Early Echocardiographic Markers in Heart Failure with Preserved Ejection Fraction
Abstract
1. Introduction
2. Limitations in the Diagnosis of HFpEF
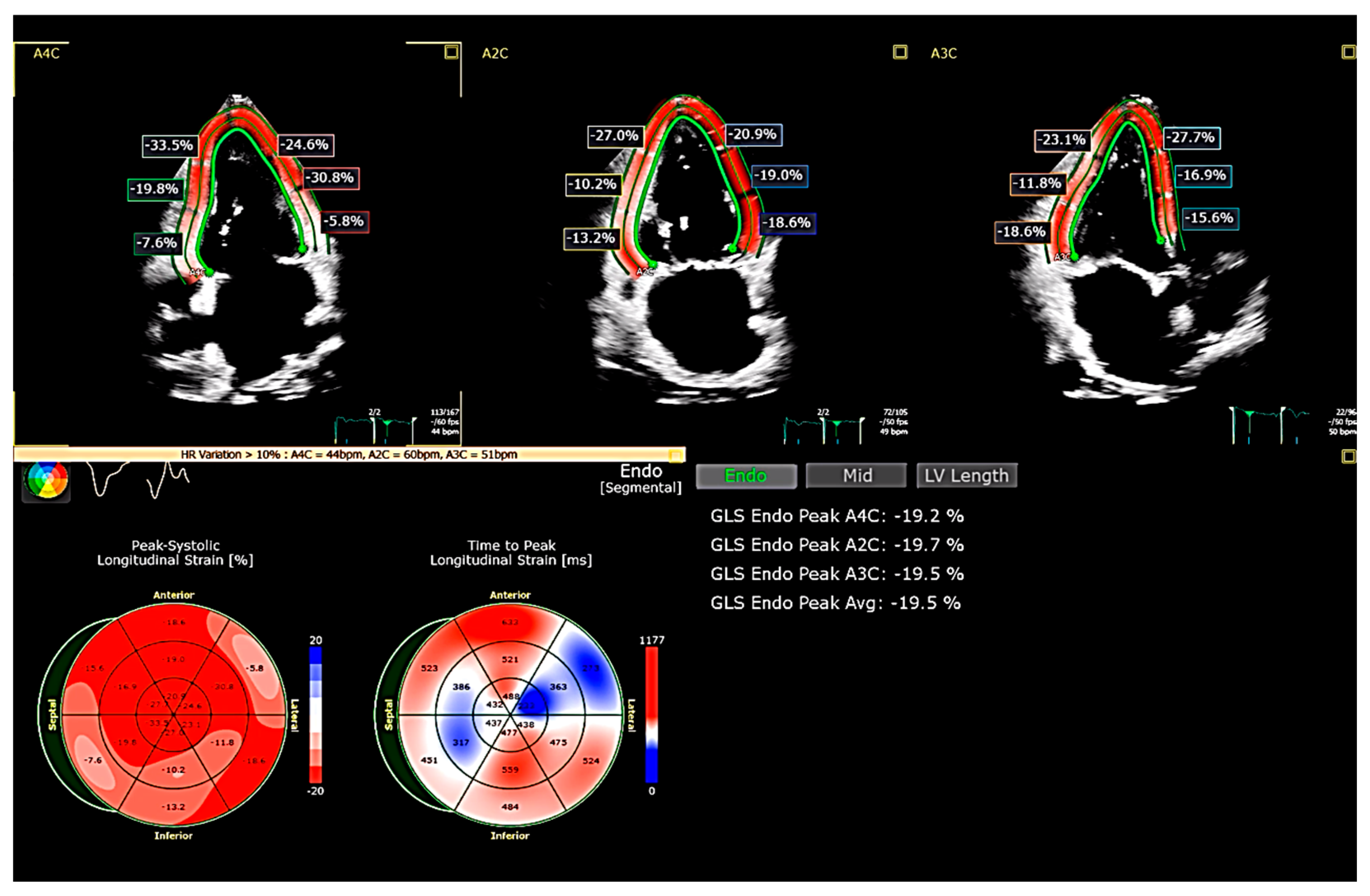
3. Global Longitudinal Strain (GLS)
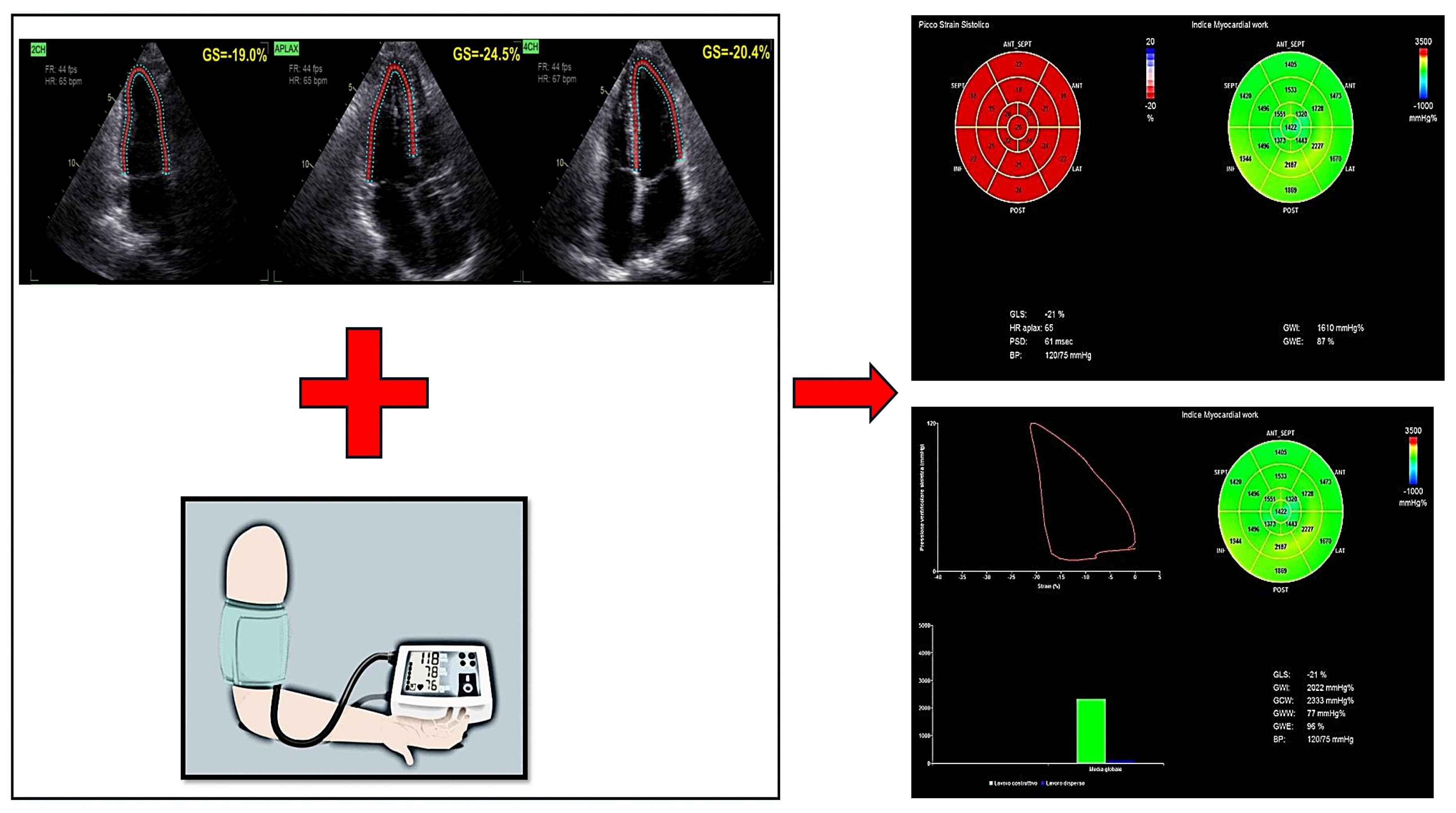
4. Myocardial Work (MW)
- -
- Global Work Index (GWI)—reflects the total myocardial effort performed by the LV.
- -
- Global Constructive Work (GCW)—represents the portion of myocardial effort contributing to effective contraction.
- -
- Global Wasted Work (GWW)—indicates inefficient myocardial effort due to dyssynchronous or ineffective contraction.
- -
- Myocardial Work Efficiency (MWE)—evaluates the ratio of constructive to total myocardial effort, serving as an indicator of LV contractile performance.
