Extracellular Volume Fraction Analysis on Cardiac Computed Tomography Is Useful for Predicting the Prognosis of Hypertrophic Cardiomyopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol for Computed Tomography
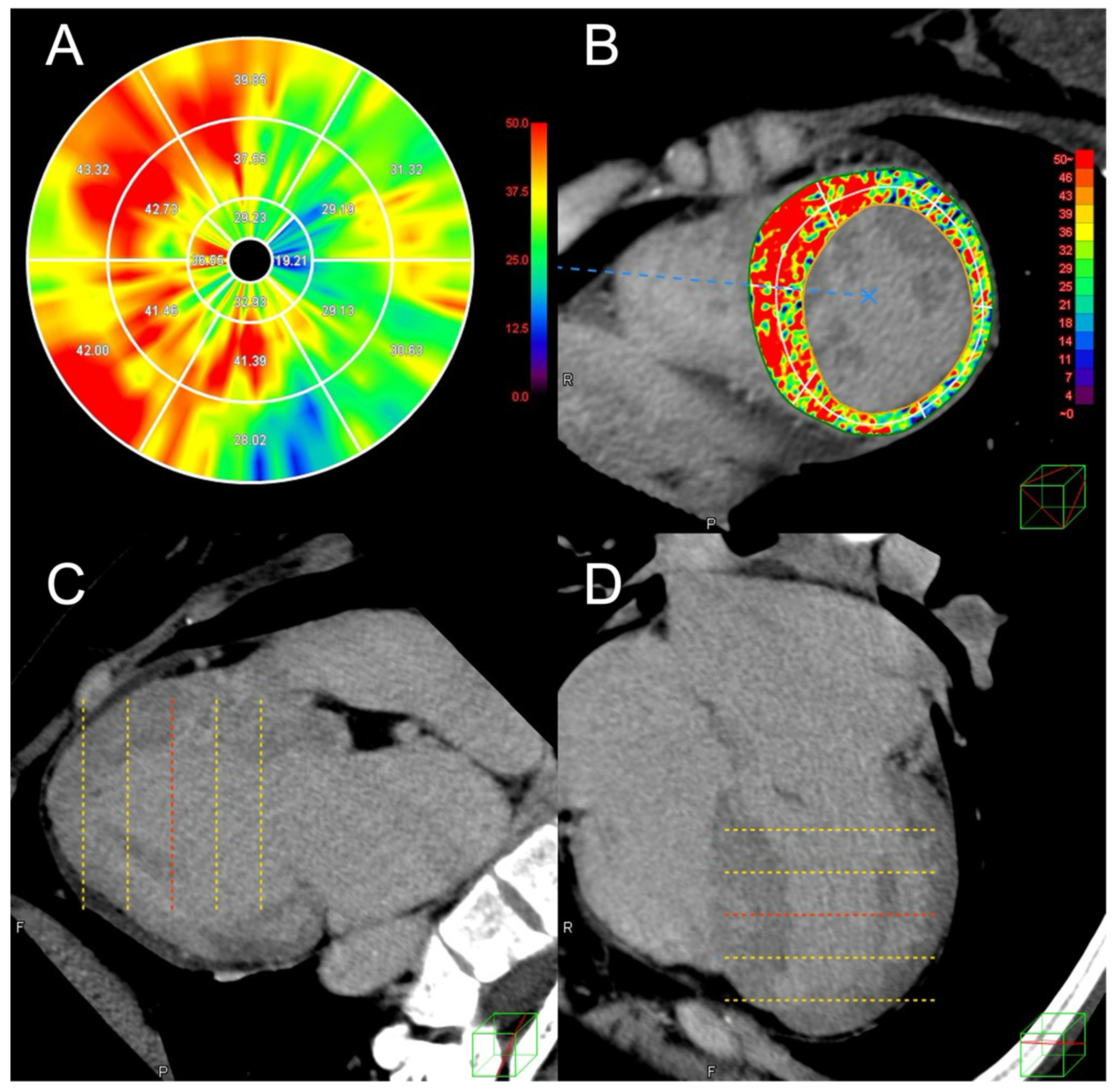
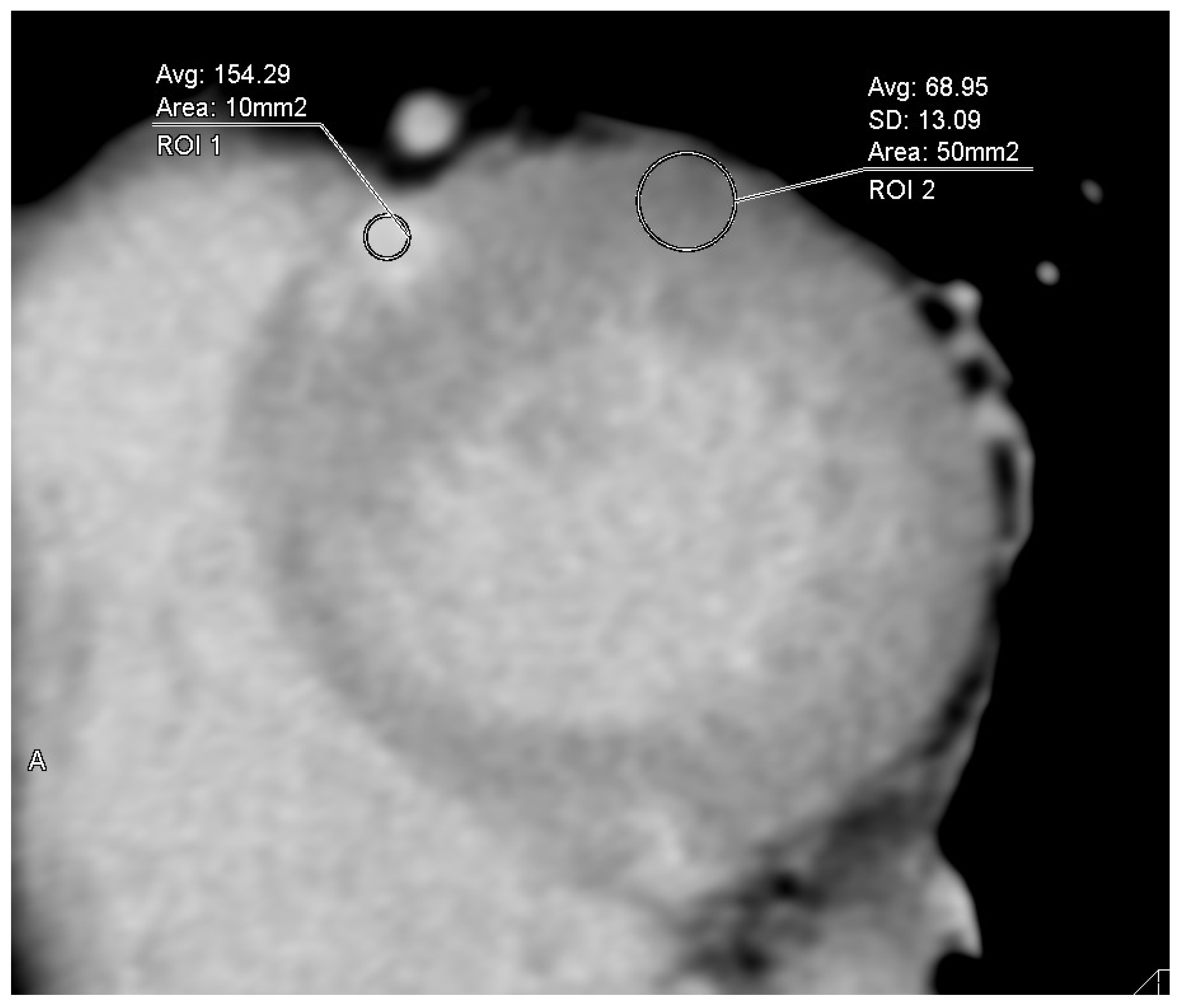
2.2. Analysis of Cardiac CT Images Including ECV
2.3. Echocardiographic Measurement
2.4. Statistical Analysis
3. Results
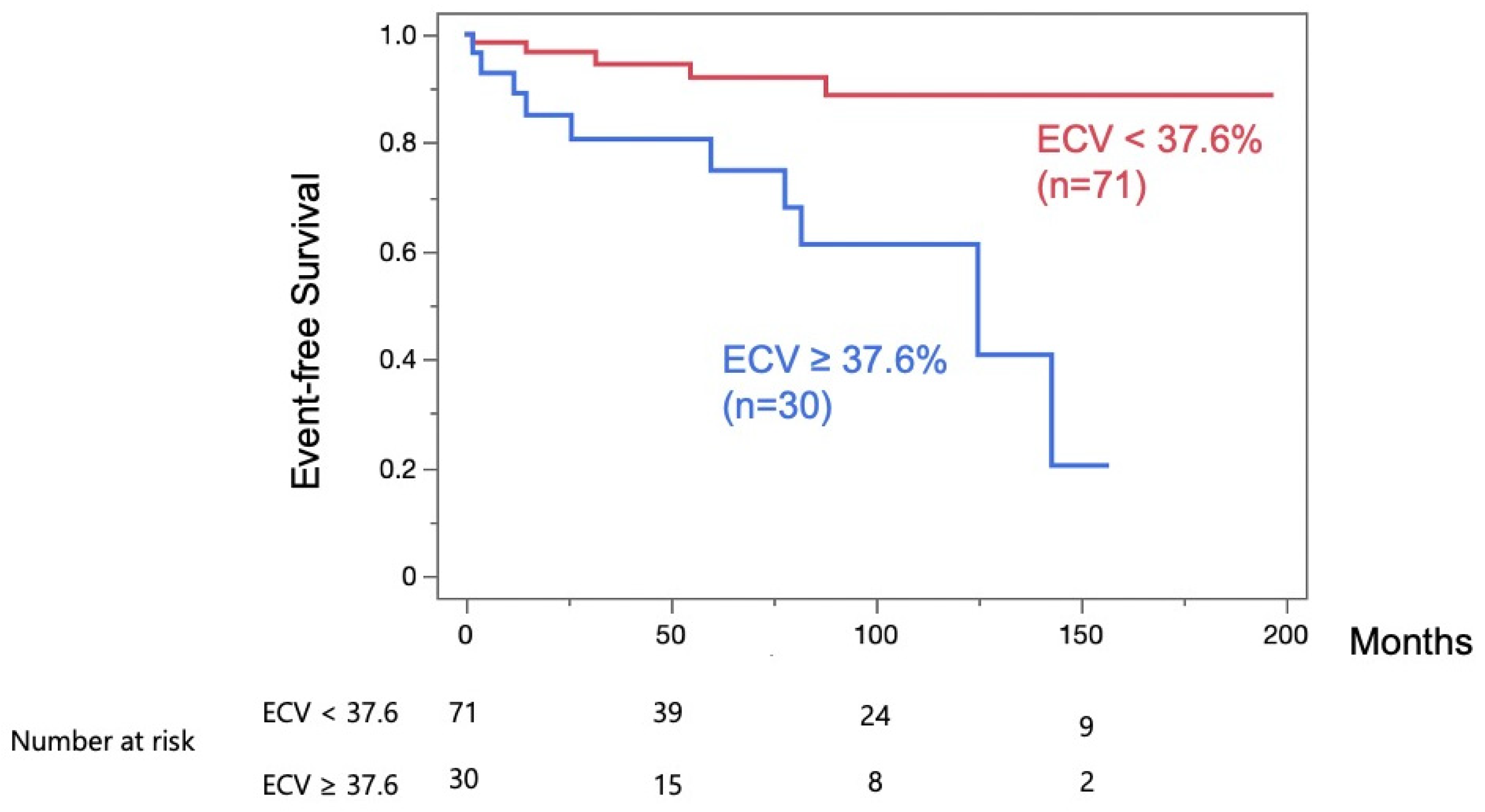
4. Discussion
4.1. ECV Analysis in HCM
4.2. Comparison of CT and MRI in ECV Analysis
4.3. Differences from Established Risk Factors and Clinical Utility of ECV on CT
4.4. Decreased Radiation Dose and Increased Image Quality for LIE Analysis on CT
4.5. ECV Analysis in Cases with LVH
4.6. Screening for Intracardiac Thrombus in Cases with AF on Late-Phase CT Images
4.7. Future Perspectives
4.8. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECV | Extracellular volume fraction |
| CT | Computed tomography |
| HCM | Hypertrophic cardiomyopathy |
| LV | Left ventricular |
| LV-ECV | Left ventricular extracellular volume fraction |
| MACE | Major adverse cardiac events |
| LAD | Left atrial diameter |
| LVDd | LV end-diastolic diameter |
| LVDs | LV end-systolic diameter |
| LVEF | Left ventricular ejection fraction |
| TTE | Transthoracic echocardiography |
| MRI | Magnetic resonance imaging |
| LGE | Late gadolinium enhancement |
| LIE | Late iodine enhancement |
| LVM | Left ventricular myocardium |
| ECG | Electrocardiogram |
| HU | Hounsfield units |
| Hct | Hematocrit |
| ROI | Regions of interest |
| CNR | Contrast-to-noise ratio |
| SD | Standard deviation |
| LVOT | Left ventricular outflow tract |
| ROC | Receiver operating characteristic |
| CI | Confidence interval |
| AUC | Area under the curve |
| CTDI | Computed Tomography Dose Index |
| AF | Atrial fibrillation |
| DCM | Dilated cardiomyopathy |
| LVH | Left ventricular hypertrophy |
| SCD | Sudden cardiac death |
| NSVT | Non-sustained ventricular tachycardia |
| eGFR | Estimated glomerular filtration rate |
| DHCM | Dilated phase hypertrophic cardiomyopathy |
References
- Maron, B.J. Sudden death in young athletes. N. Engl. J. Med. 2003, 349, 1064–1075. [Google Scholar] [CrossRef]
- Teraoka, K.; Hirano, M.; Ookubo, H.; Sasaki, K.; Katsuyama, H.; Amino, M.; Abe, Y.; Yamashina, A. Delayed contrast enhancement of MRI in hyper-trophic cardiomyopathy. Magn. Reason. Imaging 2004, 22, 155–161. [Google Scholar] [CrossRef]
