Carotid and Femoral Atherosclerotic Plaques in Asymptomatic and Non-Treated Subjects: Cardiovascular Risk Factors, 10-Years Risk Scores, and Lipid Ratios’ Capability to Detect Plaque Presence, Burden, Fibro-Lipid Composition and Geometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Interview and Anthropometric Measurements
2.3. Lipid Ratios and 10. y./CVR Scores Quantification
2.4. Cardiovascular Evaluation
2.4.1. Heart Rate, Brachial, and Aortic Blood Pressure
2.4.2. Carotid and Femoral Artery Ultrasound
2.4.3. Plaques’ Composition and Geometry
2.5. Data and Statistical Analysis
2.5.1. Association between Subject Characteristics and Plaques Presence, Burden, and Characteristics
2.5.2. Capability of Subject’s Characteristics to Detect Plaque Presence and/or Burden
3. Results
3.1. Subjects’ Characteristics
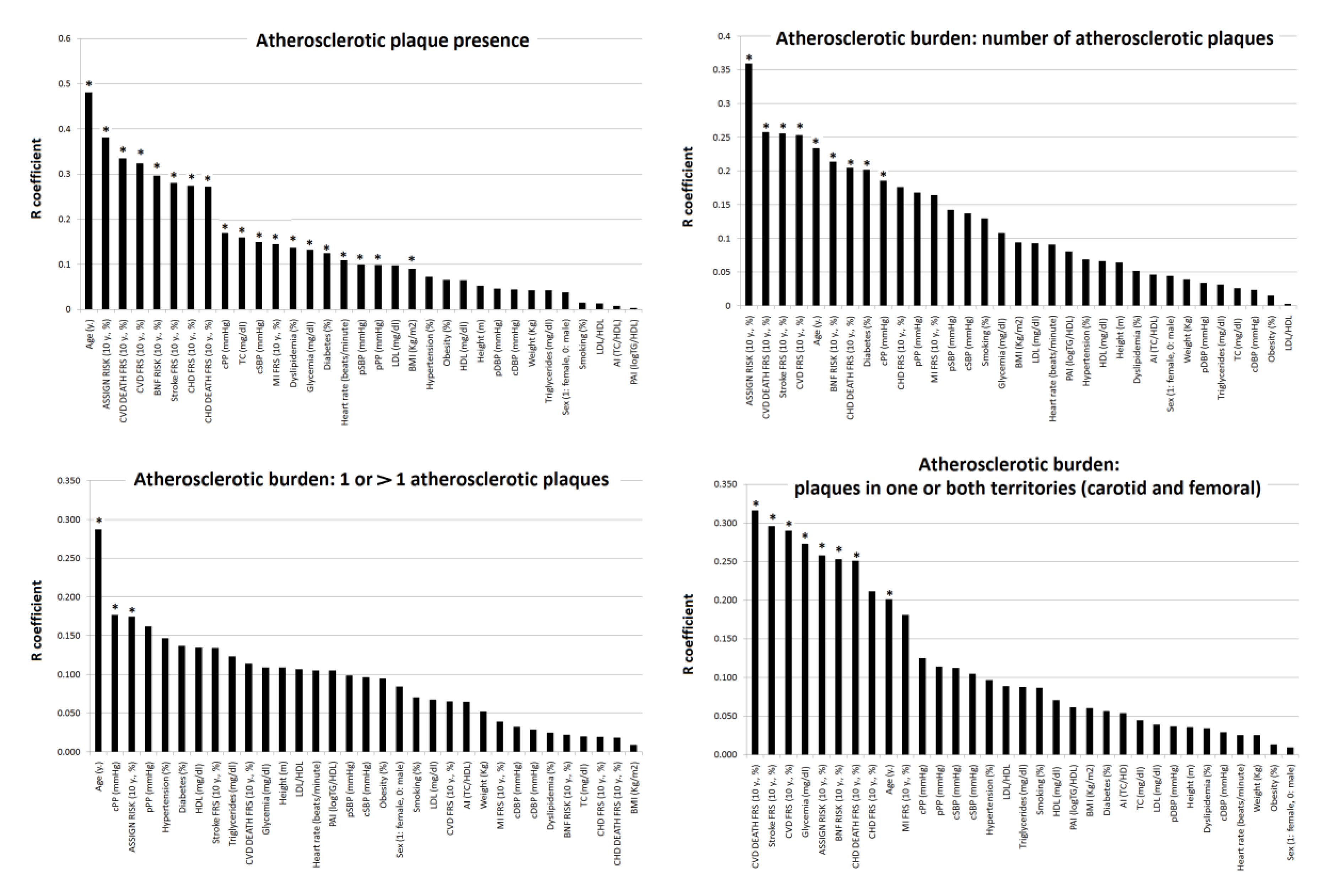
3.2. Subjects’ Characteristics: Association with Plaque Presence and Atherosclerotic Burden
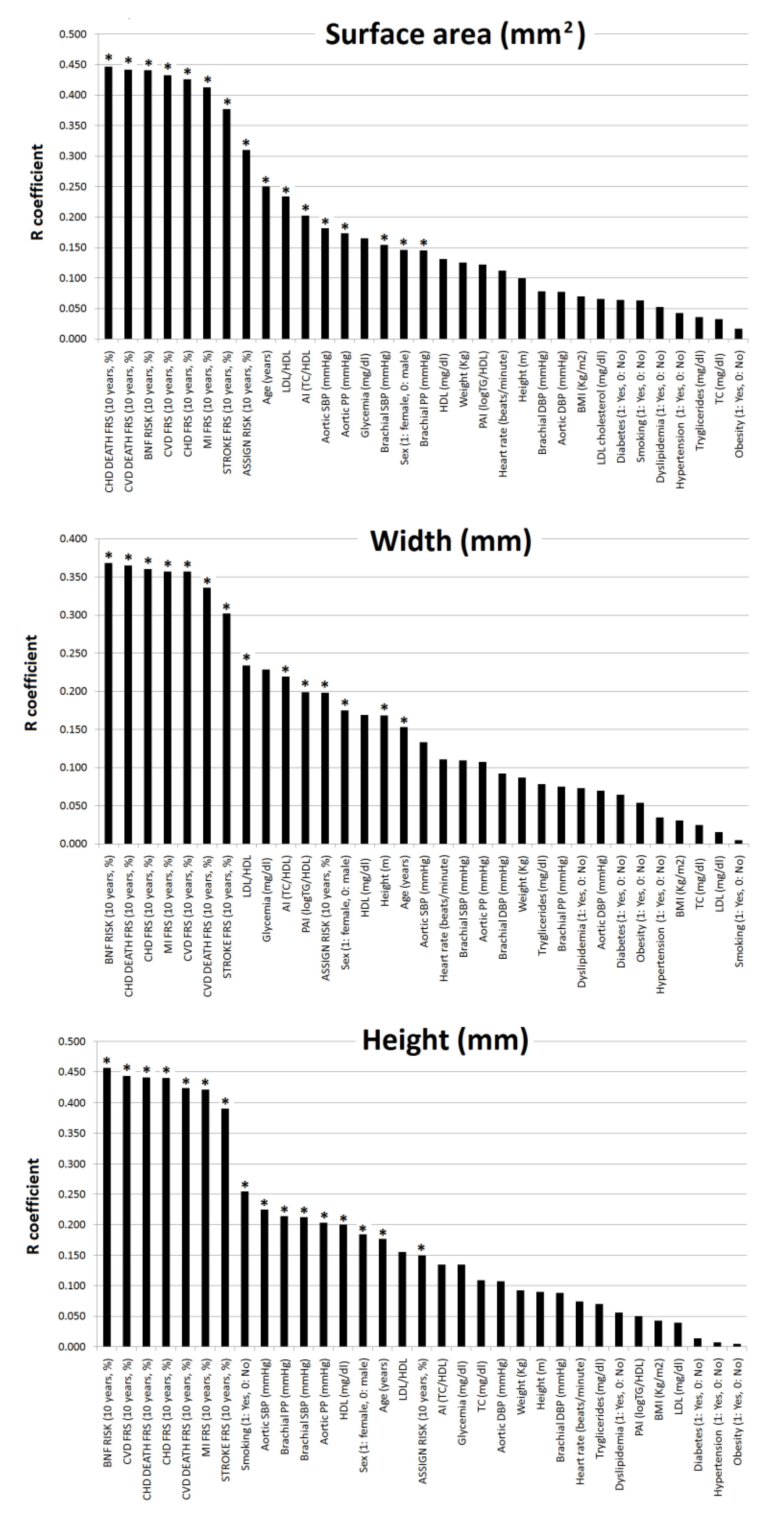
3.3. Subjects’ Characteristics: Association with Geometric Characteristics of Atherosclerotic Plaques
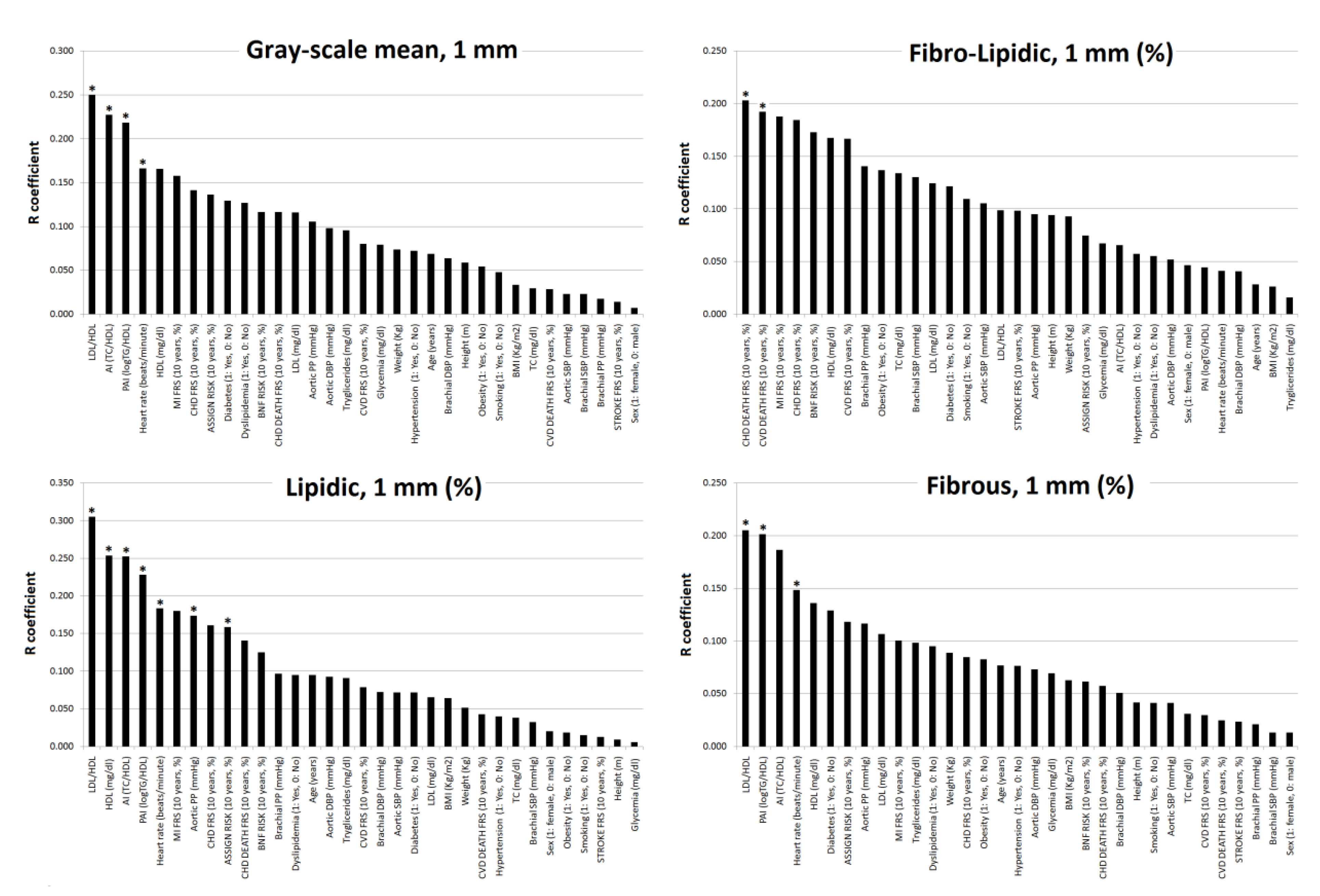
3.4. Subjects’ Characteristics: Association with Plaque Composition (Fibro-Lipid Content)
3.5. CRFs, 10-y./CVR Scores and Lipid Ratios Capability to Detect Plaque Presence and Burden
3.6. CRFs, 10-y./CVR Scores and Lipid Ratios Levels Capability to Predict Plaque Geometry and Composition
3.7. Association between Plaques’ Geometry and Composition
4. Discussion
4.1. Main Findings
- First, the variables most strongly associated with the presence of plaques, and which could be used to identify sub-populations that could benefit from imaging studies to individualize risk, were: age, 10-y./CVR and (although weakly) cSBP or cPP (rather than pSBP or pPP). Individually, classical CRFs and lipid ratios (TC/HDL, LDL/HDL, LogTG/HDL) showed almost no association with plaques presence. Thus, their capability to detect plaques presence would be limited.
- Second, regarding atherosclerotic burden, age, and 10-y./CVR levels showed the highest levels of association. The associations with 10-y./CVR varied depending on the CVR-equation and on the definition of atherosclerotic burden considered.
- Third, plaques geometry parameters (surface, width and height) showed associations with different variables, but only 10-y./CVR followed by age and BP (mainly cBP) would allow identification of the presence of plaques with large surfaces (˃p75th).
- Fourth, the greatest association with plaque composition was obtained for lipid ratios. Higher TC/HDL, LDL/HDL and/or LogTG/HDL levels associated higher lipid content and lower fibrous content. LDL/HDL and LogTG/HDL, but not TC/HDL, CRFs alone, nor 10-y./CVR were able to detect plaque composition.
- Fifth, the association between plaques’ geometry and composition was limited or non-existent.
4.2. Clinical Implications
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| +LR: -LR | Positive and negative likelihood ratio: respectively |
| AI | Atherogenic Index |
| ANCOVA | Analysis of Covariance |
| ASSIGN | Scottish Intercollegiate Guidelines Network (SIGN), Cardiovascular Risk Score |
| AUC | Area Under Curve or Area under ROC Curve |
| BMI | Body Mass Index |
| BNF | British National Formulary Cardiovascular Risk Score |
| BP | Blood Pressure |
| CCA | Common Carotid Artery |
| CFA | Common Femoral Artery |
| CHD | Coronary Heart Disease |
| CRFs | Cardiovascular Risk Factors |
| CUiiDARTE | Centro Universitario de Investigación, Innovación y Diagnóstico Arterial |