5. Right Ventricular Free Wall Longitudinal Strain (RVFWLS)
6. Left Atrial Strain (LAS)
7. Future Perspectives
8. Integration with Other Diagnostic Modalities
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Owan, T.E.; Hodge, D.O.; Herges, R.M.; Jacobsen, S.J.; Roger, V.L.; Redfield, M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N. Engl. J. Med. 2006, 355, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.S.; Xu, H.; Matsouaka, R.A.; Bhatt, D.L.; Heidenreich, P.A.; Hernandez, A.F.; Devore, A.D.; Yancy, C.W.; Fonarow, G.C. Heart Failure with Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J. Am. Coll. Cardiol. 2017, 70, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317, Erratum in Eur. Heart J. 2021, 42, 1274. https://doi.org/10.1093/eurheartj/ehaa1016. [Google Scholar] [CrossRef] [PubMed]
- Westermann, D.; Kasner, M.; Steendijk, P.; Spillmann, F.; Riad, A.; Weitmann, K.; Hoffmann, W.; Poller, W.; Pauschinger, M.; Schultheiss, H.P.; et al. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation 2008, 117, 2051–2060. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd BF3rd Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; Marino, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Carter, R.E.; Obokata, M.; Redfield, M.M.; Borlaug, B.A. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure with Preserved Ejection Fraction. Circulation 2018, 138, 861–870. [Google Scholar] [CrossRef]
- Morris, D.A.; Ma, X.X.; Belyavskiy, E.; Aravind Kumar, R.; Kropf, M.; Kraft, R.; Frydas, A.; Osmanoglou, E.; Marquez, E.; Donal, E.; et al. Left ventricular longitudinal systolic function analysed by 2D speckle-tracking echocardiography in heart failure with preserved ejection fraction: A meta-analysis. Open Heart 2017, 4, e000630. [Google Scholar] [CrossRef]
- Silva, M.R.; Sampaio, F.; Braga, J.; Ribeiro, J.; Fontes-Carvalho, R. Left atrial strain evaluation to assess left ventricle diastolic dysfunction and heart failure with preserved ejection fraction: A guide to clinical practice: Left atrial strain and diastolic function. Int. J. Cardiovasc. Imaging 2023, 39, 1083–1096. [Google Scholar] [CrossRef]
- Marzlin, N.; Hays, A.G.; Peters, M.; Kaminski, A.; Roemer, S.; O’Leary, P.; Kroboth, S.; Harland, D.R.; Khandheria, B.K.; Tajik, A.J.; et al. Myocardial Work in Echocardiography. Circ. Cardiovasc. Imaging 2023, 16, e014419. [Google Scholar] [CrossRef]
- Tadic, M.; Pieske-Kraigher, E.; Cuspidi, C.; Morris, D.A.; Burkhardt, F.; Baudisch, A.; Haßfeld, S.; Tschöpe, C.; Pieske, B. Right ventricular strain in heart failure: Clinical perspective. Arch. Cardiovasc. Dis. 2017, 110, 562–571. [Google Scholar] [CrossRef]
- Caenen, A.; Bézy, S.; Pernot, M.; Nightingale, K.R.; Vos, H.J.; Voigt, J.U.; Segers, P.; D’hooge, J. Ultrasound Shear Wave Elastography in Cardiology. JACC Cardiovasc. Imaging 2024, 17, 314–329. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726, Erratum in Eur. Heart J. 2021, 42, 4901. https://doi.org/10.1093/eurheartj/ehab670. [Google Scholar] [CrossRef] [PubMed]
- Setti, M.; Benfari, G.; Mele, D.; Rossi, A.; Ballo, P.; Galderisi, M.; Henein, M.; Nistri, S. Discrepancies in Assessing Diastolic Function in Pre-Clinical Heart Failure Using Different Algorithms—A Primary Care Study. Diagnostics 2020, 10, 850. [Google Scholar] [CrossRef]
- Mele, D.; Andrade, A.; Bettencourt, P.; Moura, B.; Pestelli, G.; Ferrari, R. From left ventricular ejection fraction to cardiac hemodynamics: Role of echocardiography in evaluating patients with heart failure. Heart Fail. Rev. 2020, 25, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Marwick, T.H. Ejection Fraction Pros and Cons: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2360–2379. [Google Scholar] [CrossRef]