- Cummings, K.W.; Bhalla, S.; Javidan-Nejad, C.; Bierhals, A.J.; Gutierrez, F.R.; Woodard, P.K. A Pattern-based approach to assessment of delayed enhancement in nonischemic cardiomyopathy at MR imaging. RadioGraphics 2009, 29, 89–103. [Google Scholar] [CrossRef]
- Nakamori, S.; Dohi, K.; Ishida, M.; Goto, Y.; Imanaka-Yoshida, K.; Omori, T.; Goto, I.; Kumagai, N.; Fujimoto, N.; Ichikawa, Y.; et al. Native T1 mapping and extracellular volume mapping for the assessment of diffuse myocardial fibrosis in dilated cardiomyopathy. JACC Cardiovasc. Imaging 2018, 11, 48–59. [Google Scholar] [CrossRef]
- Grobner, T.; Prischl, F. Gadolinium and nephrogenic systemic fibrosis. Kidney Int. 2007, 72, 260–264. [Google Scholar] [CrossRef]
- Taylor, A.J.; Cerqueira, M.; Hodgson, J.M.; Mark, D.; Min, J.; O’GAra, P.; Rubin, G.D. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. JACC 2010, 56, 1864–1894. [Google Scholar] [CrossRef]
- Uehara, M.; Takaoka, H.; Kobayashi, Y.; Funabashi, N. Diagnostic accuracy of 320-slice computed-tomography for detection of significant coronary artery stenosis in patients with various heart rates and heart rhythms compared with conventional coronary-angiography. Int. J. Cardiol. 2012, 167, 809–815. [Google Scholar] [CrossRef]
- Takaoka, H.; Uehara, M.; Saito, Y.; Ota, J.; Iida, Y.; Takahashi, M.; Sano, K.; Komuro, I.; Kobayashi, Y. Improved diagnostic performance of new-generation 320-slice computed tomography with forward-projected model-based iterative reconstruction solution for the assessment of late enhancement in left ventricular myocardium. Intern. Med. 2020, 59, 2095–2103. [Google Scholar] [CrossRef]
- Nacif, M.S.; Kawel, N.; Lee, J.J.; Chen, X.; Yao, J.; Zavodni, A.; Sibley, C.T.; Lima, J.A.C.; Liu, S.; Bluemke, D.A. Interstitial myocardial fibrosis assessed as extracellular volume fraction with low-radiation-dose cardiac CT. Radiology 2012, 264, 876–883. [Google Scholar] [CrossRef]
- Avanesov, M.; Münch, J.; Weinrich, J.; Well, L.; Säring, D.; Stehning, C.; Tahir, E.; Bohnen, S.; Radunski, U.K.; Muellerleile, K.; et al. Prediction of the estimated 5-year risk of sudden cardiac death and syncope or non-sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy using late gadolinium enhancement and extracellular volume CMR. Eur. Radiol. 2017, 27, 5136–5145. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; Boer, R.A.d.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef]
