Modeling GATAD1-Associated Dilated Cardiomyopathy in Adult Zebrafish
Abstract
:1. Introduction
2. Experimental Section
2.1. Zebrafish Lines and Maintenance
2.2. Bioinformatics Analysis
2.3. Whole-Mount in Situ Hybridization
2.4. Immunostaining
2.5. Real-Time Reverse Transcription PCR
2.6. Generation of TALEN Mutants
2.7. Generation of Transgenic Fish Lines
2.8. Stressing Fish with a High-Cholesterol Diet
2.9. Stressing Zebrafish Embryos with Ethanol
2.10. Swimming Capacity Test
2.11. Assessment of Fish Survival Curve And Ventricle Area
2.12. Statistical Analysis
3. Results
3.1. Comparison of Gatad1 Gene Sequences between Zebrafish and Humans

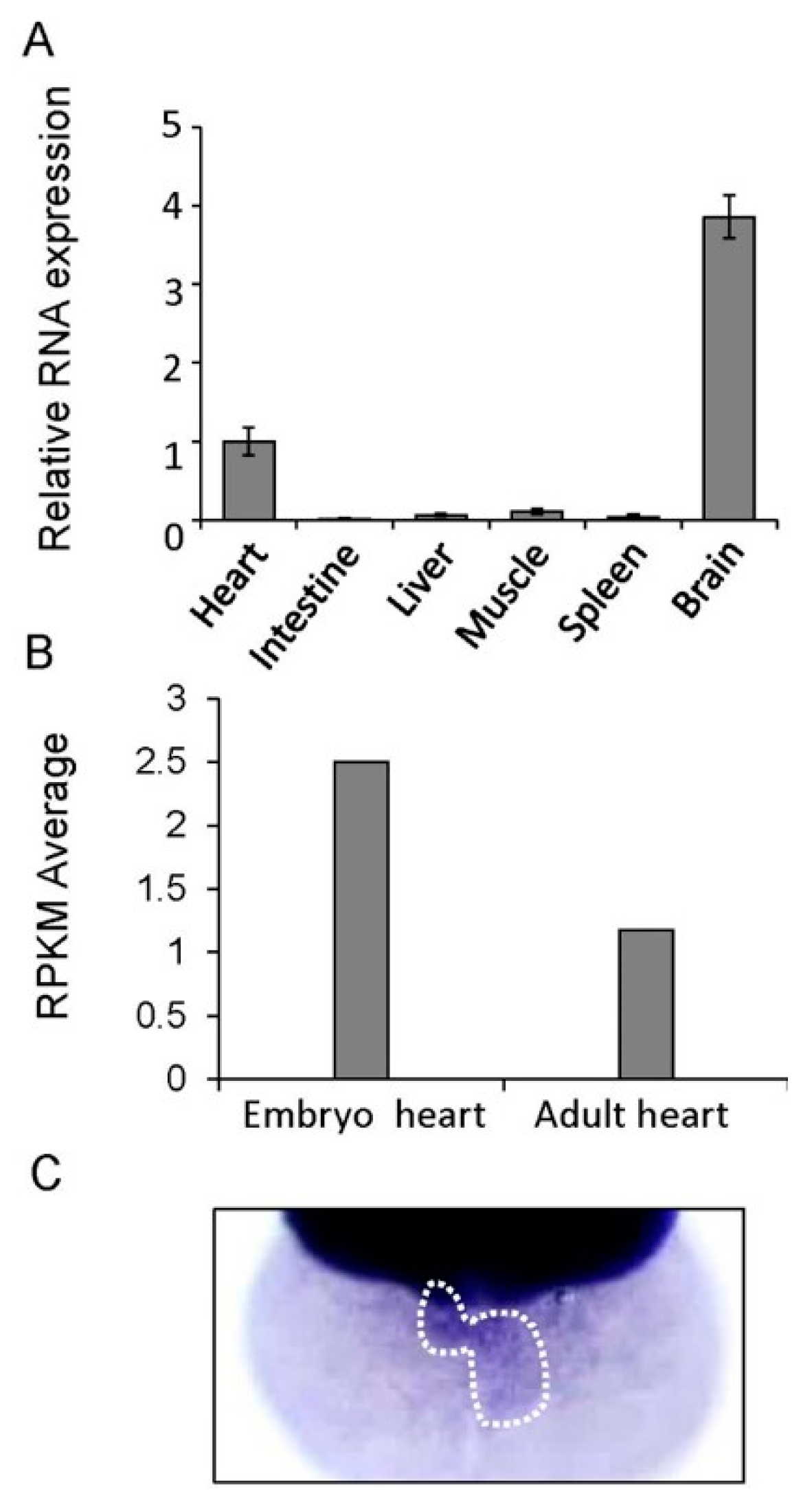
3.2. Tissue Distribution of the Gatad1 mRNA
3.3. Subcellular Expression Pattern of Gatad1 Protein in the Nucleus and Sarcomeric I Band

3.4. Heart Failure-Like Phenotypes in Gatad1 Knock-out Fish

3.5. Phenotypes in Transgenic Fish Line Expressing GATAD1 Containing the S102P Mutation

4. Discussion
4.1. Conservation of Zebrafish as a Model for Cardiomyopathy Genes
4.2. Gene Expression Can be Profiled in Zebrafish
4.3. Genetic Engineering Tools are Available in Zebrafish for Modeling Cardiomyopathy
4.4. More Cardiomyopathy Phenotyping Tools are Needed in Adult Zebrafish
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ahmad, F.; Seidman, J.G.; Seidman, C.E. The genetic basis for cardiac remodeling. Annu. Rev. Genom. Hum. Genet. 2005, 6, 185–216. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Maron, M.S.; Semsarian, C. Genetics of hypertrophic cardiomyopathy after 20 years: Clinical perspectives. J. Am. Coll. Cardiol. 2012, 60, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A. Molecular etiology and pathogenesis of hereditary cardiomyopathy. Circ. J. 2008, 72 (Suppl. A), A38–A48. [Google Scholar] [CrossRef] [PubMed]
- Norton, N.; Li, D.; Hershberger, R.E. Next-generation sequencing to identify genetic causes of cardiomyopathies. Curr. Opin. Cardiol. 2012, 27, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.E.; Frangakis, S.; Katsanis, N. Interpreting human genetic variation with in vivo zebrafish assays. Biochim. Biophys. Acta 2014, 1842, 1960–1970. [Google Scholar] [CrossRef] [PubMed]
- Staudt, D.; Stainier, D. Uncovering the molecular and cellular mechanisms of heart development using the zebrafish. Annu. Rev. Genet. 2012, 46, 397–418. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.H.; Zhang, Y.; Ding, Y.; Ross, C.A.; Li, H.; Olson, T.M.; Xu, X. The Cardiac Transcriptome and Dilated Cardiomyopathy Genes in Zebrafish. Circ. Cardiovasc. Genet. 2015, 8, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Meiler, S.E.; Zhong, T.P.; Mohideen, M.; Crossley, D.A.; Burggren, W.W.; Fishman, M.C. Cardiomyopathy in zebrafish due to mutation in an alternatively spliced exon of titin. Nat. Genet. 2002, 30, 205–209. [Google Scholar] [PubMed]
- Sehnert, A.J.; Huq, A.; Weinstein, B.M.; Walker, C.; Fishman, M.; Stainier, D.Y. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nat. Genet. 2002, 31, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Ruparelia, A.A.; Oorschot, V.; Vaz, R.; Ramm, G.; Bryson-Richardson, R.J. Zebrafish models of BAG3 myofibrillar myopathy suggest a toxic gain of function leading to BAG3 insufficiency. Acta Neuropathol. 2014, 128, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Bendig, G.; Grimmler, M.; Huttner, I.G.; Wessels, G.; Dahme, T.; Just, S.; Trano, N.; Katus, H.A.; Fishman, M.C.; Rottbauer, W. Integrin-linked kinase, a novel component of the cardiac mechanical stretch sensor, controls contractility in the zebrafish heart. Genes Dev. 2006, 20, 2361–2372. [Google Scholar] [CrossRef] [PubMed]