- Ross, J., Jr. Mechanisms of cardiac contraction. What roles for preload, afterload and inotropic state in heart failure? Eur. Heart J. 1983, 4 (Suppl. A), 19–28. [Google Scholar] [CrossRef]
- Vancheri, F.; Longo, G.; Henein, M.Y. Left ventricular ejection fraction: Clinical, pathophysiological, and technical limitations. Front. Cardiovasc. Med. 2024, 11, 1340708. [Google Scholar] [CrossRef]
- Campbell, R.T.; McMurray, J.J. Comorbidities and differential diagnosis in heart failure with preserved ejection fraction. Heart Fail. Clin. 2014, 10, 481–501. [Google Scholar] [CrossRef] [PubMed]
- Ather, S.; Chan, W.; Bozkurt, B.; Aguilar, D.; Ramasubbu, K.; Zachariah, A.A.; Wehrens, X.H.; Deswal, A. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J. Am. Coll. Cardiol. 2012, 59, 998–1005. [Google Scholar] [CrossRef]
- Bachmann, K.N.; Gupta, D.K.; Xu, M.; Brittain, E.; Farber-Eger, E.; Arora, P.; Collins, S.; Wells, Q.S.; Wang, T.J. Unexpectedly Low Natriuretic Peptide Levels in Patients with Heart Failure. JACC Heart Fail. 2021, 9, 192–200. [Google Scholar] [CrossRef]
- Sartipy, U.; Dahlström, U.; Fu, M.; Lund, L.H. Atrial Fibrillation in Heart Failure With Preserved, Mid-Range, and Reduced Ejection Fraction. JACC Heart Fail. 2017, 5, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Urheim, S.; Edvardsen, T.; Torp, H.; Angelsen, B.; Smiseth, O.A. Myocardial strain by Doppler echocardiography. Validation of a new method to quantify regional myocardial function. Circulation 2000, 102, 1158–1164. [Google Scholar] [CrossRef]
- Amundsen, B.H.; Helle-Valle, T.; Edvardsen, T.; Torp, H.; Crosby, J.; Lyseggen, E.; Støylen, A.; Ihlen, H.; Lima, J.A.; Smiseth, O.A.; et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: Validation against sonomicrometry and tagged magnetic resonance Imaging. J. Am. Coll. Cardiol. 2006, 47, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270, Erratum in Eur. Heart J. Cardiovasc. Imaging 2016, 17, 412. https://doi.org/10.1093/ehjci/jew041. Erratum in Eur. Heart J. Cardiovasc. Imaging 2016, 17, 969. https://doi.org/10.1093/ehjci/jew124. [Google Scholar] [CrossRef] [PubMed]
- Smiseth, O.A.; Rider, O.; Cvijic, M.; Valkovič, L.; Remme, E.W.; Voigt, J.U. Myocardial Strain Imaging: Theory, Current Practice, and the Future. JACC Cardiovasc. Imaging 2025, 18, 340–381. [Google Scholar] [CrossRef] [PubMed]
- Stokke, T.M.; Hasselberg, N.E.; Smedsrud, M.K.; Sarvari, S.I.; Haugaa, K.H.; Smiseth, O.A.; Edvardsen, T.; Remme, E.W. Geometry as a Confounder When Assessing Ventricular Systolic Function: Comparison Between Ejection Fraction and Strain. J. Am. Coll. Cardiol. 2017, 70, 942–954. [Google Scholar] [CrossRef]
- Tower-Rader, A.; Mohananey, D.; To, A.; Lever, H.M.; Popovic, Z.B.; Desai, M.Y. Prognostic Value of Global Longitudinal Strain in Hypertrophic Cardiomyopathy: A Systematic Review of Existing Literature. JACC Cardiovasc. Imaging 2019, 12, 1930–1942. [Google Scholar] [CrossRef]
- Minamisawa, M.; Inciardi, R.M.; Claggett, B.; Cikes, M.; Liu, L.; Prasad, N.; Biering-Sørensen, T.; Lam, C.S.P.; Shah, S.J.; Zile, M.R.; et al. Clinical implications of subclinical left ventricular dysfunction in heart failure with preserved ejection fraction: The PARAGON-HF study. Eur. J. Heart Fail. 2024, 26, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Claggett, B.; Sweitzer, N.K.; Shah, S.J.; Anand, I.S.; Liu, L.; Pitt, B.; Pfeffer, M.A.; Solomon, S.D. Prognostic Importance of Impaired Systolic Function in Heart Failure with Preserved Ejection Fraction and the Impact of Spironolactone. Circulation 2015, 132, 402–414. [Google Scholar] [CrossRef]