- Nakano, M.; Kondo, Y.; Nakano, M.; Kajiyama, T.; Miyazawa, K.; Hayashi, T.; Ito, R.; Takahira, H.; Kobayashi, Y. Predicting therapies in Japanese hypertrophic cardiomyopathy patients with an implantable cardioverter-defibrillator using the 2014 European Society of Cardiology guidelines. Heart Vessel. 2021, 36, 99–104. [Google Scholar] [CrossRef]
- Eriksson, M.J.; Sonnenberg, B.; Woo, A.; Rakowski, P.; Parker, T.G.; Wigle, E.D.; Rakowski, H. Long-term outcome in patients with apical hy-pertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2002, 39, 638–645. [Google Scholar] [CrossRef]
- O’Mahony, C.; Jichi, F.; Pavlou, M.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Gimeno, J.R.; Limongelli, G.; McKenna, W.J.; et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM Risk-SCD). Eur. Hear. J. 2013, 35, 2010–2020. [Google Scholar] [CrossRef] [PubMed]
- Yashima, S.; Takaoka, H.; Iwahana, T.; Nishikawa, Y.; Ota, J.; Aoki, S.; Kinoshita, M.; Takahashi, M.; Sasaki, H.; Suzuki-Eguchi, N.; et al. Evaluation of extracellular volume by computed tomography is useful for prediction of prognosis in dilated cardiomyopathy. Heart Vessel. 2022, 38, 185–194. [Google Scholar] [CrossRef]
- Takaoka, H.; Funabashi, N.; Uehara, M.; Fujimoto, Y.; Kobayashi, Y. Diagnostic accuracy of coronary 320 slice CT angiography using retrospective electrocardiogram gated acquisition compared with virtual prospective electrocardiogram gated acquisition with and without padding. Int. J. Cardiol. 2013, 168, 2811–2815. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, A.; Kitagawa, K.; Goto, Y.; Yamada, A.; Nakamura, S.; Takafuji, M.; Nagasawa, N.; Sakuma, H. Comparison of the different imaging time points in delayed phase cardiac CT for myocardial scar assessment and extracellular volume fraction estimation in patients with old myocardial infarction. Int. J. Cardiovasc. Imaging 2018, 35, 917–926. [Google Scholar] [CrossRef]
- Nieman, K.; Shapiro, M.D.; Ferencik, M.; Nomura, C.H.; Abbara, S.; Hoffmann, U.; Gold, H.K.; Jang, I.-K.; Brady, T.J.; Cury, R.C. Reperfused myocardial infarction: Contrast-enhanced 64-section CT in comparison to MR imaging. Radiology 2008, 247, 49–56. [Google Scholar] [CrossRef]
