patel, P. Effectiveness of transesophageal echocardiography in preventing thromboembolic complications before cardioversion: A narrative review. Cureus 2023, 15, e48149. [Google Scholar] [CrossRef]
- Grant, M.C.; Crisafi, C.; Alvarez, A.; Arora, R.C.; Brindle, M.E.; Chatterjee, S.; Ender, J.; Fletcher, N.; Gregory, A.J.; Gunaydin, S.; et al. Perioperative care in cardiac surgery: A joint consensus statement by the Enhanced Recovery After Surgery (ERAS) Cardiac Society, ERAS International Society, and The Society of Thoracic Surgeons (STS). Ann. Thorac. Surg. 2024, 117, 669–689. [Google Scholar] [CrossRef]
- Perpétuo, L.; Barros, A.S.; Dalsuco, J.; Nogueira-Ferreira, R.; Resende-Gonçalves, P.; Falcão-Pires, I.; Ferreira, R.; Leite-Moreira, A.; Trindade, F.; Vitorino, R. Coronary Artery Disease and Aortic Valve Stenosis: A Urine Proteomics Study. Int. J. Mol. Sci. 2022, 23, 13579. [Google Scholar] [CrossRef] [PubMed]
- Horvath, B.; Kloesel, B.; Todd, M.M.; Cole, D.J.; Prielipp, R.C. The Evolution, Current Value, and Future of the American Society of Anesthesiologists Physical Status Classification System. Anesthesiology 2021, 135, 904–919. [Google Scholar] [CrossRef]
- The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels, 9th ed.; Little, Brown & Co.: Boston, MA, USA, 1994; pp. 253–256. [Google Scholar]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 16, 233–271. [Google Scholar]
- GlobalPRh. Available online: https://globalrph.com/medcalcs/beta-blockers/ (accessed on 15 December 2024).
- Matyal, R.; Mahmood, F.; Hess, P.; Zhao, X.; Mitchell, J.; Maslow, A.; Gangadharan, S.; Decamp, M. Right ventricular echocardiographic predictors of postoperative supraventricular arrhythmias after thoracic surgery: A pilot study. Ann. Thorac. Surg. 2010, 90, 1080–1086. [Google Scholar] [CrossRef]
- Shimony, A.; Afilalo, J.; Flynn, A.W.; Langleben, D.; Agnihotri, A.K.; Morin, J.F.; Shahian, D.M.; Picard, M.H.; Rudski, L.G. Usefulness of right ventricular dysfunction to predict new-onset atrial fibrillation following coronary artery bypass grafting. Am. J. Cardiol. 2014, 113, 913–918. [Google Scholar] [CrossRef]