- Hassel, D.; Dahme, T.; Erdmann, J.; Meder, B.; Huge, A.; Stoll, M.; Just, S.; Hess, A.; Ehlermann, P.; Weichenhan, D.; et al. Nexilin mutations destabilize cardiac Z-disks and lead to dilated cardiomyopathy. Nat. Med. 2009, 15, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.; Rottbauer, W.; Just, S. Recent progress in the use of zebrafish for novel cardiac drug discovery. Expert Opin. Drug Discov. 2015, 10, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.R.; Robinson, T.Y.; Sachidanandan, C.; Kelly, A.E.; Coy, S.; Peterson, R.T.; MacRae, C.A. In vivo natriuretic peptide reporter assay identifies chemical modifiers of hypertrophic cardiomyopathy signalling. Cardiovasc. Res. 2012, 93, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Amsterdam, A.; Nissen, R.M.; Sun, Z.; Swindell, E.C.; Farrington, S.; Hopkins, N. Identification of 315 genes essential for early zebrafish development. Proc. Natl. Acad. Sci. USA 2004, 101, 12792–12797. [Google Scholar] [CrossRef] [PubMed]
- Kok, F.O.; Shin, M.; Ni, C.W.; Gupta, A.; Grosse, A.S.; van Impel, A.; Kirchmaier, B.C.; Peterson-Maduro, J.; Kourkoulis, G.; Male, I.; et al. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev. Cell 2015, 32, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Kontarakis, Z.; Gerri, C.; Nolte, H.; Holper, S.; Kruger, M.; Stainier, D.Y. Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 2015, 524, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Hoage, T.; Bai, P.; Ding, Y.; Chen, Z.; Zhang, R.; Huang, W.; Jahangir, A.; Paw, B.; Li, Y.G.; et al. Cardiac hypertrophy involves both myocyte hypertrophy and hyperplasia in anemic zebrafish. PLoS ONE 2009, 4, e6596. [Google Scholar] [CrossRef] [PubMed]
- Paw, B.H.; Davidson, A.J.; Zhou, Y.; Li, R.; Pratt, S.J.; Lee, C.; Trede, N.S.; Brownlie, A.; Donovan, A.; Liao, E.C.; et al. Cell-specific mitotic defect and dyserythropoiesis associated with erythroid band 3 deficiency. Nat. Genet. 2003, 34, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xu, X. Anemic Zebrafish Models of Cardiomyopathy. In Methods Pharmacol. Toxicol.; 2012; pp. 41–54. [Google Scholar]
- Hayward, R.; Hydock, D.S. Doxorubicin cardiotoxicity in the rat: An in vivo characterization. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 20–32. [Google Scholar] [PubMed]
- Christiansen, S.; Autschbach, R. Doxorubicin in experimental and clinical heart failure. Eur. J. Cardiothorac. Surg. 2006, 30, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Sun, X.; Huang, W.; Hoage, T.; Redfield, M.; Kushwaha, S.; Sivasubbu, S.; Lin, X.; Ekker, S.; Xu, X. Haploinsufficiency of target of rapamycin attenuates cardiomyopathies in adult zebrafish. Circ. Res. 2011, 109, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Asimaki, A.; Kapoor, S.; Plovie, E.; Karin Arndt, A.; Adams, E.; Liu, Z.; James, C.A.; Judge, D.P.; Calkins, H.; Churko, J.; et al. Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy. Sci. Transl. Med. 2014, 6, 240ra274. [Google Scholar] [CrossRef] [PubMed]