- Donal, E.; Lund, L.H.; Oger, E.; Hage, C.; Persson, H.; Reynaud, A.; Ennezat, P.V.; Bauer, F.; Drouet, E.; Linde, C.; et al. New echocardiographic predictors of clinical outcome in patients presenting with heart failure and a preserved left ventricular ejection fraction: A subanalysis of the Ka (Karolinska) Ren (Rennes) Study. Eur. J. Heart Fail. 2015, 17, 680–688. [Google Scholar] [CrossRef]
- Lin, Y.; Xie, M.; Zhang, L.; Zhang, Y.; Zhang, P.; Chen, X.; Ji, M.; Gao, L.; He, Q.; Wu, Z.; et al. Prognostic Value of LV Global Longitudinal Strain by 2D and 3D Speckle-Tracking Echocardiography in Patients with HFpEF. Circ. Cardiovasc. Imaging 2025, 18, e016975. [Google Scholar] [CrossRef]
- Gil, J.; Abreu, L.; Antunes, H.; Gonçalves, M.L.; Pires, M.I.; Moreira, D.; Correia, E.; Santos, L.S.; Cabral, J.C. Apical sparing of longitudinal strain in speckle-tracking echocardiography: A sensitive an specific finding in cardiac amyloidosis. Neth. Heart J. 2018, 26, 635. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Mohamed, A.A.; Hammoda, M.A.; Sabry, A.M. Role of Speckle Tracking Echocardiography in Differentiating between Ischemic and Non-ischemic Cardiomyopathy. Int. J. Cardiovasc. Acad. 2024, 10, 1–6. [Google Scholar] [CrossRef]
- Li, L.; Jiang, X.; Xie, Q. Prognostic value of left ventricular global longitudinal strain on speckle echocardiography for predicting chemotherapy-induced cardiotoxicity in breast cancer patients: A systematic review and meta-analysis. Echocardiography 2023, 40, 306–317. [Google Scholar] [CrossRef]
- Heggemann, F.; Hamm, K.; Kaelsch, T.; Sueselbeck, T.; Papavassiliu, T.; Borggrefe, M.; Haghi, D. Global and regional myocardial function quantification in Takotsubo cardiomyopathy in comparison to acute anterior myocardial infarction using two-dimensional (2D) strain echocardiography. Echocardiography 2011, 28, 715–719. [Google Scholar] [CrossRef]
- Alnaimat, S.; Mascara, M.; Lygouris, G.; Biederman, R.W.W. Blueberry-on-Top Phenomenon in Apical Variant Hypertrophic Cardiomyopathy. CASE 2024, 8, 296–302. [Google Scholar] [CrossRef]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A novel clinical method for quantification of regional left ventricular pressure-strain loop area: A non-invasive index of myocardial work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Manganaro, R.; Marchetta, S.; Dulgheru, R.; Ilardi, F.; Sugimoto, T.; Robinet, S.; Cimino, S.; Go, Y.Y.; Bernard, A.; Kacharava, G.; et al. Echocardiographic reference ranges for normal non-invasive myocardial work indices: Results from the EACVI NORRE study. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 582–590. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Ilardi, F.; D’Ascenzi, F.; Bandera, F.; Benfari, G.; Esposito, R.; Malagoli, A.; Mandoli, G.E.; Santoro, C.; Russo, V.; et al. Impaired myocardial work efficiency in heart failure with preserved ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1312–1320. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Yang, C.G.; Meng, X.Y.; Li, Y.; Xia, C.X.; Xu, T.; Weng, S.X.; Zhong, Y.; Zhang, R.S.; et al. Noninvasive assessment of myocardial work during left ventricular isovolumic relaxation in patients with diastolic dysfunction. BMC Cardiovasc. Disord. 2023, 23, 129. [Google Scholar] [CrossRef]