- Zaman, J.A.; Harling, L.; Ashrafian, H.; Darzi, A.; Gooderham, N.; Athanasiou, T.; Peters, N.S. Post-operative atrial fibrillation is associated with a pre-existing structural and electrical substrate in human right atrial myocardium. Int. J. Cardiol. 2016, 220, 580–588. [Google Scholar] [CrossRef]
- Lomivorotov, V.V.; Efremov, S.M.; Pokushalov, E.A.; Karaskov, A.M. New-Onset Atrial Fibrillation After Cardiac Surgery: Pathophysiology, Prophylaxis, and Treatment. J. Cardiothorac. Vasc. Anesth. 2016, 30, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Aksu, U.; Kalkan, K.; Gulcu, O.; Aksakal, E.; Öztürk, M.; Topcu, S. The role of the right atrium in development of postoperative atrial fibrillation: A speckle tracking echocardiography study. J. Clin. Ultrasound 2019, 47, 470–476. [Google Scholar] [CrossRef]
- Hopman, L.H.G.A.; Mulder, M.J.; van der Laan, A.M.; Demirkiran, A.; Bhagirath, P.; van Rossum, A.C.; Allaart, C.P.; Götte, M.J.W. Impaired left atrial reservoir and conduit strain in patients with atrial fibrillation and extensive left atrial fibrosis. J. Cardiovasc. Magn. Reson. 2021, 23, 131. [Google Scholar] [CrossRef]
- Nardi, F.; Diena, M.; Caimmi, P.P.; Iraghi, G.; Lazzero, M.; Cerin, G.; Rossi, L.; Bongo, A.S.; Cernigliaro, C.; Lupi, A. Relationship between left atrial volume and atrial fibrillation following coronary artery bypass grafting. J. Card. Surg. 2012, 27, 128–135. [Google Scholar] [CrossRef]
- Sardana, M.; Lessard, D.; Tsao, C.W.; Parikh, N.I.; Barton, B.A.; Nah, G.; Thomas, R.C.; Cheng, S.; Schiller, N.B.; Aragam, J.R.; et al. Association of left atrial function index with atrial fibrillation and cardiovascular disease: The Framingham Offspring Study. J. Am. Heart Assoc. 2018, 7, e008435. [Google Scholar] [CrossRef] [PubMed]
- Çamcı, S.; Arı, H.; Arı, S.; Melek, M.; Bozat, T. The predictive value of the left atrial kinetic energy for atrial fibrillation recurrence. Cureus 2022, 14, e28714. [Google Scholar] [CrossRef] [PubMed]
- Kishima, H.; Mine, T.; Takahashi, S.; Ashida, K.; Ishihara, M.; Masuyama, T. Left atrial ejection force predicts the outcome after catheter ablation for paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2018, 29, 264–271. [Google Scholar] [CrossRef]
