Lipoprotein(a): Assessing the Current Knowledge and Gaps in Screening and Treatment—A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection
3. Lipoprotein(a): Structure and Pathophysiology
4. Cardiovascular Risk
5. Prevention
6. Lp(a) Assessment
7. Treatment Options
7.1. Niacin
7.2. Statins
7.3. Lp(a) Apheresis
7.4. PCSK9-i
7.5. Nucleic Acid-Based Gene Silencing
7.5.1. Pelacarsen
7.5.2. Inclisiran
7.5.3. Olpasiran
7.5.4. Lepodisiran
7.5.5. Small-Molecule Inhibitors
8. Discussions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nedkoff, L.; Briffa, T.; Zemedikun, D.; Herrington, S.; Wright, F.L. Global Trends in Atherosclerotic Cardiovascular Disease. Clin. Ther. 2023, 45, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Lyngbakken, M.N.; Myhre, P.L.; Røsjø, H.; Omland, T. Novel Biomarkers of Cardiovascular Disease: Applications in Clinical Practice. Crit. Rev. Clin. Lab. Sci. 2019, 56, 33–60. [Google Scholar] [CrossRef] [PubMed]
- Geovanini, G.R.; Libby, P. Atherosclerosis and Inflammation: Overview and Updates. Clin. Sci. 2018, 132, 1243–1252. [Google Scholar] [CrossRef]
- Tasdighi, E.; Adhikari, R.; Almaadawy, O.; Leucker, T.M.; Blaha, M.J. LP(a): Structure, Genetics, Associated Cardiovascular Risk, and Emerging Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2024, 64, 135–157. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Edelberg, J.M.; Goodman, S.G.; Hanotin, C.; Harrington, R.A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef]
- Malick, W.A.; Goonewardena, S.N.; Koenig, W.; Rosenson, R.S. Clinical Trial Design for Lipoprotein(a)-Lowering Therapies: JACC Focus Seminar 2/3. J. Am. Coll. Cardiol. 2023, 81, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Wolski, K.; Cho, L.; Nicholls, S.J.; Kastelein, J.; Leitersdorf, E.; Landmesser, U.; Blaha, M.; Lincoff, A.M.; Morishita, R.; et al. Lipoprotein(a) Levels in a Global Population with Established Atherosclerotic Cardiovascular Disease. Open Heart 2022, 9, e002060. [Google Scholar] [CrossRef]
- Catapano, A.L.; Tokgözoğlu, L.; Banach, M.; Gazzotti, M.; Olmastroni, E.; Casula, M.; Ray, K.K. Lipid Clinics Network Group Evaluation of Lipoprotein(a) in the Prevention and Management of Atherosclerotic Cardiovascular Disease: A Survey among the Lipid Clinics Network. Atherosclerosis 2023, 370, 5–11. [Google Scholar] [CrossRef]
- Boffa, M.B. Beyond Fibrinolysis: The Confounding Role of Lp(a) in Thrombosis. Atherosclerosis 2022, 349, 72–81. [Google Scholar] [CrossRef]
- Vuorio, A.; Watts, G.F.; Schneider, W.J.; Tsimikas, S.; Kovanen, P.T. Familial Hypercholesterolemia and Elevated Lipoprotein(a): Double Heritable Risk and New Therapeutic Opportunities. J. Intern. Med. 2020, 287, 2–18. [Google Scholar] [CrossRef]
- Duarte Lau, F.; Giugliano, R.P. Lipoprotein(a) and Its Significance in Cardiovascular Disease: A Review. JAMA Cardiol. 2022, 7, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F.; Mora, S.; Stroes, E.S.G.; Ference, B.A.; Arsenault, B.J.; Berglund, L.; Dweck, M.R.; Koschinsky, M.; Lambert, G.; Mach, F.; et al. Lipoprotein(a) in Atherosclerotic Cardiovascular Disease and Aortic Stenosis: A European Atherosclerosis Society Consensus Statement. Eur. Heart J. 2022, 43, 3925–3946. [Google Scholar] [CrossRef] [PubMed]