| CV | Cardiovascular |
| CVD | Cardiovascular Disease |
| CVR | Cardiovascular risk |
| FRS | Framingham Risk Score |
| GSM | Grayscale Median |
| HDL | High-density Lipoprotein Cholesterol |
| HR | Heart Rate |
| HT | Arterial Hypertension |
| IMT | Intima-media Thickness |
| LDL | Low-density Lipoprotein Cholesterol |
| LogRM | Logistic Regression Models |
| MI | Myocardial Infarction |
| PAI | Plasma Atherogenic Index |
| pBP, cBP | Peripheral (Brachial) and Central (Aortic) Blood Pressure, respectively. |
| pDBP, cDBP | Peripheral (Brachial) and Central (Aortic) Diastolic Blood Pressure, respectively. |
| pMBP | Peripheral (Brachial) Mean Blood Pressure |
| pPP, cPP | Peripheral (Brachial) and Central (Aortic) Pulse Pressure, respectively. |
| pSBP, cSBP | Peripheral (Brachial) and Central (Aortic) Systolic Blood Pressure, respectively. |
| R | Correlation Coefficient |
| ROC | Receiver Operating Characteristic Curve |
| SE | Sensibility |
| SP | Specificity |
| TC | Total Cholesterol |
| TG | Triglycerides |
References
- Laclaustra, M.; Casasnovas, J.A.; Fernandez-Ortiz, A.; Fuster, V.; León-Latre, M.; Jiménez-Borreguero, L.J.; Pocovi, M.; Hurtado-Roca, Y.; Ordovás, J.M.; Jarauta, E.; et al. Femoral and Carotid Subclinical Atherosclerosis Association With Risk Factors and Coronary Calcium. J. Am. Coll. Cardiol. 2016, 67, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, D.; Gurses, K.M.; Taydas, O.; Poker, A.; Ozer, N.; Hazirolan, T.; Tokgozoglu, L. Role of femoral artery ultrasound imaging in cardiovascular event risk prediction in a primary prevention cohort at a medium-term follow-up. J. Cardiol. 2019. [Google Scholar] [CrossRef]
- Baber, U.; Mehran, R.; Sartori, S.; Schoos, M.; Sillesen, H.; Muntendam, P.; Garcia, M.J.; Gregson, J.; Pocock, S.; Falk, E.; et al. Prevalence, Impact, and Predictive Value of Detecting Subclinical Coronary and Carotid Atherosclerosis in Asymptomatic Adults. J. Am. Coll. Cardiol. 2015, 65, 1065–1074. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Alvira, J.M.; Fuster, V.; Pocock, S.; Sanz, J.; Friera, L.F.; Laclaustra, M.; Fernández-Jiménez, R.; Mendiguren, J.; Fernandez-Ortiz, A.; Ibañez, B.; et al. Predicting Subclinical Atherosclerosis in Low-Risk Individuals. J. Am. Coll. Cardiol. 2017, 70, 2463–2473. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Sillesen, H.; Muntendam, P.; Adourian, A.; Entrekin, R.; Garcia, M.; Falk, E.; Fuster, V. Carotid Plaque Burden as a Measure of Subclinical Atherosclerosis. JACC: Cardiovasc. Imaging 2012, 5, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Sillesen, H.; Sartori, S.; Sandholt, B.; Baber, U.; Mehran, R.; Fuster, V. Carotid plaque thickness and carotid plaque burden predict future cardiovascular events in asymptomatic adult Americans. Eur. Heart J. -Cardiovasc. Imaging 2017, 19, 1042–1050. [Google Scholar] [CrossRef]
- Toutouzas, K.; Benetos, G.; Karanasos, A.; Chatzizisis, Y.S.; Giannopoulos, A.A.; Tousoulis, D. Vulnerable plaque imaging: Updates on new pathobiological mechanisms. Eur. Heart J. 2015, 36, 3147–3154. [Google Scholar] [CrossRef] [Green Version]
- Naghavi, M.; Falk, E.; Hecht, H.S.; Jamieson, M.J.; Kaul, S.; Berman, D.; Fayad, Z.; Budoff, M.J.; Rumberger, J.; Naqvi, T.Z.; et al. From Vulnerable Plaque to Vulnerable Patient—Part III: Executive Summary of the Screening for Heart Attack Prevention and Education (SHAPE) Task Force Report. Am. J. Cardiol. 2006, 98, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Zubair, S. American College of Cardiology. Available online: https://www.acc.org/latest-in-cardiology/articles/2015/06/16/10/57/what-is-the-future-of-asymptomatic-carotid-artery-disease (accessed on 18 March 2020).