- Narula, J.; Chandrashekhar, Y.; Ahmadi, A.; Abbara, S.; Berman, D.S.; Blankstein, R.; Leipsic, J.; Newby, D.; Nicol, E.D.; Nieman, K.; et al. SCCT 2021 expert consensus document on coronary computed tomographic angiography: A report of the society of cardiovascular computed tomography. J. Cardiovasc. Comput. Tomogr. 2020, 15, 192–217. [Google Scholar] [CrossRef]
- Austen, W.; Edwards, J.; Frye, R.; Gensini, G.; Gott, V.; Griffith, L.; McGoon, D.; Murphy, M.; Roe, B. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975, 51, 5–40. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quanti-fication by echocardiography in adults: An update from the American society of echocardiography and the european asso-ciation of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Waggoner, A.D.; Flachskampf, F.A.; Pellikka, P.A.; Evangelista, A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J. Am. Soc. Echocardiogr. 2009, 22, 107–133. [Google Scholar] [CrossRef]
- Peduzzi, P.; Concato, J.; Kemper, E.; Holford, T.R.; Feinstein, A.R. A simulation study of the number of events per variable in logistic regression analysis. J. Clin. Epidemiol. 1996, 49, 1373–1379. [Google Scholar] [CrossRef]
- Austin, P.C.; Steyerberg, E.W. Events per variable (EPV) and the relative performance of different strategies for estimating the out-of-sample validity of logistic regression models. Stat. Methods Med. Res. 2014, 26, 796–808. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Yang, F.; Wang, J.; Xu, Y.; Fang, T.; Pu, L.; Zhou, X.; Han, Y.; Chen, Y. Prognostic value of myocardial extracellular volume fraction evaluation based on cardiac magnetic resonance T1 mapping with T1 long and short in hypertrophic cardiomyopathy. Eur. Radiol. 2021, 31, 4557–4567. [Google Scholar] [CrossRef]
- Takaoka, H.; Funabashi, N.; Uehara, M.; Ozawa, K.; Kobayashi, Y. Successful prediction of MACE by myocardial fibrosis on CT in hypertrophic cardiomyopathy patients without obstructed coronary arteries. Int. J. Cardiol. 2015, 199, 34–37. [Google Scholar] [CrossRef]
- Aus dem Siepen, F.; Buss, S.J.; Messroghli, D.; Andre, F.; Lossnitzer, D.; Seitz, S.; Keller, M.; Schnabel, P.A.; Giannitsis, E.; Korosoglou, G.; et al. T1 mapping in dilated cardiomyopathy with cardiac magnetic resonance: Quantification of diffuse myocardial fibrosis and comparison with endomyocardial biopsy. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 210–216. [Google Scholar] [CrossRef]