- Vinci, P.; Di Girolamo, F.G.; Panizon, E.; Tosoni, L.M.; Cerrato, C.; Pellicori, F.; Altamura, N.; Pirulli, A.; Zaccari, M.; Biasinutto, C.; et al. Lipoprotein(a) as a Risk Factor for Cardiovascular Diseases: Pathophysiology and Treatment Perspectives. Int. J. Environ. Res. Public health 2023, 20, 6721. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F. Lipoprotein(a). In Prevention and Treatment of Atherosclerosis: Improving State-of-the-Art Management and Search for Novel Targets; von Eckardstein, A., Binder, C.J., Eds.; Springer: Cham, Switzerland, 2022; ISBN 978-3-030-86075-2. [Google Scholar]
- Coassin, S.; Kronenberg, F. Lipoprotein(a) beyond the Kringle IV Repeat Polymorphism: The Complexity of Genetic Variation in the LPA Gene. Atherosclerosis 2022, 349, 17–35. [Google Scholar] [CrossRef]
- Enkhmaa, B.; Anuurad, E.; Berglund, L. Lipoprotein (a): Impact by Ethnicity and Environmental and Medical Conditions. J. Lipid Res. 2016, 57, 1111–1125. [Google Scholar] [CrossRef]
- Awad, K.; Mahmoud, A.K.; Abbas, M.T.; Alsidawi, S.; Ayoub, C.; Arsanjani, R.; Farina, J.M. Intra-Individual Variability in Lipoprotein(a) Levels: Findings from a Large Academic Health System Population. Eur. J. Prev. Cardiol. 2024, zwae341. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Langsted, A. Lipoprotein(a) and Cardiovascular Disease. Lancet 2024, 404, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Orsó, E.; Schmitz, G. Lipoprotein(a) and Its Role in Inflammation, Atherosclerosis and Malignancies. Clin. Res. Cardiol. Suppl. 2017, 12, 31–37. [Google Scholar] [CrossRef]
- Labudovic, D.; Kostovska, I.; Tosheska Trajkovska, K.; Cekovska, S.; Brezovska Kavrakova, J.; Topuzovska, S. Lipoprotein(a)—Link between Atherogenesis and Thrombosis. Prague Med. Rep. 2019, 120, 39–51. [Google Scholar] [CrossRef]
- Ugovšek, S.; Šebeštjen, M. Lipoprotein(a)—The Crossroads of Atherosclerosis, Atherothrombosis and Inflammation. Biomolecules 2021, 12, 26. [Google Scholar] [CrossRef]
- Enas, E.A.; Varkey, B.; Dharmarajan, T.S.; Pare, G.; Bahl, V.K. Lipoprotein(a): An Independent, Genetic, and Causal Factor for Cardiovascular Disease and Acute Myocardial Infarction. Indian Heart J. 2019, 71, 99–112. [Google Scholar] [CrossRef]
- Bu, L.-L.; Yuan, H.-H.; Xie, L.-L.; Guo, M.-H.; Liao, D.-F.; Zheng, X.-L. New Dawn for Atherosclerosis: Vascular Endothelial Cell Senescence and Death. Int. J. Mol. Sci. 2023, 24, 15160. [Google Scholar] [CrossRef]
- Emdin, C.A.; Khera, A.V.; Natarajan, P.; Klarin, D.; Won, H.-H.; Peloso, G.M.; Stitziel, N.O.; Nomura, A.; Zekavat, S.M.; Bick, A.G.; et al. Phenotypic Characterization of Genetically Lowered Human Lipoprotein(a) Levels. J. Am. Coll. Cardiol. 2016, 68, 2761–2772. [Google Scholar] [CrossRef]