- Liang, M.; Puri, A.; Devlin, G. The Vulnerable Plaque: The Real Villain in Acute Coronary Syndromes. Open Cardiovasc. Med. J. 2011, 5, 123–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinjikji, W.; Rabinstein, A.A.; Lanzino, G.; Murad, M.H.; Williamson, E.E.; Demarco, J.K.; Iii, J.H. Ultrasound Characteristics of Symptomatic Carotid Plaques: A Systematic Review and Meta-Analysis. Cerebrovasc. Dis. 2015, 40, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Sztajzel, R.; Momjian, S.; Momjian-Mayor, I.; Murith, N.; Djebaili, K.; Boissard, G.; Comelli, M.; Pizolatto, G. Stratified Gray-Scale Median Analysis and Color Mapping of the Carotid Plaque. Stroke 2005, 36, 741–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sztajzel, R. Ultrasonographic assessment of the morphological characteristics of the carotid plaque. Swiss Med. Wkly. 2005, 135, 635–643. [Google Scholar] [PubMed]
- Mitchell, C.C.; Korcarz, C.E.; Gepner, A.D.; Nye, R.; Young, R.L.; Matsuzaki, M.; Post, W.S.; Kaufman, J.D.; McClelland, R.L.; Stein, J.H. Carotid Artery Echolucency, Texture Features, and Incident Cardiovascular Disease Events: The MESA Study. J. Am. Heart Assoc. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zinoveev, A.; Castro, J.M.; García-Espinosa, V.; Marin, M.; Chiesa, P.; Bia, D.; Zócalo, Y. Aortic pressure and forward and backward wave components in children, adolescents and young-adults: Agreement between brachial oscillometry, radial and carotid tonometry data and analysis of factors associated with their differences. PLoS ONE 2019, 14, e0226709. [Google Scholar] [CrossRef] [Green Version]
- Castro, J.M.; García-Espinosa, V.; Zinoveev, A.; Marin, M.; Severi, C.; Chiesa, P.; Bia, D.; Zócalo, Y. Arterial Structural and Functional Characteristics at End of Early Childhood and Beginning of Adulthood: Impact of Body Size Gain during Early, Intermediate, Late and Global Growth. J. Cardiovasc. Dev. Dis. 2019. [Google Scholar] [CrossRef] [Green Version]
- Santana, D.B.; Zocalo, Y.A.; Ventura, I.F.; Arrosa, J.F.T.; Florio, L.; Lluberas, R.; Armentano, R.L. Health Informatics Design for Assisted Diagnosis of Subclinical Atherosclerosis, Structural, and Functional Arterial Age Calculus and Patient-Specific Cardiovascular Risk Evaluation. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 943–951. [Google Scholar] [CrossRef]
- Santana, D.B.; Zocalo, Y.A.; Armentano, R.L. Integrated e-Health Approach Based on Vascular Ultrasound and Pulse Wave Analysis for Asymptomatic Atherosclerosis Detection and Cardiovascular Risk Stratification in the Community. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 287–294. [Google Scholar] [CrossRef]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 2016, 253, 281–344. [Google Scholar] [CrossRef] [Green Version]
- Chamberlain, J.J.; Johnson, E.L.; Leal, S.; Rhinehart, A.S.; Shubrook, J.H.; Peterson, L. Cardiovascular Disease and Risk Management: Review of the American Diabetes Association Standards of Medical Care in Diabetes 2018. Ann. Intern. Med. 2018, 168, 640–650. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.F.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar] [CrossRef] [Green Version]
- Payne, R. Risk, The University of Edinburgh. Available online: https://www.bloodpressureclinic.ed.ac.uk/calculating-cardiovascular-risk (accessed on 18 March 2020).