- Ravenstein, C.d.M.d.; Bouzin, C.; Lazam, S.; Boulif, J.; Amzulescu, M.; Melchior, J.; Pasquet, A.; Vancraeynest, D.; Pouleur, A.-C.; Vanoverschelde, J.-L.J.; et al. Histological Validation of measurement of diffuse interstitial myocardial fibrosis by myocardial extravascular volume fraction from Modified Look-Locker imaging (MOLLI) T1 mapping at 3 T. J. Cardiovasc. Magn. Reson. 2015, 17, 48. [Google Scholar] [CrossRef]
- Treibel, T.A.; Fridman, Y.; Bering, P.; Sayeed, A.; Maanja, M.; Frojdh, F.; Niklasson, L.; Olausson, E.; Wong, T.C.; Kellman, P.; et al. Extracellular volume associates with outcomes more strongly than native or post-contrast myocardial T1. JACC Cardiovasc. Imaging 2020, 13, 44–54. [Google Scholar] [CrossRef]
- Mirelis, J.G.; Sánchez-González, J.; Zorio, E.; Ripoll-Vera, T.; Salguero-Bodes, R.; Filgueiras-Rama, D.; González-López, E.; Gallego-Delgado, M.; Fernández-Jiménez, R.; Soleto, M.J.; et al. Myocardial extracellular volume is not associated with malignant ventricular arrhythmias in high-risk hypertrophic cardiomyopathy. Rev. Esp. Cardiol. 2017, 70, 933–940. [Google Scholar] [CrossRef]
- Han, D.; Lin, A.; Kuronuma, K.; Gransar, H.; Dey, D.; Friedman, J.D.; Berman, D.S.; Tamarappoo, B.K. Cardiac computed tomography for quantification of myocardial extracellular volume fraction: A systematic review and meta-analysis. JACC Cardiovasc. Imaging 2023, 16, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, H.; Liu, G.; Wu, C.; Ma, Y.; Li, S.; Zheng, Y.; Zhang, J. CT for the evaluation of myocardial extracellular volume with MRI as reference: A systematic review and meta-analysis. Eur. Radiol. 2023, 33, 8464–8476. [Google Scholar] [CrossRef]
- Aoki, S.; Takaoka, H.; Ota, J.; Kanaeda, T.; Sakai, T.; Matsumoto, K.; Noguchi, Y.; Nishikawa, Y.; Yashima, S.; Suzuki, K.; et al. Strong diagnostic performance of single energy 256-row multidetector computed tomography with deep learning image reconstruction in the assessment of myocardial fibrosis. Intern. Med. 2024, 63, 2499–2507. [Google Scholar] [CrossRef]
- Hayashi, H.; Oda, S.; Kidoh, M.; Yamaguchi, S.; Yoshimura, F.; Takashio, S.; Usuku, H.; Nagayama, Y.; Nakaura, T.; Ueda, M.; et al. Myocardial extracellular volume quantification in cardiac amyloidosis: A comparative study between cardiac computed tomography and magnetic resonance imaging. Eur. Radiol. 2023, 34, 1016–1025. [Google Scholar] [CrossRef]