- Kamstrup, P.R.; Nordestgaard, B.G. Elevated Lipoprotein(a) Levels, LPA Risk Genotypes, and Increased Risk of Heart Failure in the General Population. JACC Heart Fail. 2016, 4, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.M.; Kamstrup, P.R.; Langsted, A.; Varbo, A.; Nordestgaard, B.G. Lipoprotein(a)-Lowering by 50 Mg/dL (105 Nmol/L) May Be Needed to Reduce Cardiovascular Disease 20% in Secondary Prevention. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Nurmohamed, N.S.; Moriarty, P.M.; Stroes, E.S. Considerations for Routinely Testing for High Lipoprotein(a). Curr. Opin. Lipidol. 2023, 34, 174. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Ridker, P.M.; Nestel, P.J.; Simes, J.; Tonkin, A.M.; Pedersen, T.R.; Schwartz, G.G.; Olsson, A.G.; Colhoun, H.M.; Kronenberg, F.; et al. Baseline and On-Statin Treatment Lipoprotein(a) Levels for Prediction of Cardiovascular Events: Individual Patient-Data Meta-Analysis of Statin Outcome Trials. Lancet 2018, 392, 1311–1320. [Google Scholar] [CrossRef]
- Tsimikas, S. Lipoprotein(a) and Coronary Calcium. JACC 2022, 79, 769–771. [Google Scholar] [CrossRef]
- Jackson, C.L.; Garg, P.K.; Guan, W.; Tsai, M.Y.; Criqui, M.H.; Tsimikas, S.; Bhatia, H.S. Lipoprotein(a) and Coronary Artery Calcium in Comparison with Other Lipid Biomarkers: The Multi-Ethnic Study of Atherosclerosis. J. Clin. Lipidol. 2023, 17, 538–548. [Google Scholar] [CrossRef]
- Bhatia, H.S. Aspirin and Lipoprotein(a) in Primary Prevention. Curr. Opin. Lipidol. 2023, 34, 214–220. [Google Scholar] [CrossRef]
- Cui, K.; Wang, H.-Y.; Yin, D.; Zhu, C.; Song, W.; Wang, H.; Jia, L.; Zhang, D.; Song, C.; Feng, L.; et al. Benefit and Risk of Prolonged Dual Antiplatelet Therapy After Percutaneous Coronary Intervention With Drug-Eluting Stents in Patients With Elevated Lipoprotein(a) Concentrations. Front. Cardiovasc. Med. 2021, 8, 807925. [Google Scholar] [CrossRef] [PubMed]
- Marcovina, S.M.; Koschinsky, M.L.; Albers, J.J.; Skarlatos, S. Report of the National Heart, Lung, and Blood Institute Workshop on Lipoprotein(a) and Cardiovascular Disease: Recent Advances and Future Directions. Clin. Chem. 2003, 49, 1785–1796. [Google Scholar] [CrossRef]
- Minelli, S.; Minelli, P.; Montinari, M.R. Reflections on Atherosclerosis: Lesson from the Past and Future Research Directions. J. Multidiscip. Healthc. 2020, 13, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, S.; Tan, J.; Wei, L.; Wu, D.; Gao, S.; Weng, Y.; Chen, J. Recent Advance in Treatment of Atherosclerosis: Key Targets and Plaque-Positioned Delivery Strategies. J. Tissue Eng. 2022, 13, 20417314221088509. [Google Scholar] [CrossRef]
- Pirillo, A.; Bonacina, F.; Norata, G.D.; Catapano, A.L. The Interplay of Lipids, Lipoproteins, and Immunity in Atherosclerosis. Curr. Atheroscler. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Marcovina, S.M.; Albers, J.J.; Scanu, A.M.; Kennedy, H.; Giaculli, F.; Berg, K.; Couderc, R.; Dati, F.; Rifai, N.; Sakurabayashi, I.; et al. Use of a Reference Material Proposed by the International Federation of Clinical Chemistry and Laboratory Medicine to Evaluate Analytical Methods for the Determination of Plasma Lipoprotein(a). Clin. Chem. 2000, 46, 1956–1967. [Google Scholar] [CrossRef] [PubMed]
- Lampsas, S.; Xenou, M.; Oikonomou, E.; Pantelidis, P.; Lysandrou, A.; Sarantos, S.; Goliopoulou, A.; Kalogeras, K.; Tsigkou, V.; Kalpis, A.; et al. Lipoprotein(a) in Atherosclerotic Diseases: From Pathophysiology to Diagnosis and Treatment. Molecules 2023, 28, 969. [Google Scholar] [CrossRef]