- Paolisso, P.; Gallinoro, E.; Mileva, N.; Moya, A.; Fabbricatore, D.; Esposito, G.; De Colle, C.; Beles, M.; Spapen, J.; Heggermont, W.; et al. Performance of non-invasive myocardial work to predict the first hospitalization for de novo heart failure with preserved ejection fraction. ESC Heart Fail. 2022, 9, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Qin, Y.; Ding, X.; Zhang, M.; Zhu, W.; Wang, J.; Leng, C.; Lu, X.; Cai, Q. Association between left ventricular geometry and global myocardial work in patients with heart failure with preserved ejection fraction: Assessment using strain-pressure loop. Int. J. Cardiovasc. Imaging 2023, 39, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Wang, Y.; Zhang, R.; Li, J.; Yu, T.; Yin, L.; Shao, T.; Lu, H.; Wang, C.; Xue, L. The value of speckle-tracking stratified strain combined with myocardial work measurement in evaluating left ventricular function in patients with heart failure with preserved ejection fraction. Quant. Imaging Med. Surg. 2024, 14, 2514–2527. [Google Scholar] [CrossRef]
- Galli, E.; Leclercq, C.; Hubert, A.; Bernard, A.; Smiseth, O.A.; Mabo, P.; Samset, E.; Hernandez, A.; Donal, E. Role of myocardial constructive work in the identification of responders to CRT. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1010–1018. [Google Scholar] [CrossRef]
- Muraru, D.; Haugaa, K.; Donal, E.; Stankovic, I.; Voigt, J.U.; Petersen, S.E.; Popescu, B.A.; Marwick, T. Right ventricular longitudinal strain in the clinical routine: A state-of-the-art review. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 898–912. [Google Scholar] [CrossRef]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation Imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600, Erratum in Eur. Heart J. Cardiovasc. Imaging 2018, 19, 830–833. https://doi.org/10.1093/ehjci/jey071. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choi, J.O.; Park, S.W.; Cho, G.Y.; Oh, J.K.; Lee, J.H.; Seong, I.W. Normal references of right ventricular strain values by two-dimensional strain echocardiography according to the age and gender. Int. J. Cardiovasc. Imaging 2018, 34, 177–183. [Google Scholar] [CrossRef]
- Addetia, K.; Miyoshi, T.; Citro, R.; Daimon, M.; Gutierrez Fajardo, P.; Kasliwal, R.R.; Kirkpatrick, J.N.; Monaghan, M.J.; Muraru, D.; Ogunyankin, K.O.; et al. Two-Dimensional Echocardiographic Right Ventricular Size and Systolic Function Measurements Stratified by Sex, Age, and Ethnicity: Results of the World Alliance of Societies of Echocardiography Study. J. Am. Soc. Echocardiogr. 2021, 34, 1148–1157.e1. [Google Scholar] [CrossRef]
- Nyberg, J.; Jakobsen, E.O.; Østvik, A.; Holte, E.; Stølen, S.; Lovstakken, L.; Grenne, B.; Dalen, H. Echocardiographic Reference Ranges of Global Longitudinal Strain for All Cardiac Chambers Using Guideline-Directed Dedicated Views. JACC Cardiovasc. Imaging 2023, 16, 1516–1531. [Google Scholar] [CrossRef]
- Guazzi, M.; Naeije, R. Right Heart Phenotype in Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2021, 14, e007840. [Google Scholar] [CrossRef]
- Houston, B.A.; Brittain, E.L.; Tedford, R.J. Right Ventricular Failure. N. Engl. J. Med. 2023, 388, 1111–1125. [Google Scholar] [CrossRef] [PubMed]
- Inciardi, R.M.; Abanda, M.; Shah, A.M.; Cikes, M.; Claggett, B.; Skali, H.; Vaduganathan, M.; Prasad, N.; Litwin, S.; Merkely, B.; et al. Right Ventricular Function and Pulmonary Coupling in Patients with Heart Failure and Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2023, 82, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.B.; Kraigher-Krainer, E.; Gupta, D.K.; Claggett, B.; Zile, M.R.; Pieske, B.; Voors, A.A.; Lefkowitz, M.; Bransford, T.; Shi, V.; et al. Impaired left atrial function in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2014, 16, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Sparla, S.; Losito, M.; Righini, F.M.; Menci, D.; Lisi, M.; D’Ascenzi, F.; Focardi, M.; Favilli, R.; Pierli, C.; et al. Correlation of Left Atrial Strain and Doppler Measurements with Invasive Measurement of Left Ventricular End-Diastolic Pressure in Patients Stratified for Different Values of Ejection Fraction. Echocardiography 2016, 33, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.A.; Takeuchi, M.; Krisper, M.; Köhncke, C.; Bekfani, T.; Carstensen, T.; Hassfeld, S.; Dorenkamp, M.; Otani, K.; Takigiku, K.; et al. Normal values and clinical relevance of left atrial myocardial function analysed by speckle-tracking echocardiography: Multicentre study. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 364–372. [Google Scholar] [CrossRef]