- Ommen, S.R.; Ho, C.Y.; Asif, I.M.; Balaji, S.; Burke, M.A.; Day, S.M.; Dearani, J.A.; Epps, K.C.; Evanovich, L.; Ferrari, V.A. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR guideline for the management of Hypertrophic Cardiomyopathy: A report of the American heart association/American college of cardiology joint committee on clinical practice guidelines. Circulation 2024, 149, e1239–e1311. [Google Scholar]
- Lopes, L.R.; Losi, M.-A.; Sheikh, N.; Laroche, C.; Charron, P.; Gimeno, J.; Kaski, J.P.; Maggioni, A.P.; Tavazzi, L.; Arbustini, E.; et al. Association between common cardiovascular risk factors and clinical phenotype in patients with hypertrophic cardiomyopathy from the European Society of Cardiology (ESC) EurObservational Research Programme (EORP) Cardiomyopathy/Myocarditis registry. Eur. Heart J.—Qual. Care Clin. Outcomes 2022, 9, 42–53. [Google Scholar] [CrossRef]
- De Stefano, D.; Vaccarino, F.; Santucci, D.; Parillo, M.; Nenna, A.; Loreni, F.; Ferrisi, C.; Giacinto, O.; Barbato, R.; Mastroianni, C.; et al. Delayed enhancement in cardiac CT: A potential alternative to cardiac MRI? technical updates and clinical considerations. Appl. Sci. 2024, 14, 4275. [Google Scholar] [CrossRef]
- Oyama-Manabe, N.; Oda, S.; Ohta, Y.; Takagi, H.; Kitagawa, K.; Jinzaki, M. Myocardial late enhancement and extracellular volume with single-energy, dual-energy, and photon-counting computed tomography. J. Cardiovasc. Comput. Tomogr. 2024, 18, 3–10. [Google Scholar] [CrossRef]
- Matsunaga, Y.; Chida, K.; Kondo, Y.; Kobayashi, K.; Kobayashi, M.; Minami, K.; Suzuki, S.; Asada, Y. Diagnostic reference levels and achievable doses for common computed tomography examinations: Results from the japanese nationwide dose survey. Br. J. Radiol. 2019, 92, 20180290. [Google Scholar] [CrossRef]
- Regita, K.; Choirul, A.; Heri, S.; Dito Adi, R. Effects of tube voltage and phantom diameter on noise inhomogeneity of CT image. Int. J. Innov. Sci. Res. Technol. 2024, 9, 2529–2533. [Google Scholar]
- Chang, S.; Han, K.; Youn, J.-C.; Im, D.J.; Kim, J.Y.; Suh, Y.J.; Hong, Y.J.; Hur, J.; Kim, Y.J.; Choi, B.W.; et al. Utility of dual-energy ct-based monochromatic imaging in the assessment of myocardial delayed enhancement in patients with cardiomyopathy. Radiology 2018, 287, 442–451. [Google Scholar] [CrossRef]
- Kitaoka, H.; Izumi, C.; Izumiya, Y.; Inomata, T.; Ueda, M.; Kubo, T.; Koyama, J.; Sano, M.; Sekijima, Y.; Tahara, N.; et al. JCS 2020 guideline on diagnosis and treatment of cardiac amyloidosis. Circ. J. 2020, 84, 1610–1671. [Google Scholar] [CrossRef] [PubMed]