- Millan, J.; Pinto, X.; Munoz, A.; Rubies-Prat, J.; Pallardo, L.F.; Masana, L.; Mangas, A.; Hernandez-Mijares, A.; Hernández-Mijares, A.; González-Santos, P. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc. Health Risk Manag. 2009, 5, 757–765. [Google Scholar]
- Zócalo, Y.; Bia, D. Presión aórtica central y parámetros clínicos derivados de la onda del pulso: Evaluación no invasivaen la práctica clínica. Importancia clínica y análisis de las bases metodológicas de los equipos disponibles para suevaluación. Rev. Urug. Cardiol. 2014, 29, 215–230. [Google Scholar]
- Zócalo, Y.; Bia, D. Ultrasonografía carotídea para detección de placas de ateroma y medición del espesor íntima-media; índice tobillo-brazo: Evaluación no invasivaen la práctica clínica: Importancia clínica y análisis de las bases metodológicas para su evaluación. Rev. Urug. Cardiol. 2016, 31, 47–60. [Google Scholar]
- Farro, F.; Farro, I.; Torrado, J.; Zócalo, Y.; Armentano, R.L.; Lluberas, R.; Bia, D. Composición y biomecánica de placas de ateroma carotídeas. Análisis comparativo de la distensibilidad arterial a lo largo del eje longitudinal en arterias carótidas control y con placas de ateroma de diferente composición. Rev. Urug. Cardiol. 2014, 29, 17–31. [Google Scholar]
- Craiem, D.; Chironi, G.; Graf, S.; Denarié, N.; Armentano, R.L.; Simon, A. Atheromatous plaque: Quantitative analysis of the echogenicity of different layers. Rev. Esp. Cardiol. 2009, 62, 984–991. [Google Scholar] [CrossRef]
- Momjian-Mayor, I.; Kuzmanovic, I.; Momjian, S.; Bonvin, C.; Albanese, S.; Bichsel, D.; Comelli, M.; Pereira, V.M.; Lövblad, K.-O.; Sztajzel, R. Accuracy of a Novel Risk Index Combining Degree of Stenosis of the Carotid Artery and Plaque Surface Echogenicity. Stroke 2012, 43, 1260–1265. [Google Scholar] [CrossRef]
- Metz, C.E. Basic principles of ROC analysis. Semin. Nucl. Med. 1978, 8, 283–298. [Google Scholar] [CrossRef]
- Delong, E.R.; Delong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Lumley, T.; Diehr, P.; Emerson, S.; Chen, L. The Importance of the Normality Assumption in Large Public Health Data Sets. Annu. Rev. Public Health 2002, 23, 151–169. [Google Scholar] [CrossRef]
- Protogerou, A.; Argyris, A.A.; Papaioannou, T.G.; Kollias, G.; Konstantonis, G.D.; Nasothimiou, E.; Achimastos, A.; Blacher, J.; Safar, M.; Sfikakis, P.P. Left-ventricular hypertrophy is associated better with 24-h aortic pressure than 24-h brachial pressure in hypertensive patients. J. Hypertens. 2014, 32, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Negishi, K.; Yang, H.; Wang, Y.; Nolan, M.; Negishi, T.; Pathan, F.; Marwick, T.H.; Sharman, J.E. Importance of Calibration Method in Central Blood Pressure for Cardiac Structural Abnormalities. Am. J. Hypertens. 2016, 29, 1070–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wassertheurer, S.; Baumann, M. Assessment of systolic aortic pressure and its association to all cause mortality critically depends on waveform calibration. J. Hypertens. 2015, 33, 1884–1889. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Bia, D.; Zócalo, Y. Impact of Methodological and Calibration Approach on the Association of Central and Peripheral Systolic Blood Pressure with Cardiac Structure and Function in Children, Adolescents and Adults. High Blood Press. Cardiovasc. Prev. 2019, 26, 509–534. [Google Scholar] [CrossRef]
- Feig, J.E.; Feig, J.L.; Dangas, G.D. The role of HDL in plaque stabilization and regression: Basic mechanisms and clinical implications. Coron. Artery Dis. 2016, 27, 592–603. [Google Scholar] [CrossRef]
- Elshazly, M.; Nicholls, S.; Nissen, S.E.; John, J.S.; Martin, S.S.; Jones, S.R.; Quispe, R.; Stegman, B.; Kapadia, S.; Tuzcu, E.M.; et al. Implications of Total to High-Density Lipoprotein Cholesterol Ratio Discordance With Alternative Lipid Parameters for Coronary Atheroma Progression and Cardiovascular Events. Am. J. Cardiol. 2016, 118, 647–655. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Cook, N.R.; Bradwin, G.; Buring, J.E. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA 2005, 294, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Kastelein, J.J.; Van Der Steeg, W.A.; Holme, I.; Gaffney, M.; Cater, N.B.; Barter, P.J.; Deedwania, P.; Olsson, A.G.; Boekholdt, S.M.; Demicco, D.A.; et al. Lipids, Apolipoproteins, and Their Ratios in Relation to Cardiovascular Events With Statin Treatment. Circulation 2008, 117, 3002–3009. [Google Scholar] [CrossRef] [Green Version]
- Baradaran, H.; Al-Dasuqi, K.; Knight-Greenfield, A.; Giambrone, A.; Delgado, D.; Ebani, E.; Kamel, H.; Gupta, A. Association between Carotid Plaque Features on CTA and Cerebrovascular Ischemia: A Systematic Review and Meta-Analysis. Am. J. Neuroradiol. 2017, 38, 2321–2326. [Google Scholar] [CrossRef] [Green Version]
- Mann, J.M.; Davies, M.J. Vulnerable plaque. Relation of characteristics to degree of stenosis in human coronary arteries. Circulation 1996, 94, 928–931. [Google Scholar] [CrossRef]