- Abdelrazek, G.; Mandour, K.; Osama, M.; Elkhashab, K. Strain and strain rate echocardiographic imaging predict occurrence of atrial fibrillation in post-coronary artery bypass grafting patients. Egypt. Heart J. 2021, 73, 62. [Google Scholar] [CrossRef] [PubMed]
- Kawczynski, M.J.; Gilbers, M.; Van De Walle, S.; Schalla, S.; Crijns, H.J.; Maessen, J.G.; Schotten, U.; Maesen, B.; Bidar, E. Role of pre-operative transthoracic echocardiography in predicting post-operative atrial fibrillation after cardiac surgery: A systematic review of the literature and meta-analysis. Europace 2021, 23, 1731–1743. [Google Scholar] [CrossRef]
- Sánchez, F.J.; Pueyo, E.; Diez, E.R. Strain Echocardiography to Predict Postoperative Atrial Fibrillation. Int. J. Mol. Sci. 2022, 23, 1355. [Google Scholar] [CrossRef]
| Parameters | Explanation |
|---|---|
| Left and right atrial kinetic energy (LAKE; RAKE) | Kinetic energy = ½ × stroke volume of each atrium × density of the blood × A − wave velocity. Stroke volume is calculated as the difference between pre-atrial contraction volume and the minimal volume of the corresponding atrium. For blood density, the accepted value is 1.06 g/cm3. |
| Left and right atrial ejection force (LAEF; RAEF) | Ejection force of each atrium is calculated as follows: ejection force = 0.53 × annular orifice area of corresponding atrioventricular valve × (A − wave velocity)2. |
| Left and right atrial active emptying fractions (LAAEF; RAAEF). | Active emptying fraction of each atrium is calculated as the difference between pre-atrial contraction volume and minimal volume divided by the pre-atrial contraction volume of the corresponding atrium. |
| Left and right atrial passive emptying fractions (LAPEF; RAPEF) | Passive emptying fraction of each atrium is calculated as the difference between maximal volume and pre-atrial contraction volume divided by the maximal volume of the corresponding atrium. |
| Left and right atrial total emptying fractions (LATEF; RATEF) | Total emptying fraction of each atrium is calculated as the difference between maximal volume and minimal volume divided by the maximal volume of the corresponding atrium. |
| Total atrial conduction time (TACT) | TACT = time from P-wave onset (ECG) to the peak of A-wave (TDI-derived atrial contraction wave) at the lateral mitral annulus. |
| Variable | With | Without | p |