- Gama, F.; Rosmini, S.; Bandula, S.; Patel, K.P.; Massa, P.; Tobon-Gomez, C.; Ecke, K.; Stroud, T.; Condron, M.; Thornton, G.D.; et al. Extracellular volume fraction by computed tomography predicts long-term prognosis among patients with cardiac amyloidosis. JACC Cardiovasc. Imaging 2022, 15, 2082–2094. [Google Scholar] [CrossRef] [PubMed]
- Scully, P.R.; Patel, K.P.; Saberwal, B.; Klotz, E.; Augusto, J.B.; Thornton, G.D.; Hughes, R.K.; Manisty, C.; Lloyd, G.; Newton, J.D. Identifying cardiac amyloid in aortic stenosis: ECV quantification by CT in TAVR patients. JACC Cardiovasc. Imaging 2020, 13, 2177–2189. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.K.; Knott, K.D.; Malcolmson, J.; Augusto, J.B.; Mohiddin, S.A.; Kellman, P.; Moon, J.C.; Captur, G. Apical hypertrophic cardiomyopathy: The variant less known. J. Am. Heart Assoc. 2020, 9, e015294. [Google Scholar] [CrossRef]

| MACE (+) (n = 15) | MACE (−) (n = 86) | p-Value | |
|---|---|---|---|
| Age, years | 63 ± 16 | 66 ± 11 | 0.42 |
| Male, n (%) | 8 (53) | 58 (67) | 0.38 |
| Body mass index, kg/m2 | 24 ± 4 | 25 ± 4 | 0.67 |
| Atrial fibrillation, n (%) | 7 (47) | 20 (23) | 0.11 |
| Hypertension, n (%) | 6 (40) | 57 (66) | 0.08 |
| Dyslipidemia, n (%) | 5 (33) | 31 (36) | 1.0 |
| Diabetes, n (%) | 2 (13) | 17 (20) | 0.73 |
| Family history of SCD, n (%) | 1 (7) | 2 (2) | 0.40 |
| Syncope, n (%) | 0 (0) | 8 (9) | 0.60 |
| Previous NSVT, n (%) | 5 (33) | 23 (27) | 0.76 |
| eGFR, mL/min/1.73 m2 | 63 ± 21 | 69 ± 16 | 0.32 |
| Administration of β-blocker, n (%) | 7 (50) | 36 (43) | 0.77 |
| Administration of statin, n (%) | 5 (36) | 22 (26) | 0.52 |
| Administration of ACE inhibitor or ARB, n (%) | 4 (29) | 40 (48) | 0.25 |
| Follow-up period (month) | 49 ± 46 | 67 ± 56 | 0.19 |
| MACE (+) (n = 15) | MACE (−) (n = 86) | p-Value | |
|---|---|---|---|
| LVEF on TTE, % | 56 ± 13 | 67 ± 7 | 0.007 ** |
| Maximum LV wall thickness on TTE, mm | 19 ± 5 | 17 ± 4 | 0.18 |
| LA diameter on TTE, mm | 48 ± 9 | 42 ± 7 | 0.04 * |
| LVOT gradient on TTE, mmHg | 7 ± 4 | 9 ± 14 | 0.31 |
| LVOT gradient > 30 mmHg on TTE, n (%) | 1 (7) | 5 (6) | 1.0 |
| LVDd on TTE, mm | 50 ± 10 | 46 ± 6 | 0.15 |
| LVDs on TTE, mm | 35 ± 10 | 28 ± 5 | 0.025 * |
| Valvular heart disease (≥2+) | 1 (7) | 2 (2) | 0.38 |
| HCM SCD risk score, % | 2.4 ± 1.1 | 2.0 ± 1.5 | 0.26 |