- Haffter, P.; Granato, M.; Brand, M.; Mullins, M.C.; Hammerschmidt, M.; Kane, D.A.; Odenthal, J.; van Eeden, F.J.; Jiang, Y.J.; Heisenberg, C.P.; et al. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development 1996, 123, 1–36. [Google Scholar] [PubMed]
- Driever, W.; Solnica-Krezel, L.; Schier, A.F.; Neuhauss, S.C.; Malicki, J.; Stemple, D.L.; Stainier, D.Y.; Zwartkruis, F.; Abdelilah, S.; Rangini, Z.; et al. A genetic screen for mutations affecting embryogenesis in zebrafish. Development 1996, 123, 37–46. [Google Scholar] [PubMed]
- Wang, D.; Jao, L.E.; Zheng, N.; Dolan, K.; Ivey, J.; Zonies, S.; Wu, X.; Wu, K.; Yang, H.; Meng, Q.; et al. Efficient genome-wide mutagenesis of zebrafish genes by retroviral insertions. Proc. Natl. Acad. Sci. USA 2007, 104, 12428–12433. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Hartjes, K.A.; Nelson, T.J.; Xu, X.; Ekker, S.C. New and TALENted Genome Engineering Toolbox. Circ. Res. 2013, 113, 571–587. [Google Scholar] [CrossRef] [PubMed]
- Cade, L.; Reyon, D.; Hwang, W.Y.; Tsai, S.Q.; Patel, S.; Khayter, C.; Joung, J.K.; Sander, J.D.; Peterson, R.T.; Yeh, J.R. Highly efficient generation of heritable zebrafish gene mutations using homo- and heterodimeric TALENs. Nucleic Acids Res. 2012, 40, 8001–8010. [Google Scholar] [CrossRef] [PubMed]
- Dahlem, T.J.; Hoshijima, K.; Jurynec, M.J.; Gunther, D.; Starker, C.G.; Locke, A.S.; Weis, A.M.; Voytas, D.F.; Grunwald, D.J. Simple methods for generating and detecting locus-specific mutations induced with TALENs in the zebrafish genome. PLoS Genet. 2012, 8, e1002861. [Google Scholar] [CrossRef] [PubMed]
- Auer, T.O.; Duroure, K.; de Cian, A.; Concordet, J.P.; del Bene, F. Highly efficient CRISPR/Cas9-mediated knock-in in zebrafish by homology-independent DNA repair. Genome Res. 2014, 24, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Chen, J.; Solnica-Krezel, L. Efficient homologous recombination-mediated genome engineering in zebrafish using TALE nucleases. Development 2014, 141, 3807–3818. [Google Scholar] [CrossRef] [PubMed]
- Kwan, K.M.; Fujimoto, E.; Grabher, C.; Mangum, B.D.; Hardy, M.E.; Campbell, D.S.; Parant, J.M.; Yost, H.J.; Kanki, J.P.; Chien, C.B. The Tol2kit: A multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 2007, 236, 3088–3099. [Google Scholar] [CrossRef] [PubMed]
- Tsuruga, T.; Kanamoto, T.; Kato, T.; Yamashita, H.; Miyagawa, K.; Mishima, H.K. Ocular development-associated gene (ODAG), a novel gene highly expressed in ocular development. Gene 2002, 290, 125–130. [Google Scholar] [CrossRef]
- Sasaki, T.; Watanabe, W.; Muranishi, Y.; Kanamoto, T.; Aihara, M.; Miyazaki, K.; Tamura, H.; Saeki, T.; Oda, H.; Souchelnytskyi, N.; et al. Elevated intraocular pressure, optic nerve atrophy, and impaired retinal development in ODAG transgenic mice. Investig. Ophthalmol. Vis. Sci. 2009, 50, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Theis, J.L.; Sharpe, K.M.; Matsumoto, M.E.; Chai, H.S.; Nair, A.A.; Theis, J.D.; de Andrade, M.; Wieben, E.D.; Michels, V.V.; Olson, T.M. Homozygosity mapping and exome sequencing reveal GATAD1 mutation in autosomal recessive dilated cardiomyopathy. Circ. Cardiovasc. Genet. 