|---|---|---|---|
| Age (years) | 68.70 ± 7.64 | 63.86 ± 7.91 | 0.001 |
| Gender (male) | 36 (76.6%) | 63 (75.0%) | 1.000 |
| Weight (kg) | 80.94 ± 13.68 | 80.40 ± 13.92 | 0.829 |
| Height (cm) | 168.28 ± 8.57 | 167.75 ± 8.92 | 0.740 |
| BSA (m2) | 1.94 ± 0.19 | 1.92 ± 0.20 | 0.607 |
| BMI (kg/m2) | 28.57 ± 4.35 | 28.60 ± 4.70 | 0.973 |
| HATCH score | 1.79 ± 0.88 | 1.56 ± 1.09 | 0.197 |
| CHA2DS2-VASc score | 2.91 ± 1.02 | 2.56 ± 1.21 | 0.076 |
| POAF score | 2.13 ± 1.15 | 1.54 ± 1.10 | 0.005 |
| AFRI | 2.02 ± 0.94 | 1.74 ± 0.78 | 0.084 |
| P (V1 lead) (mV) | −0.03 ± 0.07 | −0.04 ± 0.09 | 0.356 |
| P (avR lead) (mV) | −0.04 ± 0.12 | −0.06 ± 0.12 | 0.437 |
| P axis (°) | 41.81 ± 32.26 | 44.75 ± 36.22 | 0.633 |
| PR(Q) (ms) | 174.28 ± 22.23 | 164.23 ± 27.89 | 0.026 |
| Stable angina | 0.17 ± 0.38 | 0.22 ± 0.41 | 0.517 |
| Unstable angina | 28 (59.57%) | 41 (48.81%) | 0.32 |
| LMCA stenosis | 14 (29.79%) | 19 (22.62%) | 0.49 |
| Prior myocardial infarction | 35 (74.47%) | 55 (65.48%) | 0.39 |
| Prior PCI | 12 (25.53%) | 13 (15.48%) | 0.24 |
| Time from cardiac event (months) | 22.74 ± 61.67 | 14.76 ± 46.04 | 0.441 |
| Hypertension | 45 (95.74%) | 77 (91.67%) | 0.339 |
| Diabetes mellitus (OAD) | 12 (25.53%) | 27 (32.14%) | 0.55 |
| DMID | 8 (17.02%) | 7 (8.33%) | 0.23 |
| Hyperlipidemia | 26 (55.32%) | 50 (59.52%) | 0.78 |
| Smoking | 26 (55.32%) | 28 (33.33%) | 0.02 |
| Family history of cardiovascular diseases | 33 (70.21%) | 59 (70.24%) | 0.40 |
| Chronic kidney disease | 4 (8.51%) | 5 (5.95%) | 0.85 |
| Malignancy | 1 (2.13%) | 4 (4.76%) | 1.00 |
| Hypothyroidism | 4 (8.51%) | 4 (4.76%) | 0.78 |
| COPD | 15 (31.91%) | 10 (11.9%) | 0.63 |
| Peripheral vascular disease | 8 (17.02%) | 6 (7.14%) | 0.01 |
| Carotid stenosis | 6 (12.77%) | 10 (11.9%) | 0.14 |
| ARB | 0.02 ± 0.15 | 0.10 ± 0.30 | 1.00 |
| Beta-blocker dose (mg) | 8.16 ± 17.53 | 3.05 ± 2.40 | 0.052 |
| ACE inhibitor | 34 (72.34%) | 55 (65.48%) | 0.54 |
| Calcium antagonist | 16 (34.04%) | 29 (34.52%) | 1.00 |
| Statin | 37 (78.72%) | 62 (73.81%) | 0.68 |
| Nitroglycerin | 27 (57.45%) | 33 (39.29%) | 0.07 |
| SGLT 2 inhibitors | 9 (19.15%) | 19 (22.62%) | 0.81 |
| ASA | 41 (87.23%) | 70 (83.33%) | 0.73 |
| P2Y12 inhibitors | 18 (38.3%) | 39 (46.43%) | 0.47 |