- Borque, L.; Rus, A.; del Cura, J.; Maside, C.; Escanero, J. Automated Latex Nephelometric Immunoassay for the Measurement of Serum Lipoprotein (a). J. Clin. Lab. Anal. 1993, 7, 105–110. [Google Scholar] [CrossRef]
- Cegla, J.; France, M.; Marcovina, S.M.; Neely, R.D.G. Lp(a): When and How to Measure It. Ann. Clin. Biochem. 2021, 58, 16–21. [Google Scholar] [CrossRef]
- Heydari, M.; Rezayi, M.; Ruscica, M.; Jpamialahamdi, T.; Johnston, T.P.; Sahebkar, A. The Ins and Outs of Lipoprotein(a) Assay Methods. Arch. Med. Sci. Atheroscler. Dis. 2023, 8, e128–e139. [Google Scholar] [CrossRef]
- Szarek, M.; Reijnders, E.; Jukema, J.W.; Bhatt, D.L.; Bittner, V.A.; Diaz, R.; Fazio, S.; Garon, G.; Goodman, S.G.; Harrington, R.A.; et al. Relating Lipoprotein(a) Concentrations to Cardiovascular Event Risk After Acute Coronary Syndrome: A Comparison of 3 Tests. Circulation 2024, 149, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Kamstrup, P.R. Lipoprotein(a) and Cardiovascular Disease. Clin. Chem. 2021, 67, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Shahjehan, R.D.; Sharma, S.; Bhutta, B.S. Coronary Artery Disease; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- O’Donoghue, M.L.; Rosenson, R.S.; Gencer, B.; López, J.A.G.; Lepor, N.E.; Baum, S.J.; Stout, E.; Gaudet, D.; Knusel, B.; Kuder, J.F.; et al. Small Interfering RNA to Reduce Lipoprotein(a) in Cardiovascular Disease. N. Engl. J. Med. 2022, 387, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C.; Watts, G.F. The Promise of PCSK9 and Lipoprotein(a) as Targets for Gene Silencing Therapies. Clin. Ther. 2023, 45, 1034–1046. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Sahebkar, A.; Reiner, Ž.; Simental-Mendia, L.E.; Ferretti, G.; Cicero, A.F. Effect of Extended-Release Niacin on Plasma Lipoprotein(a) Levels: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials. Metab. Clin. Exp. 2016, 65, 1664–1678. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Ballantyne, C.M. Existing and Emerging Strategies to Lower Lipoprotein(a). Atherosclerosis 2022, 349, 110–122. [Google Scholar] [CrossRef]
- Tsaban, G. Statins and Lipoprotein(a); Facing the Residual Risk. Eur. J. Prev. Cardiol. 2022, 29, 777–778. [Google Scholar] [CrossRef]
- de Boer, L.M.; Oorthuys, A.O.J.; Wiegman, A.; Langendam, M.W.; Kroon, J.; Spijker, R.; Zwinderman, A.H.; Hutten, B.A. Statin Therapy and Lipoprotein(a) Levels: A Systematic Review and Meta-Analysis. Eur. J. Prev. Cardiol. 2022, 29, 779–792. [Google Scholar] [CrossRef]
- Safarova, M.S.; Moriarty, P.M. Lipoprotein Apheresis: Current Recommendations for Treating Familial Hypercholesterolemia and Elevated Lipoprotein(a). Curr. Atheroscler. Rep. 2023, 25, 391–404. [Google Scholar] [CrossRef]
- Víšek, J.; Bláha, M.; Bláha, V.; Lášticová, M.; Lánska, M.; Andrýs, C.; Tebbens, J.D.; Igreja e Sá, I.C.; Tripská, K.; Vicen, M.; et al. Monitoring of up to 15 Years Effects of Lipoprotein Apheresis on Lipids, Biomarkers of Inflammation, and Soluble Endoglin in Familial Hypercholesterolemia Patients. Orphanet. J. Rare Dis. 2021, 16, 110. [Google Scholar] [CrossRef] [PubMed]