- Narula, J.; Garg, P.; Achenbach, S.; Motoyama, S.; Virmani, R.; Strauss, H.W. Arithmetic of vulnerable plaques for noninvasive imaging. Nat. Clin. Pract. Neurol. 2008, 5, S2–S10. [Google Scholar] [CrossRef] [PubMed]
- Redgrave, J.; Lovett, J.; Rothwell, P.; Gallagher, P. Response to Letter Regarding Article, “Histological Assessment of 526 Symptomatic Carotid Plaques in Relation to the Nature and Timing of Ischemic Symptoms: The Oxford Plaque Study”. Circulation 2006. [Google Scholar] [CrossRef] [Green Version]
- Aboyans, V.; Ricco, J.; Bartelink, M.-L.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.-P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2017, 39, 763–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brott, T.G.; Halperin, J.L.; Abbara, S.; Bacharach, J.M.; Barr, J.D.; Bush, R.L.; Cates, C.U.; Creager, M.A.; Fowler, S.B.; Friday, G.; et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: Executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Circulation 2011, 124, 489–532. [Google Scholar] [PubMed] [Green Version]
- Hafiane, A. Vulnerable Plaque, Characteristics, Detection, and Potential Therapies. J. Cardiovasc. Dev. Dis. 2019, 6, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
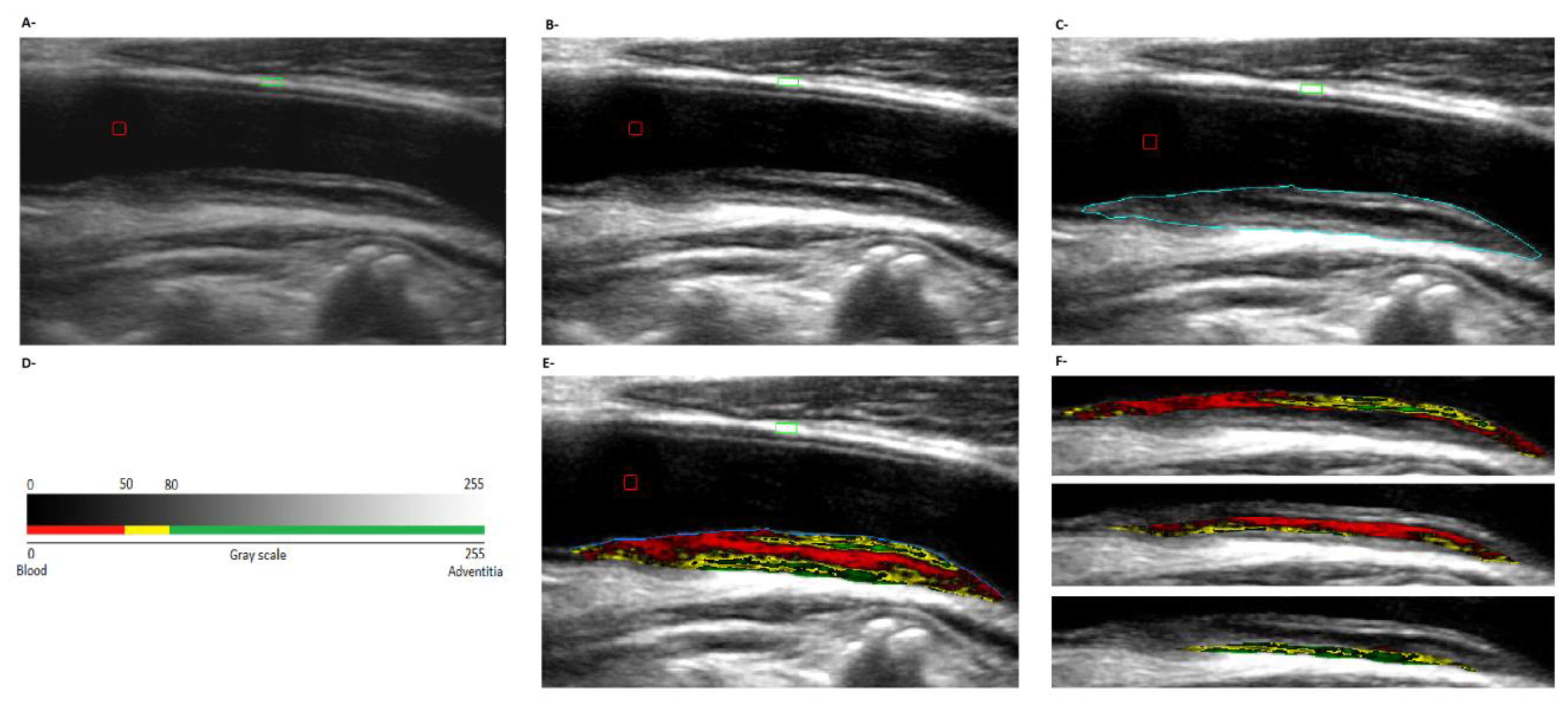

| Entire Group | Atherosclerotic Plaques | Non-Atherosclerotic Plaques | |||
|---|---|---|---|---|---|
| MV ± SD | MV ± SD | MV ± SD | p | p* | |
| n (% females) | 581 (47.0) | 144 (43.4) | 437 (48.1) | 0.867 | - |
| Age (years) | 51.4 ± 12.4 | 61.4 ± 10.0 | 48 ± 11.1 | <0.001 | - |
| Body weight (Kg) | 76.2 ± 15.6 | 77.4 ± 15.9 | 75.9 ± 15.4 | 0.316 | 0.599 |
| Body height (m) | 1.68 ± 0.10 | 1.67 ± 0.10 | 1.68 ± 0.1 | 0.213 | 0.389 |
| BMI (Kg/m2) | 26.8 ± 4.5 | 27.5 ± 4.7 | 26.5 ± 4.4 | 0.032 | 0.354 |
| Heart rate (b.p.m.) | 67.04 ± 10.30 | 65.08 ± 9.44 | 67.68 ± 10.49 | 0.009 | 0.580 |
| pSBP (mmHg) | 125.78 ± 15.02 | 128.37 ± 14.68 | 124.92 ± 15.05 | 0.017 | 0.432 |
| pDBP (mmHg) | 74.75 ± 9.52 | 75.51 ± 9.04 | 74.5 ± 9.66 | 0.272 | 0.440 |
| pPP (mmHg) | 51.03 ± 10.78 | 52.86 ± 10.57 | 50.42 ± 10.8 | 0.018 | 0.694 |
| cSBP (mmHg) | 112.11 ± 13.37 | 115.56 ± 12.99 | 110.96 ± 13.31 | <0.001 | 0.437 |
| cDBP (mmHg) | 75.74 ± 9.50 | 76.45 ± 9.09 | 75.5 ± 9.64 | 0.296 | 0.372 |
| cPP (mmHg) | 36.25 ± 8.87 | 38.86 ± 9.05 | 35.38 ± 8.65 | <0.001 | 0.735 |
| Hypertension (%) | 14.5 | 18.6 | 12.2 | 0.083 | 0.265 |
| Dyslipidemia (%) | 37.1 | 48.3 | 33.2 | 0.001 | 0.056 |
| Diabetes (%) | 1.9 | 4.8 | 0.9 | 0.003 | 0.050 |
| Obesity (%) | 19.1 | 23.4 | 17.6 | 0.122 | 0.464 |
| Smoking (%) | 36.9 | 37.6 | 34.2 | 0.647 | 0.125 |
| TC (mg/dL) | 213.88 ± 36.96 | 224.71 ± 39.97 | 210.78 ± 35.48 | 0.001 | 0.033 |
| HDL (mg/dL) | 54.32 ± 13.04 | 55.63 ± 13.13 | 53.83 ± 13 | 0.255 | 0.256 |
| LDL (mg/dL) | 138.34 ± 33.20 | 143.94 ± 31.87 | 136.53 ± 33.41 | 0.074 | 0.163 |
| AI (TC/HDL) | 4.14 ± 1.11 | 4.16 ± 1.04 | 4.13 ± 1.13 | 0.850 | 0.136 |
| LDL/HDL | 2.66 ± 0.92 | 2.69 ± 0.79 | 2.66 ± 0.96 | 0.790 | 0.299 |
| PAI (LogTG/HDL) | 0.28 ± 0.26 | 0.28 ± 0.27 | 0.28 ± 0.26 | 0.949 | 0.121 |
| TG (mg/dL) | 114.00 ± 59.00 | 119.06 ± 76.50 | 112.61 ± 52.31 | 0.384 | 0.025 |
| Glycemia (mg/dL) | 90.54 ± 12.17 | 93.77 ± 9.70 | 89.74 ± 12.59 | 0.014 | 0.814 |
| 10-y./FRS-CHD (%) | 7.03 ± 5.63 | 9.79 ± 5.44 | 6.17 ± 5.41 | <0.001 | 0.573 |
| 10-y./FRS-MI (%) | 3.22 ± 3.70 | 4.17 ± 3.39 | 2.92 ± 3.74 | 0.006 | 0.162 |
| 10-y./FRS-Stroke (%) | 1.66 ± 2.01 | 2.67 ± 1.95 | 1.35 ± 1.93 | <0.001 | 0.015 |
| 10-y./FRS-CVD (%) | 10.42 ± 8.54 | 15.35 ± 7.77 | 8.88 ± 8.19 | <0.001 | 0.302 |
| 10-y./FRS-CHD DEATH (%) | 1.25 ± 2.01 | 2.23 ± 2.39 | 0.95 ± 1.77 | <0.001 | 0.333 |
| 10-y./FRS-CVDDEATH (%) | 2.06 ± 3.29 | 4.04 ± 4.02 | 1.45 ± 2.77 | <0.001 | 0.466 |
| 10-y./BNF Risk Score (%) | 8.69 ± 7.10 | 12.46 ± 6.82 | 7.52 ± 6.78 | <0.001 | 0.252 |
| 10-y./ASSIGN Risk Score (%) | 10.23 ± 9.59 | 16.70 ± 10.79 | 8.17 ± 8.18 | <0.001 | 0.405 |
| Presence (1) | Burden (2) | Burden (3) | Burden (4) | Correlation Comparison (William’s Test) | |||||||
| (n = 581) | (n = 144) | (n = 144) | (n = 144) | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | |||||
| 1: yes, 0: no | Nº of AP | Nº AP, 1 or >1 | Nº territories | ΔR | p | ΔR | p | ΔR | p | ||
| Sex (1: female, 0: male) | R | −0.037 | 0.045 | 0.085 | 0.009 | −0.082 | 0.002 | −0.122 | <0.001 | −0.047 | 0.004 |
| P | 0.370 | 0.596 | 0.314 | 0.911 | |||||||
| Age (y.) | R | 0.481 | 0.234 | 0.287 | 0.201 | 0.247 | 0.000 | 0.194 | <0.001 | 0.280 | <0.001 |
| P | <0.001 | 0.005 | <0.001 | 0.015 | |||||||
| Weight (Kg) | R | 0.042 | 0.040 | −0.052 | 0.025 | 0.003 | 0.940 | 0.095 | <0.001 | 0.017 | 0.287 |
| P | 0.314 | 0.639 | 0.539 | 0.768 | |||||||
| Height (m) | R | −0.053 | −0.065 | −0.109 | −0.036 | 0.012 | 0.649 | 0.056 | <0.001 | −0.017 | 0.287 |
| P | 0.208 | 0.447 | 0.198 | 0.674 | |||||||
| BMI (Kg/m2) | R | 0.090 | 0.094 | 0.009 | 0.060 | −0.004 | 0.879 | 0.081 | <0.001 | 0.030 | 0.060 |