| Oral anticoagulants | 2 (4.26%) | 3 (3.57%) | 1.00 |
| Amiodarone | 4 (8.51%) | 7 (8.33%) | 1.00 |
| Trimetazidine | 15 (31.91%) | 29 (34.52%) | 0.91 |
| Loop diuretic | 11 (23.4%) | 21 (25.0%) | 1.00 |
| Thiazide diuretics | 10 (21.28%) | 13 (15.48%) | 0.55 |
| Aldosterone receptor antagonists | 15 (31.91%) | 20 (23.81%) | 0.42 |
| Levosimendan | 8 (17.02%) | 8 (9.52%) | 0.33 |
| Variable | With | Without | p |
|---|---|---|---|
| maxLAV (mL) | 75.65 ± 14.79 | 68.86 ± 16.05 | 0.0183 |
| maxLAVi (mL/m2) | 39.09 ± 7.26 | 36.08 ± 9.06 | 0.0439 |
| minLAV(mL) | 54.48 ± 13.05 | 39.62 ± 15.81 | <0.001 |
| minLAVi (mL/m2) | 28.19 ± 6.52 | 20.73 ± 8.55 | <0.001 |
| pacLAV (mL) | 60.24 ± 13.83 | 52.30 ± 15.12 | 0.0035 |
| pacLAVi (mL/m2) | 31.17 ± 6.91 | 27.37 ± 8.20 | 0.0067 |
| MI E-wave (cm/s) | 0.59 ± 0.15 | 0.81 ± 0.23 | <0.001 |
| MI A-wave (cm/s) | 0.42 ± 0.25 | 0.84 ± 0.20 | <0.001 |
| MI E/A ratio | 1.78 ± 0.69 | 1.00 ± 0.36 | <0.001 |
| MAOA (cm2) | 4.07 ± 0.12 | 4.63 ± 0.21 | <0.001 |
| TACT (ms) | 141.93 ± 15.23 | 103.59 ± 7.73 | <0.001 |
| maxRAV (mL) | 58.89 ± 10.61 | 46.03 ± 11.87 | <0.001 |
| maxRAVi (mL/m2) | 30.52 ± 5.31 | 24.03 ± 6.21 | <0.001 |
| minRAV (mL) | 42.48 ± 10.26 | 23.94 ± 7.06 | <0.001 |
| minRAVi (mL/m2) | 22.00 ± 5.12 | 12.50 ± 3.71 | <0.001 |
| pacRAV (mL) | 47.39 ± 9.88 | 33.41 ± 8.29 | <0.001 |
| pacRAVi (mL/m2) | 24.57 ± 4.98 | 17.48 ± 4.57 | <0.001 |
| TAOA (cm2) | 7.19 ± 0.31 | 8.13 ± 0.34 | <0.001 |
| MAPSE (mm) | 13.41 ± 5.08 | 14.75 ± 3.61 | 0.12 |
| TAPSE (mm) | 16.78 ± 4.17 | 20.09 ± 3.09 | <0.001 |
| TI E-wave(cm/s) | 0.51 ± 0.08 | 0.54 ± 0.15 | 0.15 |
| TI A-wave (cm/s) | 0.31 ± 0.15 | 0.47 ± 0.08 | <0.001 |
| TI E/A ratio | 1.91 ± 0.64 | 1.17 ± 0.30 | <0.001 |
| LATEV (mL) | 21.17 ± 8.20 | 29.24 ± 6.60 | <0.001 |
| LATEVi (mL/m2) | 10.68 ± 4.30 | 14.98 ± 4.38 | <0.001 |
| LATEF (%) | 0.28 ± 0.10 | 0.44 ± 0.11 | <0.001 |
| LAPEV (mL) | 15.41 ± 7.63 | 16.56 ± 6.79 | 0.40 |
| LAPEVi (mL/m2) | 7.76 ± 3.89 | 8.50 ± 4.09 | 0.31 |
| LAPEF (%) | 0.20 ± 0.09 | 0.24 ± 0.09 | 0.02 |
| LAAEV (mL) | 5.76 ± 2.09 | 12.68 ± 5.83 | <0.001 |
| LAAEVi (mL/m2) | 2.92 ± 1.15 | 6.25 ± 3.43 | <0.001 |
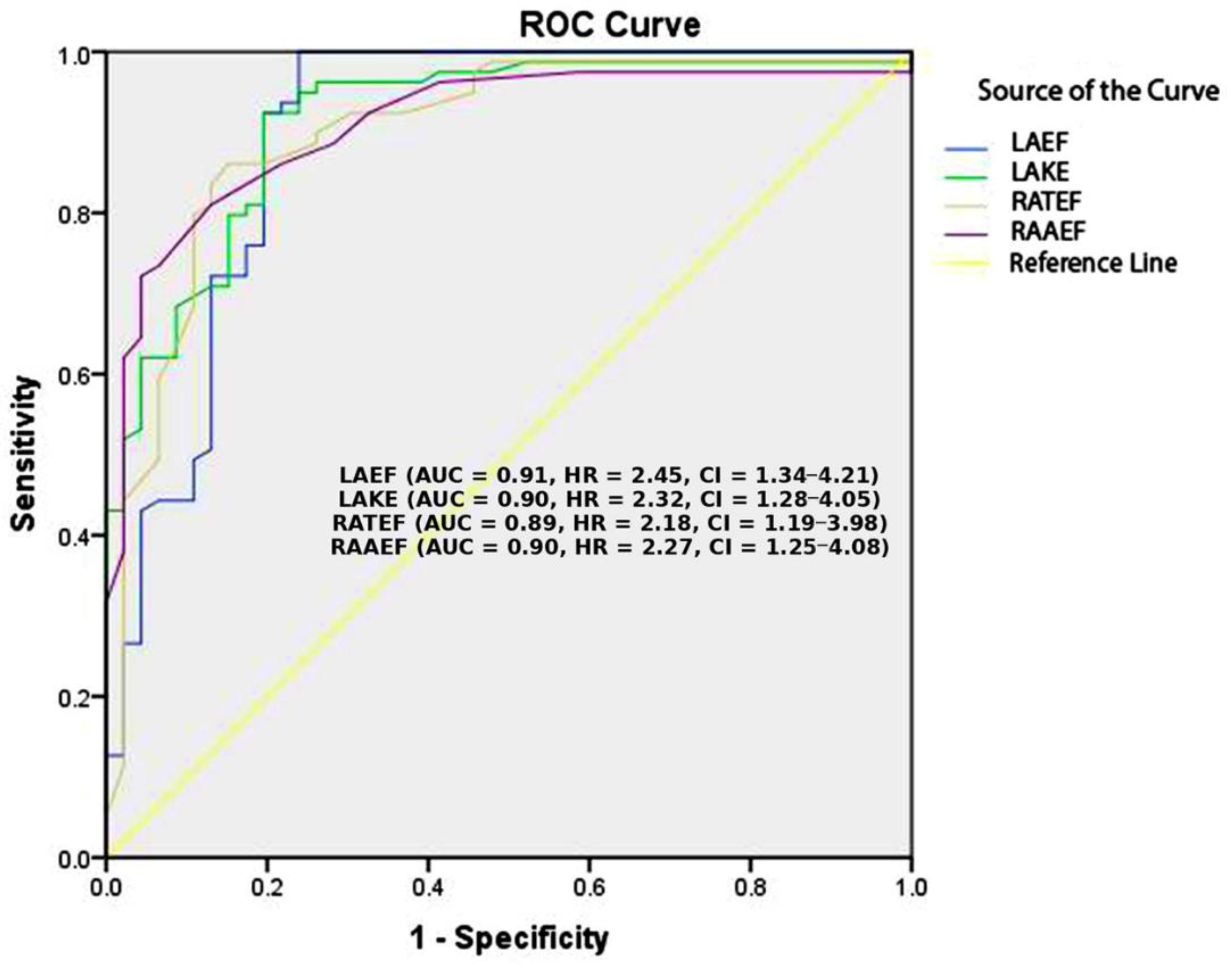
| LAAEF (%) | 0.10 ± 0.03 | 0.26 ± 0.12 | <0.001 |