2011, 4, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Heidecker, B.; Lamirault, G.; Kasper, E.K.; Wittstein, I.S.; Champion, H.C.; Breton, E.; Russell, S.D.; Hall, J.; Kittleson, M.M.; Baughman, K.L.; et al. The gene expression profile of patients with new-onset heart failure reveals important gender-specific differences. Eur. Heart J. 2010, 31, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Cermak, T.; Doyle, E.L.; Christian, M.; Wang, L.; Zhang, Y.; Schmidt, C.; Baller, J.A.; Somia, N.V.; Bogdanove, A.J.; Voytas, D.F. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011, 39, e82. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.C.; Lee, H.B.; Clark, K.J.; Ekker, S.C. High efficiency In vivo genome engineering with a simplified 15-RVD GoldyTALEN design. PLoS ONE 2013, 8, e65259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Zhang, R.; Lin, X.; Xu, X. Wnt3a regulates the development of cardiac neural crest cells by modulating expression of cysteine-rich intestinal protein 2 in rhombomere 6. Circ. Res. 2008, 102, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, X. Immunostaining of dissected zebrafish embryonic heart. J. Vis. Exp. JoVE 2012, e3510. [Google Scholar] [CrossRef] [PubMed]
- Sander, J.D.; Zaback, P.; Joung, J.K.; Voytas, D.F.; Dobbs, D. Zinc Finger Targeter (ZiFiT): An engineered zinc finger/target site design tool. Nucleic Acids Res. 2007, 35, W599–W605. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Green, S.R.; Baek, J.S.; Lee, S.H.; Ellett, F.; Deer, E.; Lieschke, G.J.; Witztum, J.L.; Tsimikas, S.; Miller, Y.I. In vivo visualization and attenuation of oxidized lipid accumulation in hypercholesterolemic zebrafish. J. Clin. Investig. 2011, 121, 4861–4869. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Yoon, J.; Jang, M.Y.; Na, Y.; Ko, Y.; Choi, J.H.; Seok, S.H. High cholesterol diet induces IL-1beta expression in adult but not larval zebrafish. PLoS ONE 2013, 8, e66970. [Google Scholar]
- Sarmah, S.; Marrs, J.A. Complex cardiac defects after ethanol exposure during discrete cardiogenic events in zebrafish: Prevention with folic acid. Dev. Dyn. 2013, 242, 1184–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Fang, Y.; Xu, X.; Lu, G.; Chen, Z. Evidence of an Association between Age-Related Functional Modifications and Pathophysiological Changes in Zebrafish Heart. Gerontology 2015, 61, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Panakova, D.; Kikuchi, K.; Holdway, J.E.; Gemberling, M.; Burris, J.S.; Singh, S.P.; Dickson, A.L.; Lin, Y.F.; Sabeh, M.K.; et al. The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development 2011, 138, 3421–3430. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; Tu, C.T.; Hsiao, C.D.; Hsieh, F.J.; Tsai, H.J. Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev. Dyn. 2003, 228, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Thisse, B.; Thisse, C. In situ hybridization on whole-mount zebrafish embryos and young larvae. Methods Mol. Biol. 2014, 1211, 53–67. [Google Scholar] [PubMed]
- Yang, J.; Xu, X. Alpha-Actinin2 is required for the lateral alignment of Z discs and ventricular chamber enlargement during zebrafish cardiogenesis. FASEB J. 2012, 26, 4230–4242. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, K.; Wu, C.C.; Kurth, T.; Weidinger, G. Regeneration of cryoinjury induced necrotic heart lesions in zebrafish is associated with epicardial activation and cardiomyocyte proliferation. PLoS ONE 2011, 6, e18503. [Google Scholar] [CrossRef] [PubMed]