- Inoue, K.; Khan, F.H.; Remme, E.W.; Ohte, N.; García-Izquierdo, E.; Chetrit, M.; Moñivas-Palomero, V.; Mingo-Santos, S.; Andersen, Ø.S.; Gude, E.; et al. Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur. Heart J. Cardiovasc. Imaging 2021, 23, 61–70, Erratum in Eur. Heart J. Cardiovasc. Imaging 2021, 23, 136. https://doi.org/10.1093/ehjci/jeab194. [Google Scholar] [CrossRef]
- Singh, A.; Carvalho Singulane, C.; Miyoshi, T.; Prado, A.D.; Addetia, K.; Bellino, M.; Daimon, M.; Gutierrez Fajardo, P.; Kasliwal, R.R.; Kirkpatrick, J.N.; et al. Normal Values of Left Atrial Size and Function and the Impact of Age: Results of the World Alliance Societies of Echocardiography Study. J. Am. Soc. Echocardiogr. 2022, 35, 154–164.e3. [Google Scholar] [CrossRef]
- Morris, D.A.; Belyavskiy, E.; Aravind-Kumar, R.; Kropf, M.; Frydas, A.; Braunauer, K.; Marquez, E.; Krisper, M.; Lindhorst, R.; Osmanoglou, E.; et al. Potential Usefulness and Clinical Relevance of Adding Left Atrial Strain to Left Atrial Volume Index in the Detection of Left Ventricular Diastolic Dysfunction. JACC Cardiovasc. Imaging 2018, 11, 1405–1415. [Google Scholar] [CrossRef]
- Singh, A.; Addetia, K.; Maffessanti, F.; Mor-Avi, V.; Lang, R.M. LA Strain for Categorization of LV Diastolic Dysfunction. JACC Cardiovasc. Imaging 2017, 10, 735–743. [Google Scholar] [CrossRef]
- Ovchinnikov, A.G.; Potekhina, A.; Belyavskiy, E.; Gvozdeva, A.; Ageev, F. Left atrial dysfunction as the major driver of heart failure with preserved ejection fraction syndrome. J. Clin. Ultrasound 2022, 50, 1073–1083. [Google Scholar] [CrossRef]
- Kagami, K.; Harada, T.; Yuasa, N.; Saito, Y.; Sorimachi, H.; Murakami, F.; Naito, A.; Tani, Y.; Kato, T.; Wada, N.; et al. Impaired Left Atrial Reserve Function in Heart Failure with Preserved Ejection Fraction. Circ. Cardiovasc. Imaging 2024, 17, e016549. [Google Scholar] [CrossRef] [PubMed]
- Bercoff, J.; Tanter, M.; Fink, M. Supersonic shear imaging: A new technique for soft tissue elasticity mapping. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 396–409, Erratum in IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 1492–1494. https://doi.org/10.1109/TUFFC.2020.2973565. [Google Scholar] [CrossRef] [PubMed]
- Villemain, O.; Correia, M.; Mousseaux, E.; Baranger, J.; Zarka, S.; Podetti, I.; Soulat, G.; Damy, T.; Hagège, A.; Tanter, M.; et al. Myocardial Stiffness Evaluation Using Noninvasive Shear Wave Imaging in Healthy and Hypertrophic Cardiomyopathic Adults. JACC Cardiovasc. Imaging 2019, 12 Pt 1, 1135–1145. [Google Scholar] [CrossRef]
- Hulot, J.S.; Janiak, P.; Boutinaud, P.; Boutouyrie, P.; Chézalviel-Guilbert, F.; Christophe, J.J.; Cohen, A.; Damy, T.; Djadi-Prat, J.; Firat, H.; et al. Rationale and design of the PACIFIC-PRESERVED (PhenomApping, ClassIFcation and Innovation for Cardiac dysfunction in patients with heart failure and PRESERVED left ventricular ejection fraction) study. Arch. Cardiovasc. Dis. 2024, 117, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.Q.; Kakkad, V.; Bradway, D.P.; LeFevre, M.; Kisslo, J.; Khouri, M.G.; Trahey, G.E. Evaluation of Myocardial Stiffness in Cardiac Amyloidosis Using Acoustic Radiation Force Impulse and Natural Shear Wave Imaging. Ultrasound Med. Biol. 2023, 49, 1719–1727. [Google Scholar] [CrossRef]