| P | 0.031 | 0.268 | 0.912 | 0.476 | |||||||
| Heart rate (b.p.m.) | R | −0.109 | −0.091 | −0.106 | −0.025 | −0.018 | 0.493 | −0.003 | 0.836 | −0.084 | <0.001 |
| P | 0.009 | 0.282 | 0.210 | 0.765 | |||||||
| pSBP (mmHg) | R | 0.099 | 0.142 | 0.099 | 0.105 | −0.043 | 0.100 | 0.000 | 1.000 | −0.006 | 0.706 |
| P | 0.017 | 0.089 | 0.238 | 0.211 | |||||||
| pDBP (mmHg) | R | 0.046 | 0.034 | −0.029 | 0.037 | 0.011 | 0.649 | 0.074 | <0.001 | 0.009 | 0.573 |
| P | 0.272 | 0.684 | 0.732 | 0.661 | |||||||
| pPP (mmHg) | R | 0.098 | 0.168 | 0.162 | 0.114 | −0.070 | 0.007 | −0.064 | <0.001 | −0.016 | 0.314 |
| P | 0.018 | 0.044 | 0.052 | 0.173 | |||||||
| cSBP (mmHg) | R | 0.149 | 0.138 | 0.097 | 0.112 | 0.011 | 0.673 | 0.052 | <0.001 | 0.037 | 0.019 |
| P | <0.001 | 0.100 | 0.249 | 0.180 | |||||||
| cDBP (mmHg) | R | 0.044 | 0.024 | −0.033 | 0.029 | 0.020 | 0.448 | 0.076 | <0.001 | 0.014 | 0.348 |
| P | 0.296 | 0.776 | 0.698 | 0.728 | |||||||
| cPP (mmHg) | R | 0.170 | 0.186 | 0.177 | 0.125 | −0.016 | 0.537 | −0.007 | 0.627 | 0.045 | 0.004 |
| P | <0.001 | 0.025 | 0.034 | 0.135 | |||||||
| Hypertension | R | 0.072 | 0.069 | 0.147 | 0.096 | 0.004 | 0.909 | −0.074 | <0.001 | −0.024 | 0.132 |
| P | 0.083 | 0.414 | 0.080 | 0.252 | |||||||
| Dyslipidemia | R | 0.137 | 0.052 | −0.025 | 0.034 | 0.085 | 0.001 | 0.162 | <0.001 | 0.103 | <0.001 |
| P | 0.001 | 0.533 | 0.767 | 0.688 | |||||||
| Diabetes | R | 0.125 | 0.202 | 0.137 | 0.057 | −0.077 | 0.003 | −0.012 | 0.408 | 0.068 | <0.001 |
| P | 0.003 | 0.015 | 0.101 | 0.500 | |||||||
| Obesity | R | 0.066 | 0.015 | −0.095 | 0.013 | 0.050 | 0.053 | 0.161 | <0.001 | 0.053 | 0.001 |
| P | 0.113 | 0.854 | 0.257 | 0.875 | |||||||
| Smoking | R | 0.015 | 0.130 | 0.070 | 0.087 | −0.114 | <0.001 | −0.055 | 0.001 | −0.071 | <0.001 |
| P | 0.715 | 0.121 | 0.402 | 0.301 | |||||||
| TC (mg/dL) | R | 0.159 | −0.027 | 0.020 | 0.045 | 0.186 | <0.001 | 0.139 | <0.001 | 0.114 | <0.001 |
| P | 0.001 | 0.800 | 0.847 | 0.671 | |||||||
| Presence (1) | Burden (2) | Burden (3) | Burden (4) | Correlation comparison (William’s Test) | |||||||
| (n = 581) | (n = 144) | (n = 144) | (n = 144) | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | |||||
| 1: yes, 0: no | Nº of AP | Nº AP, 1 or >1 | Nº territories | ΔR | p | ΔR | P | ΔR | P | ||
| HDL (mg/dL) | R | 0.065 | −0.066 | 0.135 | 0.071 | 0.131 | <0.001 | −0.070 | <0.001 | −0.006 | 0.707 |
| P | 0.219 | 0.535 | 0.206 | 0.507 | |||||||
| LDL (mg/dL) | R | 0.098 | −0.093 | −0.067 | −0.039 | 0.190 | <0.001 | 0.165 | <0.001 | 0.137 | <0.001 |
| P | 0.067 | 0.405 | 0.545 | 0.726 | |||||||
| AI (TC/HDL) | R | 0.007 | 0.046 | −0.065 | −0.054 | −0.039 | 0.139 | 0.072 | <0.001 | 0.061 | <0.001 |
| P | 0.892 | 0.667 | 0.546 | 0.616 | |||||||
| LDL/HDL | R | 0.013 | 0.003 | −0.107 | −0.089 | 0.010 | 0.851 | 0.120 | <0.001 | 0.102 | <0.001 |
| P | 0.809 | 0.977 | 0.335 | 0.425 | |||||||
| PAI (LogTG/HDL) | R | −0.003 | 0.081 | −0.105 | −0.062 | −0.084 | 0.014 | 0.102 | <0.001 | 0.059 | <0.001 |
| P | 0.949 | 0.468 | 0.343 | 0.580 | |||||||
| TG (mg/dL) | R | 0.042 | −0.032 | −0.124 | −0.088 | 0.074 | 0.005 | 0.166 | <0.001 | 0.129 | <0.001 |
| P | 0.437 | 0.772 | 0.266 | 0.431 | |||||||
| Glycemia (mg/dL) | R | 0.132 | 0.109 | 0.109 | 0.273 | 0.023 | 0.379 | 0.023 | 0.113 | −0.141 | <0.001 |
| P | 0.014 | 0.378 | 0.376 | 0.024 | |||||||
| 10-y./FRS-CHD (%) | R | 0.274 | 0.177 | −0.020 | 0.211 | 0.097 | <0.001 | 0.294 | <0.001 | 0.063 | <0.001 |
| P | <0.001 | 0.106 | 0.856 | 0.052 | |||||||
| 10-y./FRS-MI (%) | R | 0.144 | 0.164 | −0.039 | 0.181 | −0.020 | 0.442 | 0.183 | <0.001 | −0.037 | 0.019 |
| P | 0.006 | 0.133 | 0.720 | 0.098 | |||||||
| 10-y./FRS-Stroke (%) | R | 0.280 | 0.256 | 0.135 | 0.296 | 0.024 | 0.192 | 0.145 | <0.001 | −0.016 | 0.295 |
| P | <0.001 | 0.018 | 0.220 | 0.006 | |||||||
| 10-y./FRS-CVD (%) | R | 0.323 | 0.254 | 0.065 | 0.290 | 0.069 | 0.006 | 0.258 | <0.001 | 0.033 | 0.029 |
| P | <0.001 | 0.019 | 0.552 | 0.007 | |||||||
| 10-y./FRS-CHD DEATH (%) | R | 0.272 | 0.206 | 0.019 | 0.251 | 0.066 | 0.010 | 0.253 | <0.001 | 0.021 | 0.173 |
| P | <0.001 | 0.059 | 0.865 | 0.021 | |||||||
| 10-y./FRS-CVD DEATH (%) | R | 0.335 | 0.258 | 0.114 | 0.316 | 0.077 | 0.021 | 0.221 | <0.001 | 0.019 | 0.208 |
| P | <0.001 | 0.017 | 0.299 | 0.003 | |||||||
| 10-y./BNF Risk Score (%) | R | 0.296 | 0.214 | 0.023 | 0.253 | 0.082 | 0.001 | 0.273 | <0.001 | 0.043 | 0.005 |
| P | <0.001 | 0.049 | 0.838 | 0.019 | |||||||
| 10-y./ASSIGN Risk Score (%) | R | 0.381 | 0.360 | 0.175 | 0.258 | 0.021 | 0.387 | 0.206 | <0.001 | 0.123 | <0.001 |
| p | <0.001 | <0.001 | 0.041 | 0.002 | |||||||
| Surface Area (mm2) | Width (mm) | Height (mm) | Grayscale Median | Grayscale Mean | Heterogeneity % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | p | R | P | R | p | R | p | R | p | R | p | |||
| Sex (1: female, 0: male) | −0.146 | 0.044 | −0.175 | 0.016 | −0.185 | 0.009 | 0.034 | 0.643 | 0.050 | 0.500 | −0.088 | 0.227 | ||