| Significant coronary artery stenosis, n (%) | 3 (20) | 11 (13) | 0.44 |
| LIE on CT, n (%) | 10 (67) | 40 (47) | 0.17 |
| LV-ECV on CT (%) | 42 ± 8 | 34 ± 6 | 0.002 ** |
| DHCM, n (%) | 6 (40) | 2 (2) | <0.001 ** |
| Variable | Univariable | ||
|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | p-Value | |
| LVEF (%) | 0.90 | 0.86–0.94 | <0.001 ** |
| LV-ECV on CT (%) | 1.15 | 1.08–1.23 | <0.001 ** |
| LAD | 1.07 | 1.01–1.12 | 0.029 * |
| LVDs | 1.13 | 1.06–1.20 | <0.001 ** |
| DHCM | 8.12 | 2.9–23 | <0.001 ** |
| Variable | Multivariable | ||
|---|---|---|---|
| Hazard Ratio | 95% Confidence Interval | p-Value | |
| LVEF (%) | 0.93 | 0.88–0.98 | 0.006 ** |
| LV-ECV on CT (%) | 1.12 | 1.04–1.20 | 0.003 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoki, S.; Takaoka, H.; Kanaeda, T.; Asada, K.; Ota, J.; Noguchi, Y.; Matsumoto, M.; Nishikawa, Y.; Suzuki, K.; Yashima, S.; et al. Extracellular Volume Fraction Analysis on Cardiac Computed Tomography Is Useful for Predicting the Prognosis of Hypertrophic Cardiomyopathy. J. Cardiovasc. Dev. Dis. 2025, 12, 372. https://doi.org/10.3390/jcdd12090372
Aoki S, Takaoka H, Kanaeda T, Asada K, Ota J, Noguchi Y, Matsumoto M, Nishikawa Y, Suzuki K, Yashima S, et al. Extracellular Volume Fraction Analysis on Cardiac Computed Tomography Is Useful for Predicting the Prognosis of Hypertrophic Cardiomyopathy. Journal of Cardiovascular Development and Disease. 2025; 12(9):372. https://doi.org/10.3390/jcdd12090372
Chicago/Turabian StyleAoki, Shuhei, Hiroyuki Takaoka, Tomonori Kanaeda, Kazunari Asada, Joji Ota, Yoshitada Noguchi, Moe Matsumoto, Yusei Nishikawa, Katsuya Suzuki, Satomi Yashima, and et al. 2025. "Extracellular Volume Fraction Analysis on Cardiac Computed Tomography Is Useful for Predicting the Prognosis of Hypertrophic Cardiomyopathy" Journal of Cardiovascular Development and Disease 12, no. 9: 372. https://doi.org/10.3390/jcdd12090372
APA StyleAoki, S., Takaoka, H., Kanaeda, T., Asada, K., Ota, J., Noguchi, Y., Matsumoto, M., Nishikawa, Y., Suzuki, K., Yashima, S., Kinoshita, M., Suzuki-Eguchi, N., Sasaki, H., Takahashi, K., Ozawa, Y., Inaba, Y., & Kobayashi, Y. (2025). Extracellular Volume Fraction Analysis on Cardiac Computed Tomography Is Useful for Predicting the Prognosis of Hypertrophic Cardiomyopathy. Journal of Cardiovascular Development and Disease, 12(9), 372. https://doi.org/10.3390/jcdd12090372