- Schettler, V.J.; Peter, C.; Zimmermann, T.; Julius, U.; Roeseler, E.; Schlieper, G.; Heigl, F.; Grützmacher, P.; Löhlein, I.; Klingel, R.; et al. The German Lipoprotein Apheresis Registry—Summary of the Ninth Annual Report. Ther. Apher. Dial. 2022, 26, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, A.; Connelly-Smith, L.; Aqui, N.; Balogun, R.A.; Klingel, R.; Meyer, E.; Pham, H.P.; Schneiderman, J.; Witt, V.; Wu, Y.; et al. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice—Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Eighth Special Issue. J. Clin. Apher. 2019, 34, 171–354. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.R. The Scientific Basis and Future of Lipoprotein Apheresis. Ther. Apher. Dial. 2022, 26, 32–36. [Google Scholar] [CrossRef]
- Nugent, A.K.; Gray, J.V.; Gorby, L.K.; Moriarty, P.M. Lipoprotein Apheresis: First FDA Indicated Treatment for Elevated Lipoprotein(a). J. Clin. Cardiol. 2020, 1, 16–21. [Google Scholar] [CrossRef]
- Zimmermann, T.S.; Karsten, V.; Chan, A.; Chiesa, J.; Boyce, M.; Bettencourt, B.R.; Hutabarat, R.; Nochur, S.; Vaishnaw, A.; Gollob, J. Clinical Proof of Concept for a Novel Hepatocyte-Targeting GalNAc-siRNA Conjugate. Mol. Ther. 2017, 25, 71–78. [Google Scholar] [CrossRef]
- Macchi, C.; Sirtori, C.R.; Corsini, A.; Santos, R.D.; Watts, G.F.; Ruscica, M. A New Dawn for Managing Dyslipidemias: The Era of Rna-Based Therapies. Pharmacol. Res. 2019, 150, 104413. [Google Scholar] [CrossRef]
- Landmesser, U.; Poller, W.; Tsimikas, S.; Most, P.; Paneni, F.; Lüscher, T.F. From Traditional Pharmacological towards Nucleic Acid-Based Therapies for Cardiovascular Diseases. Eur. Heart J. 2020, 41, 3884–3899. [Google Scholar] [CrossRef]
- Katzmann, J.L.; Packard, C.J.; Chapman, M.J.; Katzmann, I.; Laufs, U. Targeting RNA With Antisense Oligonucleotides and Small Interfering RNA: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 563–579. [Google Scholar] [CrossRef]
- Yeang, C.; Karwatowska-Prokopczuk, E.; Su, F.; Dinh, B.; Xia, S.; Witztum, J.L.; Tsimikas, S. Effect of Pelacarsen on Lipoprotein(a) Cholesterol and Corrected Low-Density Lipoprotein Cholesterol. J. Am. Coll. Cardiol. 2022, 79, 1035–1046. [Google Scholar] [CrossRef]
- Novartis Pharmaceuticals. A Randomized Double-Blind, Placebo-Controlled, Multicenter Trial Assessing the Impact of Lipoprotein (a) Lowering with Pelacarsen (TQJ230) on Major Cardiovascular Events in Patients with Established Cardiovascular Disease. 2025. Available online: https://clinicaltrials.gov/study/NCT04023552 (accessed on 3 February 2025).
- Katsiki, N.; Vrablik, M.; Banach, M.; Gouni-Berthold, I. Inclisiran, Low-Density Lipoprotein Cholesterol and Lipoprotein (a). Pharmaceuticals 2023, 16, 577. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef]
- Eli Lilly and Company. A Phase 2, Randomized, Double-Blind, Placebo-Controlled Study to Investigate the Efficacy and Safety of LY3819469 in Adults with Elevated Lipoprotein(a). 2025. Available online: https://clinicaltrials.gov/study/NCT05565742 (accessed on 3 February 2025).