| Age (y.) | 0.250 | 0.001 | 0.153 | 0.035 | 0.177 | 0.012 | 0.076 | 0.296 | 0.110 | 0.136 | 0.075 | 0.304 | ||
| Weight (Kg) | 0.125 | 0.090 | 0.087 | 0.241 | 0.093 | 0.195 | −0.100 | 0.17 | −0.092 | 0.218 | −0.087 | 0.236 | ||
| Height (m) | 0.099 | 0.178 | 0.168 | 0.023 | 0.090 | 0.209 | −0.041 | 0.577 | −0.070 | 0.347 | −0.006 | 0.937 | ||
| BMI (Kg/m2) | 0.070 | 0.344 | −0.030 | 0.681 | 0.043 | 0.552 | −0.081 | 0.271 | −0.053 | 0.481 | −0.087 | 0.237 | ||
| Heart rate (b.p.m.) | −0.112 | 0.127 | −0.111 | 0.130 | −0.075 | 0.295 | 0.133 | 0.067 | 0.157 | 0.034 | 0.010 | 0.890 | ||
| pSBP (mmHg) | 0.154 | 0.033 | 0.109 | 0.134 | 0.213 | 0.002 | 0.013 | 0.86 | 0.010 | 0.890 | 0.087 | 0.232 | ||
| pDBP (mmHg) | 0.078 | 0.286 | 0.092 | 0.205 | 0.089 | 0.211 | 0.039 | 0.589 | 0.012 | 0.867 | 0.042 | 0.560 | ||
| pPP (mmHg) | 0.145 | 0.046 | 0.075 | 0.306 | 0.214 | 0.002 | −0.012 | 0.864 | 0.004 | 0.953 | 0.083 | 0.256 | ||
| cSBP (mmHg) | 0.182 | 0.012 | 0.133 | 0.067 | 0.225 | 0.001 | −0.037 | 0.612 | −0.055 | 0.460 | 0.062 | 0.394 | ||
| cDBP (mmHg) | 0.077 | 0.292 | 0.070 | 0.338 | 0.108 | 0.129 | 0.065 | 0.368 | 0.054 | 0.468 | 0.067 | 0.356 | ||
| cPP (mmHg) | 0.173 | 0.017 | 0.107 | 0.142 | 0.204 | 0.004 | −0.099 | 0.171 | −0.113 | 0.126 | 0.020 | 0.787 | ||
| Hypertension | 0.043 | 0.559 | 0.035 | 0.635 | 0.008 | 0.915 | −0.070 | 0.334 | −0.056 | 0.448 | −0.018 | 0.809 | ||
| Dyslipidemia | 0.052 | 0.476 | 0.073 | 0.318 | 0.056 | 0.429 | −0.096 | 0.182 | −0.148 | 0.045 | −0.132 | 0.068 | ||
| Diabetes | 0.063 | 0.384 | 0.064 | 0.379 | −0.014 | 0.846 | −0.105 | 0.148 | −0.105 | 0.154 | −0.143 | 0.048 | ||
| Obesity | −0.017 | 0.819 | −0.054 | 0.468 | 0.005 | 0.947 | −0.097 | 0.186 | −0.066 | 0.379 | −0.117 | 0.111 | ||
| Smoking | −0.063 | 0.388 | −0.005 | 0.947 | −0.255 | <0.001 | −0.007 | 0.926 | −0.013 | 0.859 | −0.178 | 0.014 | ||
| TC (mg/dL) | 0.033 | 0.734 | −0.025 | 0.798 | −0.110 | 0.240 | −0.057 | 0.548 | −0.049 | 0.607 | −0.197 | 0.038 | ||
| HDL (mg/dL) | −0.131 | 0.180 | −0.169 | 0.082 | −0.200 | 0.034 | 0.145 | 0.131 | 0.169 | 0.081 | −0.065 | 0.503 | ||
| LDL (mg/dL) | 0.065 | 0.524 | 0.015 | 0.884 | −0.040 | 0.688 | −0.126 | 0.215 | −0.152 | 0.137 | −0.222 | 0.029 | ||
| AI (TC/HDL) | 0.202 | 0.038 | 0.219 | 0.024 | 0.135 | 0.156 | −0.225 | 0.019 | −0.235 | 0.015 | −0.108 | 0.270 | ||
| LDL/HDL | 0.234 | 0.021 | 0.234 | 0.021 | 0.156 | 0.117 | −0.232 | 0.021 | −0.281 | 0.005 | −0.123 | 0.229 | ||
| PAI (LogTG/HDL) | 0.122 | 0.232 | 0.199 | 0.049 | 0.051 | 0.610 | −0.196 | 0.049 | −0.197 | 0.050 | −0.149 | 0.141 | ||
| Triglycerides (mg/dL) | 0.036 | 0.727 | 0.078 | 0.444 | −0.070 | 0.478 | −0.062 | 0.539 | −0.081 | 0.422 | −0.185 | 0.067 | ||
| Glycemia (mg/dL) | 0.165 | 0.243 | 0.228 | 0.103 | 0.135 | 0.326 | −0.053 | 0.705 | −0.106 | 0.454 | −0.133 | 0.345 | ||
| 10-y./FRS-CHD (%) | 0.426 | <0.001 | 0.36 | <0.001 | 0.44 | <0.001 | −0.142 | 0.14 | −0.132 | 0.175 | 0.042 | 0.666 | ||
| 10-y./FRS-MI (%) | 0.413 | <0.001 | 0.357 | <0.001 | 0.421 | <0.001 | −0.147 | 0.127 | −0.144 | 0.140 | 0.023 | 0.816 | ||
| 10-y./FRS-Stroke(%) | 0.377 | <0.001 | 0.302 | 0.002 | 0.391 | <0.001 | −0.021 | 0.827 | 0.001 | 0.989 | 0.118 | 0.225 | ||
| 10-y./FRS-CVD (%) | 0.433 | <0.001 | 0.357 | <0.001 | 0.444 | <0.001 | −0.089 | 0.358 | −0.071 | 0.466 | 0.083 | 0.393 | ||
| 10-y./FRS-CHD DEATH (%) | 0.447 | <0.001 | 0.365 | <0.001 | 0.441 | <0.001 | −0.125 | 0.197 | −0.116 | 0.236 | 0.047 | 0.631 | ||
| 10-y./FRS-CVD DEATH (%) | 0.442 | <0.001 | 0.336 | <0.001 | 0.424 | <0.001 | −0.052 | 0.591 | −0.033 | 0.733 | 0.087 | 0.370 | ||
| 10-y./BNF Risk Score (%) | 0.441 | <0.001 | 0.368 | <0.001 | 0.457 | <0.001 | −0.120 | 0.215 | −0.106 | 0.278 | 0.065 | 0.505 | ||
| 10-y./ASSIGN Risk Score (%) | 0.31 | <0.001 | 0.198 | 0.007 | 0.150 | 0.038 | −0.101 | 0.166 | −0.095 | 0.205 | −0.076 | 0.299 | ||
| Lipidic (%) | Fibrolipidic (%) | Fibrous (%) | Grayscale mean, 1 mm | Lipidic, 1 mm (%) | Fibrolipidic, 1 mm (%) | Fibrous, 1 mm (%) | ||||||||
| R | p | R | p | R | p | R | p | R | p | R | P | R | p | |
| Sex | −0.027 | 0.715 | 0.044 | 0.547 | 0.008 | 0.911 | 0.007 | 0.925 | −0.020 | 0.789 | 0.046 | 0.528 | −0.013 | 0.862 |
| Age (y.) | −0.083 | 0.254 | −0.041 | 0.575 | 0.090 | 0.216 | 0.068 | 0.353 | −0.094 | 0.199 | −0.028 | 0.703 | 0.076 | 0.297 |
| Weight (Kg) | 0.069 | 0.350 | 0.107 | 0.148 | −0.109 | 0.141 | −0.074 | 0.323 | 0.051 | 0.494 | 0.093 | 0.211 | −0.088 | 0.234 |
| Height (m) | −0.035 | 0.641 | 0.074 | 0.317 | −0.018 | 0.812 | −0.058 | 0.433 | −0.009 | 0.909 | 0.094 | 0.207 | −0.041 | 0.578 |
| BMI (Kg/m2) | 0.107 | 0.148 | 0.058 | 0.434 | −0.107 | 0.147 | −0.033 | 0.654 | 0.064 | 0.392 | 0.026 | 0.725 | −0.062 | 0.402 |
| HR (b.p.m.) | −0.139 | 0.058 | −0.071 | 0.332 | 0.157 | 0.031 | 0.166 | 0.024 | −0.183 | 0.013 | −0.041 | 0.578 | 0.148 | 0.044 |
| pSBP (mmHg) | 0.053 | 0.472 | −0.119 | 0.102 | 0.019 | 0.799 | 0.023 | 0.759 | 0.032 | 0.663 | −0.130 | 0.076 | 0.013 | 0.860 |