| LAEF (kdyne) | 0.51 ± 0.65 | 1.85 ± 0.94 | <0.001 |
| LAKE (kdyne·cm) | 0.91 ± 1.36 | 5.13 ± 3.40 | <0.001 |
| RATEV (mL) | 16.41 ± 5.28 | 22.09 ± 8.96 | <0.001 |
| RATEVi (mL/m2) | 8.34 ± 2.96 | 10.84 ± 5.33 | <0.001 |
| RATEF (%) | 0.28 ± 0.09 | 0.47 ± 0.11 | <0.001 |
| RAPEV (mL) | 11.50 ± 4.82 | 12.62 ± 7.72 | 0.32 |
| RAPEVi (mL/m2) | 5.83 ± 2.55 | 6.16 ± 4.16 | 0.57 |
| RAPEF (%) | 0.20 ± 0.08 | 0.26 ± 0.10 | <0.001 |
| RAAEV (mL) | 4.91 ± 1.03 | 9.47 ± 4.82 | <0.001 |
| RAAEVi (mL/m2) | 2.51 ± 0.72 | 4.68 ± 2.79 | <0.001 |
| RAAEF (%) | 0.11 ± 0.05 | 0.28 ± 0.12 | <0.001 |
| RAEF (kdyne) | 0.44 ± 0.47 | 0.84 ± 0.34 | <0.001 |
| RAKE (kdyne.cm) | 0.32 ± 0.38 | 1.11 ± 0.63 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perić, V.; Golubović, M.; Stošić, M.; Milić, D.; Lazović, L.; Stojanović, D.; Lazarević, M.; Marković, D.; Unić-Stojanović, D. Echocardiographic Predictors of Postoperative Atrial Fibrillation After Cardiac Surgery: Assessing Atrial Mechanics for Risk Stratification. J. Cardiovasc. Dev. Dis. 2025, 12, 160. https://doi.org/10.3390/jcdd12040160
Perić V, Golubović M, Stošić M, Milić D, Lazović L, Stojanović D, Lazarević M, Marković D, Unić-Stojanović D. Echocardiographic Predictors of Postoperative Atrial Fibrillation After Cardiac Surgery: Assessing Atrial Mechanics for Risk Stratification. Journal of Cardiovascular Development and Disease. 2025; 12(4):160. https://doi.org/10.3390/jcdd12040160
Chicago/Turabian StylePerić, Velimir, Mlađan Golubović, Marija Stošić, Dragan Milić, Lela Lazović, Dalibor Stojanović, Milan Lazarević, Dejan Marković, and Dragana Unić-Stojanović. 2025. "Echocardiographic Predictors of Postoperative Atrial Fibrillation After Cardiac Surgery: Assessing Atrial Mechanics for Risk Stratification" Journal of Cardiovascular Development and Disease 12, no. 4: 160. https://doi.org/10.3390/jcdd12040160
APA StylePerić, V., Golubović, M., Stošić, M., Milić, D., Lazović, L., Stojanović, D., Lazarević, M., Marković, D., & Unić-Stojanović, D. (2025). Echocardiographic Predictors of Postoperative Atrial Fibrillation After Cardiac Surgery: Assessing Atrial Mechanics for Risk Stratification. Journal of Cardiovascular Development and Disease, 12(4), 160. https://doi.org/10.3390/jcdd12040160