- Nissen, S.E.; Linnebjerg, H.; Shen, X.; Wolski, K.; Ma, X.; Lim, S.; Michael, L.F.; Ruotolo, G.; Gribble, G.; Navar, A.M.; et al. Lepodisiran, an Extended-Duration Short Interfering RNA Targeting Lipoprotein(a). JAMA 2023, 330, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Hooper, A.J.; Fernando, P.M.S.; Burnett, J.R. Potential of Muvalaplin as a Lipoprotein(a) Inhibitor. Expert Opin. Investig. Drugs 2024, 33, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Ni, W.; Rhodes, G.M.; Nissen, S.E.; Navar, A.M.; Michael, L.F.; Haupt, A.; Krege, J.H. Oral Muvalaplin for Lowering of Lipoprotein(a): A Randomized Clinical Trial. JAMA 2025, 333, 222–231. [Google Scholar] [CrossRef]
- Chakraborty, A.; Pang, J.; Chan, D.C.; Ellis, K.L.; Hooper, A.J.; Bell, D.A.; Burnett, J.R.; Moses, E.K.; Watts, G.F. Cascade Testing for Elevated Lipoprotein(a) in Relatives of Probands with Familial Hypercholesterolaemia and Elevated Lipoprotein(a). Atherosclerosis 2022, 349, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Annink, M.E.; Janssen, E.S.; Reeskamp, L.F. Effectiveness of Cascade Screening for Elevated Lipoprotein(a), an Underdiagnosed Family Disorder. Curr. Opin. Lipidol. 2024, 35, 290. [Google Scholar] [CrossRef]
- De Vries, T.I.; Cooney, M.T.; Selmer, R.M.; Hageman, S.H.; Pennells, L.A.; Wood, A.; Kaptoge, S.; Xu, Z.; Westerink, J.; Rabanal, K.S.; et al. SCORE2-OP Risk Prediction Algorithms: Estimating Incident Cardiovascular Event Risk in Older Persons in Four Geographical Risk Regions. Eur. Heart J. 2021, 42, 2455–2467. [Google Scholar] [CrossRef]


| Assesment Method | Description | Advantages | Limitations | Cut-Off Value |
|---|---|---|---|---|
| Immunoturbidimetric Assay | Measures turbidity changes due to Lp(a)–antibody complexes | Broad range; High-throughput | Influenced by apo(a) size; Variability between reagents | LOW |
| Nephelometry | Measures light scattering from antigen–antibody complexes | Automated; Reproducible; Broad detection range | Influenced by apo(a) size; Less standardization | <30 mg/dL or <75 nmol/L |
| ELISA | Uses antibodies targeting Lp(a) components | Sensitive; Adaptable | Accuracy depends on antibody used | INCREASED RISK |
| Denka Assay | Commercial assay with multiple calibrators for isoform coverage | Least affected by isoform heterogeneity; More accurate and standardized | Dependent on proper calibration; Available through specific providers | 30–50 mg/dL or 75–125 nmol/L |