- Meyer, T.; Wellge, B.; Barzen, G.; Klemmer Chandia, S.; Knebel, F.; Hahn, K.; Elgeti, T.; Fischer, T.; Braun, J.; Tzschätzsch, H.; et al. Cardiac Elastography with External Vibration for Quantification of Diastolic Myocardial Stiffness. J. Am. Soc. Echocardiogr. 2025, 38, 431–442. [Google Scholar] [CrossRef]
- Ipek, R.; Holland, J.; Cramer, M.; Rider, O. CMR to characterize myocardial structure and function in heart failure with preserved left ventricular ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 1491–1504. [Google Scholar] [CrossRef]
- Lau, C.; Elshibly, M.M.M.; Kanagala, P.; Khoo, J.P.; Arnold, J.R.; Hothi, S.S. The role of cardiac magnetic resonance imaging in the assessment of heart failure with preserved ejection fraction. Front Cardiovasc Med. 2022, 9, 922398. [Google Scholar] [CrossRef]
- Chrysohoou, C.; Konstantinou, K.; Tsioufis, K. The Role of NT-proBNP Levels in the Diagnosis and Treatment of Heart Failure with Preserved Ejection Fraction-It Is Not Always a Hide-and-Seek Game. J. Cardiovasc. Dev. Dis. 2024, 11, 225. [Google Scholar] [CrossRef]
- Hahn, V.S.; Knutsdottir, H.; Peterson, T.E.; Kikuchi, D.; Vungarala, S.; Kass, D.A.; Sharma, K. Relationship Between Myocardial NPPB Expression and Serum NT-proBNP in Heart Failure with Preserved Ejection Fraction. JACC Heart Fail. 2024, 12, 1306–1308. [Google Scholar] [CrossRef]


| GLS Pattern | Explanation | Cardiac Disease | Sensitivity and Specificity |
|---|---|---|---|
| Apical sparing | Normal or near-normal GLS values of LV apical segments associated with reduced GLS of basal and mid-ventricular segments | Cardiac amyloidosis | Se 93%, Sp 82% [32] |
| Global strain reduction | Uniformly low LV GLS values across all the ventricular segments | DCM chemotherapy-induced cardiac toxicity | Se 86%, Sp 70% [33] Se 70%, Sp 70% [34] |
| Basal accentuation | Hypercontractility of basal LV segments with higher GLS values in comparison with mid-apical segments | Takotsubo cardiomyopathy | Unknown precise Se and Sp [35] |
| “Cherry-on-top” | Hypercontractility of apical LV segments with higher GLS values in comparison with basal and mid-ventricular segments | Apical HCM | Se 93%, Sp 82% [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavernese, A.; Rizza, V.; Cammalleri, V.; Mollace, R.; Carresi, C.; Antonelli, G.; Cocco, N.; D’Antonio, L.; Gelfusa, M.; Piccirillo, F.; et al. Early Echocardiographic Markers in Heart Failure with Preserved Ejection Fraction. J. Cardiovasc. Dev. Dis. 2025, 12, 229. https://doi.org/10.3390/jcdd12060229
Tavernese A, Rizza V, Cammalleri V, Mollace R, Carresi C, Antonelli G, Cocco N, D’Antonio L, Gelfusa M, Piccirillo F, et al. Early Echocardiographic Markers in Heart Failure with Preserved Ejection Fraction. Journal of Cardiovascular Development and Disease. 2025; 12(6):229. https://doi.org/10.3390/jcdd12060229
Chicago/Turabian StyleTavernese, Annamaria, Vincenzo Rizza, Valeria Cammalleri, Rocco Mollace, Cristina Carresi, Giorgio Antonelli, Nino Cocco, Luca D’Antonio, Martina Gelfusa, Francesco Piccirillo, and et al. 2025. "Early Echocardiographic Markers in Heart Failure with Preserved Ejection Fraction" Journal of Cardiovascular Development and Disease 12, no. 6: 229. https://doi.org/10.3390/jcdd12060229
APA StyleTavernese, A., Rizza, V., Cammalleri, V., Mollace, R., Carresi, C., Antonelli, G., Cocco, N., D’Antonio, L., Gelfusa, M., Piccirillo, F., Nusca, A., & Ussia, G. P. (2025). Early Echocardiographic Markers in Heart Failure with Preserved Ejection Fraction. Journal of Cardiovascular Development and Disease, 12(6), 229. https://doi.org/10.3390/jcdd12060229