- Chablais, F.; Jazwinska, A. The regenerative capacity of the zebrafish heart is dependent on TGFbeta signaling. Development 2012, 139, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Kettleborough, R.N.; Busch-Nentwich, E.M.; Harvey, S.A.; Dooley, C.M.; de Bruijn, E.; van Eeden, F.; Sealy, I.; White, R.J.; Herd, C.; Nijman, I.J.; et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature 2013, 496, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.J.; Balciunas, D.; Pogoda, H.M.; Ding, Y.; Westcot, S.E.; Bedell, V.M.; Greenwood, T.M.; Urban, M.D.; Skuster, K.J.; Petzold, A.M.; et al. In vivo protein trapping produces a functional expression codex of the vertebrate proteome. Nat. Methods 2011, 8, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.J.; Argue, D.P.; Petzold, A.M.; Ekker, S.C. Zfishbook: Connecting you to a world of zebrafish revertible mutants. Nucleic Acids Res. 2012, 40, D907–D911. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.; Wang, Z.; Hu, Y.; Wu, Y.; Luo, Z.; Yang, Z.; Zu, Y.; Li, W.; Huang, P.; Tong, X.; et al. Chromosomal deletions and inversions mediated by TALENs and CRISPR/Cas in zebrafish. Nucleic Acids Res. 2013, 41, e141. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Hall, V.L.; Kok, F.O.; Shin, M.; McNulty, J.C.; Lawson, N.D.; Wolfe, S.A. Targeted chromosomal deletions and inversions in zebrafish. Genome Res. 2013, 23, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Bedell, V.M.; Wang, Y.; Campbell, J.M.; Poshusta, T.L.; Starker, C.G.; Krug, R.G., 2nd; Tan, W.; Penheiter, S.G.; Ma, A.C.; Leung, A.Y.; et al. In vivo genome editing using a high-efficiency TALEN system. Nature 2012, 491, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Zu, Y.; Tong, X.; Wang, Z.; Liu, D.; Pan, R.; Li, Z.; Hu, Y.; Luo, Z.; Huang, P.; Wu, Q.; et al. TALEN-mediated precise genome modification by homologous recombination in zebrafish. Nat. Methods 2013, 10, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Lawson, N.D.; Weinstein, B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002, 248, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Dong, L.; Ahn, J.; Dao, D.; Hammerschmidt, M.; Chen, J.N. FoxH1 negatively modulates flk1 gene expression and vascular formation in zebrafish. Dev. Biol. 2007, 304, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Gupta, V.; Wang, J.; Holdway, J.E.; Wills, A.A.; Fang, Y.; Poss, K.D. tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development 2011, 138, 2895–2902. [Google Scholar] [CrossRef] [PubMed]
- Hans, S.; Kaslin, J.; Freudenreich, D.; Brand, M. Temporally-controlled site-specific recombination in zebrafish. PLoS ONE 2009, 4, e4640. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Maillet, M.; Miano, J.M.; Molkentin, J.D. Lost in transgenesis: A user’s guide for genetically manipulating the mouse in cardiac research. Circ. Res. 2012, 111, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Molkentin, J.D.; Robbins, J. With great power comes great responsibility: Using mouse genetics to study cardiac hypertrophy and failure. J. Mol. Cell. Cardiol. 2009, 46, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.; Edwards, J.; Ferguson-Mignan, T.F.; Cobb, M.; Mongan, N.P.; Rutland, C.S. Genetics of Human and Canine Dilated Cardiomyopathy. Int. J. Genom. 2015, 2015, 204823. [Google Scholar] [CrossRef] [PubMed]