| Radial Immunodiffusion | Simple diffusion-based immunoassay measuring precipitate ring | Low cost; Detects low levels of Lp(a) | Low sensitivity; Slow; Cannot assess apo(a) isoform size | HIGH >50 mg/dL or >125 nmol/L |
| Mass Spectrometry | Direct quantification of apolipoprotein(a) peptides | Isoform-independent; Accurate; Better standardization | Expensive; Not widely available; Complex technique |
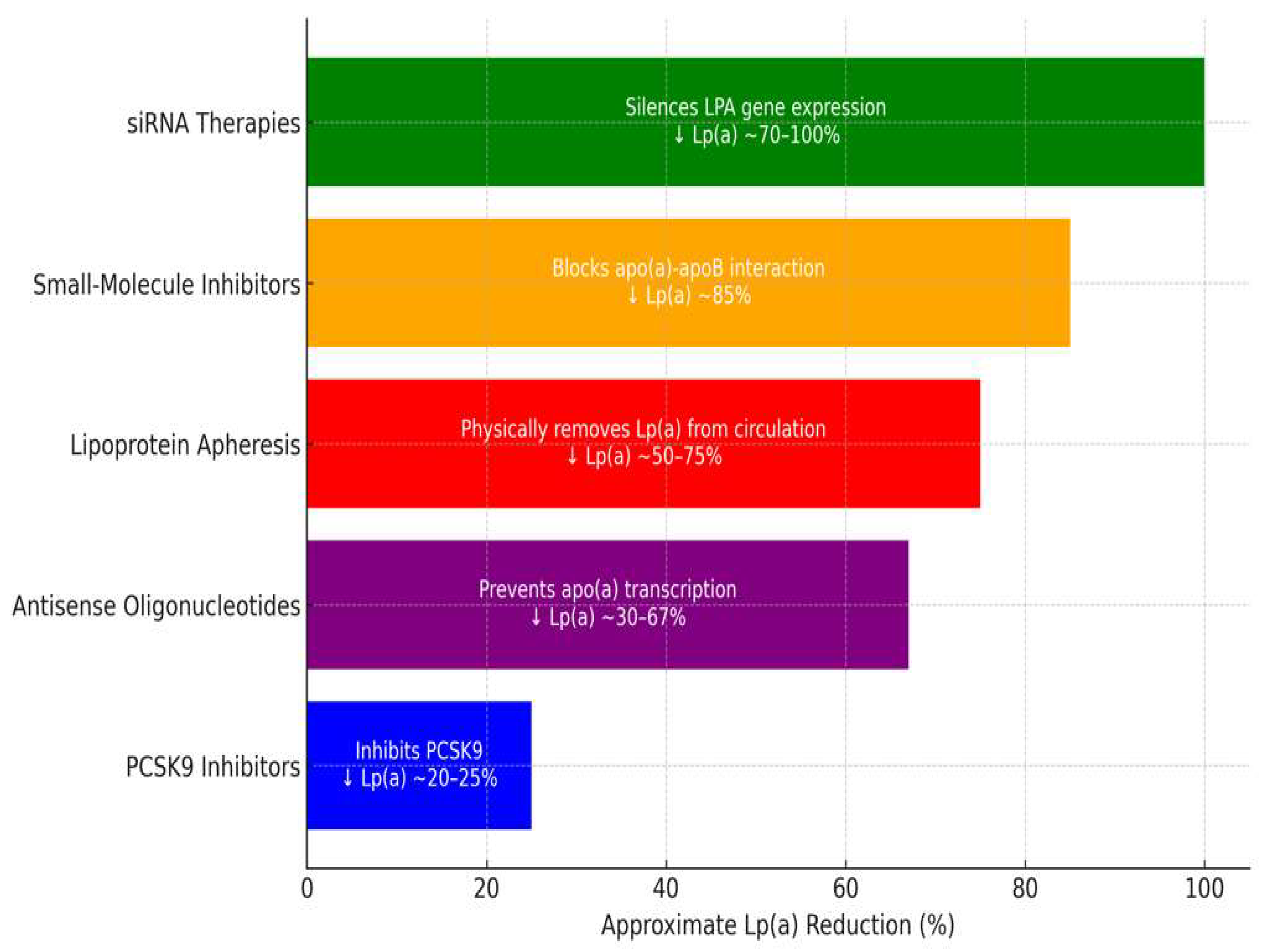
| Therapy | Mechanism of Action | Reduction in Lp(a) | Duration of Effect | Limitations |
|---|---|---|---|---|
| PCSK9-i | Increases clearance via LDLR upregulation | 20–25% | Short-term, frequent dosing | Moderate effect on Lp(a) reduction |
| ASOs (Pelacarsen) | Inhibits apo(a) mRNA translation | 29–67% | Long-term, monthly dosing | Not yet widely available |
| siRNA (Olpasiran) | Blocks apo(a) mRNA translation | 68.5–100% | Long-term, quarterly dosing | Not yet widely available |
| Apheresis | Physically removes Lp(a) from circulation | up to 73% | Immediate but transient | Invasive, expensive |
| Small-molecule inhibitors (Muvalaplin) | Disrupts Lp(a) assembly | up to 85.8% | Oral, daily dosing | Early-stage development |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaritei, O.; Mierlan, O.L.; Gutu, C.; Gurau, G. Lipoprotein(a): Assessing the Current Knowledge and Gaps in Screening and Treatment—A Narrative Review. J. Cardiovasc. Dev. Dis. 2025, 12, 169. https://doi.org/10.3390/jcdd12050169
Amaritei O, Mierlan OL, Gutu C, Gurau G. Lipoprotein(a): Assessing the Current Knowledge and Gaps in Screening and Treatment—A Narrative Review. Journal of Cardiovascular Development and Disease. 2025; 12(5):169. https://doi.org/10.3390/jcdd12050169
Chicago/Turabian StyleAmaritei, Octavian, Oana Laura Mierlan, Cristian Gutu, and Gabriela Gurau. 2025. "Lipoprotein(a): Assessing the Current Knowledge and Gaps in Screening and Treatment—A Narrative Review" Journal of Cardiovascular Development and Disease 12, no. 5: 169. https://doi.org/10.3390/jcdd12050169
APA StyleAmaritei, O., Mierlan, O. L., Gutu, C., & Gurau, G. (2025). Lipoprotein(a): Assessing the Current Knowledge and Gaps in Screening and Treatment—A Narrative Review. Journal of Cardiovascular Development and Disease, 12(5), 169. https://doi.org/10.3390/jcdd12050169