- James, J.F.; Hewett, T.E.; Robbins, J. Cardiac physiology in transgenic mice. Circ. Res. 1998, 82, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Burd, L.; Deal, E.; Rios, R.; Adickes, E.; Wynne, J.; Klug, M.G. Congenital heart defects and fetal alcohol spectrum disorders. Congenit. Heart Disease 2007, 2, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Milan, D.J.; Jones, I.L.; Ellinor, P.T.; MacRae, C.A. In vivo recording of adult zebrafish electrocardiogram and assessment of drug-induced QT prolongation. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H269–H273. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zhang, Y.; Yu, F.; Parks, E.; Lyman, A.; Wu, Q.; Ai, L.; Hu, C.H.; Zhou, Q.; Shung, K.; et al. Micro-electrocardiograms to study post-ventricular amputation of zebrafish heart. Ann. Biomed. Eng. 2009, 37, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhao, Y.; Gu, J.; Quigley, K.L.; Chi, N.C.; Tai, Y.C.; Hsiai, T.K. Flexible microelectrode arrays to interface epicardial electrical signals with intracardial calcium transients in zebrafish hearts. Biomed. Microdevices 2012, 14, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Lien, C.L.; Xu, X.; Shung, K.K. In vivo cardiac imaging of adult zebrafish using high frequency ultrasound (45–75 MHz). Ultrasound Med. Biol. 2008, 34, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cao, H.; Kang, B.J.; Jen, N.; Yu, F.; Lee, C.A.; Fei, P.; Park, J.; Bohlool, S.; Lash-Rosenberg, L.; et al. Hemodynamics and ventricular function in a zebrafish model of injury and repair. Zebrafish 2014, 11, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Lee, P.Y.; Huang, C.C.; Sun, L.; Shung, K.K. A study of the adult zebrafish ventricular function by retrospective Doppler-gated ultrahigh-frame-rate echocardiography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 1827–1837. [Google Scholar] [CrossRef] [PubMed]
- Bosisio, M.R.; Hasquenoph, J.M.; Sandrin, L.; Laugier, P.; Bridal, S.L.; Yon, S. Real-time chirp-coded imaging with a programmable ultrasound biomicroscope. IEEE Trans. Biomed. Eng. 2010, 57, 654–664. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Shah, S.; Olson, T.M.; Xu, X. Modeling GATAD1-Associated Dilated Cardiomyopathy in Adult Zebrafish. J. Cardiovasc. Dev. Dis. 2016, 3, 6. https://doi.org/10.3390/jcdd3010006
Yang J, Shah S, Olson TM, Xu X. Modeling GATAD1-Associated Dilated Cardiomyopathy in Adult Zebrafish. Journal of Cardiovascular Development and Disease. 2016; 3(1):6. https://doi.org/10.3390/jcdd3010006
Chicago/Turabian StyleYang, Jingchun, Sahrish Shah, Timothy M. Olson, and Xiaolei Xu. 2016. "Modeling GATAD1-Associated Dilated Cardiomyopathy in Adult Zebrafish" Journal of Cardiovascular Development and Disease 3, no. 1: 6. https://doi.org/10.3390/jcdd3010006
APA StyleYang, J., Shah, S., Olson, T. M., & Xu, X. (2016). Modeling GATAD1-Associated Dilated Cardiomyopathy in Adult Zebrafish. Journal of Cardiovascular Development and Disease, 3(1), 6. https://doi.org/10.3390/jcdd3010006