| pDBP (mmHg) | −0.040 | 0.584 | −0.048 | 0.508 | 0.062 | 0.396 | 0.064 | 0.387 | −0.072 | 0.327 | −0.040 | 0.582 | 0.050 | 0.492 |
| pPP (mmHg) | 0.099 | 0.174 | −0.120 | 0.099 | −0.022 | 0.766 | −0.017 | 0.813 | 0.096 | 0.19 | −0.140 | 0.055 | −0.021 | 0.776 |
| cSBP (mmHg) | 0.095 | 0.195 | −0.084 | 0.252 | −0.036 | 0.623 | −0.023 | 0.756 | 0.071 | 0.333 | −0.105 | 0.151 | −0.041 | 0.576 |
| cDBP (mmHg) | −0.061 | 0.404 | −0.067 | 0.355 | 0.089 | 0.221 | 0.098 | 0.185 | −0.092 | 0.210 | −0.052 | 0.481 | 0.073 | 0.319 |
| cPP (mmHg) | 0.178 | 0.014 | −0.061 | 0.403 | −0.120 | 0.100 | −0.106 | 0.152 | 0.173 | 0.018 | −0.095 | 0.196 | −0.116 | 0.112 |
| Hypertension | 0.049 | 0.505 | 0.033 | 0.653 | −0.055 | 0.449 | −0.072 | 0.330 | 0.039 | 0.595 | 0.057 | 0.438 | −0.076 | 0.298 |
| Dyslipidemia | 0.068 | 0.353 | 0.084 | 0.250 | −0.104 | 0.151 | −0.127 | 0.085 | 0.095 | 0.198 | 0.055 | 0.452 | −0.095 | 0.196 |
| Diabetes | 0.057 | 0.437 | 0.135 | 0.063 | −0.114 | 0.119 | −0.129 | 0.079 | 0.071 | 0.333 | 0.121 | 0.097 | −0.129 | 0.079 |
| Obesity | 0.063 | 0.395 | 0.131 | 0.076 | −0.105 | 0.156 | −0.054 | 0.468 | 0.018 | 0.810 | 0.137 | 0.065 | −0.082 | 0.268 |
| Smoking | −0.028 | 0.707 | 0.112 | 0.125 | −0.035 | 0.635 | −0.048 | 0.517 | 0.014 | 0.845 | 0.109 | 0.136 | −0.041 | 0.575 |
| TC (mg/dL) | 0.002 | 0.987 | 0.146 | 0.129 | −0.069 | 0.474 | −0.029 | 0.764 | −0.038 | 0.698 | 0.134 | 0.165 | −0.031 | 0.750 |
| HDL (mg/dL) | −0.226 | 0.019 | 0.118 | 0.225 | 0.137 | 0.159 | 0.165 | 0.092 | −0.253 | 0.009 | 0.167 | 0.087 | 0.136 | 0.165 |
| LDL (mg/dL) | 0.093 | 0.365 | 0.141 | 0.168 | −0.148 | 0.148 | −0.116 | 0.265 | 0.065 | 0.530 | 0.124 | 0.228 | −0.106 | 0.302 |
| AI (TC/HDL) | 0.249 | 0.010 | −0.005 | 0.955 | −0.211 | 0.030 | −0.227 | 0.021 | 0.252 | 0.010 | −0.065 | 0.508 | −0.186 | 0.057 |
| LDL/HDL | 0.291 | 0.004 | −0.045 | 0.661 | −0.230 | 0.024 | −0.250 | 0.014 | 0.305 | 0.003 | −0.098 | 0.340 | −0.205 | 0.045 |
| PAI (LogTG/HDL) | 0.234 | 0.020 | 0.017 | 0.870 | −0.213 | 0.036 | −0.218 | 0.033 | 0.228 | 0.024 | −0.044 | 0.667 | −0.201 | 0.048 |
| TG (mg/dL) | 0.115 | 0.258 | 0.026 | 0.803 | −0.113 | 0.266 | −0.095 | 0.356 | 0.090 | 0.378 | −0.016 | 0.879 | −0.098 | 0.338 |
| Glycemia (mg/dL) | 0.142 | 0.314 | −0.095 | 0.504 | −0.079 | 0.580 | −0.079 | 0.581 | 0.005 | 0.971 | −0.067 | 0.640 | −0.069 | 0.629 |
| 10-y./FRS-CHD (%) | 0.211 | 0.030 | −0.135 | 0.168 | −0.116 | 0.238 | −0.141 | 0.153 | 0.160 | 0.102 | −0.184 | 0.060 | −0.085 | 0.391 |
| 10-y./FRS-MI (%) | 0.224 | 0.021 | −0.139 | 0.155 | −0.126 | 0.200 | −0.157 | 0.110 | 0.179 | 0.067 | −0.188 | 0.055 | −0.100 | 0.309 |
| 10-y./FRS-Stroke ( %) | 0.031 | 0.753 | −0.085 | 0.384 | 0.0130 | 0.896 | −0.014 | 0.889 | −0.012 | 0.901 | −0.098 | 0.320 | 0.023 | 0.816 |
| 10-y./FRS-CVD (%) | 0.142 | 0.148 | −0.130 | 0.183 | −0.059 | 0.548 | −0.080 | 0.420 | 0.078 | 0.428 | −0.166 | 0.090 | −0.030 | 0.765 |
| 10-y./FRS-CHD DEATH (%) | 0.192 | 0.049 | −0.159 | 0.104 | −0.089 | 0.364 | −0.116 | 0.240 | 0.14 | 0.154 | −0.203 | 0.038 | −0.057 | 0.561 |
| 10-y./FRS-CVD DEATH (%) | 0.103 | 0.292 | −0.159 | 0.104 | −0.015 | 0.883 | −0.028 | 0.776 | 0.042 | 0.667 | −0.192 | 0.050 | 0.024 | 0.805 |
| 10-y./BNF Risk Score (%) | 0.177 | 0.070 | −0.131 | 0.182 | −0.089 | 0.364 | −0.116 | 0.240 | 0.125 | 0.205 | −0.173 | 0.078 | −0.061 | 0.534 |
| 10-y./ASSIGN Risk Score (%) | 0.156 | 0.034 | −0.032 | 0.664 | −0.112 | 0.128 | −0.136 | 0.067 | 0.158 | 0.033 | −0.075 | 0.315 | −0.118 | 0.11 |
| AUC | AUC 95% CI | AUC p | Youden Index (J) | Associated Criterion | SE | SP | +LR | -LR | |
|---|---|---|---|---|---|---|---|---|---|
| Presence of atherosclerotic plaques (1: yes, 0: no) (n = 581) | |||||||||
| Age (y.) | 0.82 | 0.78–0.85 | <0.001 | 0.519 | >54.9 | 77.78 | 74.14 | 3.01 | 0.30 |
| 10-y./FRS-CVD DEATH (%) | 0.81 | 0.76–0.84 | <0.001 | 0.474 | >0.91 | 81.18 | 64.47 | 2.28 | 0.29 |
| 10-y./ASSIGN Risk Score (%) | 0.80 | 0.76–0.83 | <0.001 | 0.4656 | >9.60 | 71.32 | 75.23 | 2.88 | 0.38 |
| 10-y./FRS-Stroke (%) | 0.79 | 0.74–0.83 | <0.001 | 0.4858 | >1.24 | 81.18 | 67.40 | 2.49 | 0.28 |
| 10-y./FRS-CHD DEATH (%) | 0.79 | 0.73–0.82 | <0.001 | 0.489 | >0.57 | 81.18 | 67.77 | 2.52 | 0.28 |
| 10-y./FRS-CVD (%) | 0.78 | 0.73–0.82 | <0.001 | 0.4802 | >7.81 | 89.41 | 58.61 | 2.16 | 0.18 |
| 10-y./BNF Risk Score (%) | 0.76 | 0.71–0.80 | <0.001 | 0.4582 | >6.45 | 89.41 | 56.41 | 2.05 | 0.19 |
| 10-y./FRS-CHD (%) | 0.74 | 0.69–0.78 | <0.001 | 0.4362 | >5.15 | 89.41 | 54.21 | 1.95 | 0.20 |
| 10-y./FRS-MI (%) | 0.69 | 0.63–0.73 | <0.001 | 0.3369 | >1.86 | 77.65 | 56.04 | 1.77 | 0.40 |
| cPP (mmHg) | 0.63 | 0.58–0.66 | <0.001 | 0.211 | >35 | 62.50 | 58.60 | 1.51 | 0.64 |
| TC (mg/dL) | 0.62 | 0.57–0.66 | <0.001 | 0.236 | >216 | 61.29 | 62.35 | 1.63 | 0.62 |
| cSBP (mmHg) | 0.62 | 0.57–0.65 | <0.001 | 0.208 | >105 | 82.64 | 38.14 | 1.34 | 0.46 |
| Glycemia (mg/dL) | 0.61 | 0.55–0.65 | 0.005 | 0.196 | >87 | 79.41 | 40.22 | 1.33 | 0.51 |
| pPP (mmHg) | 0.58 | 0.53–0.62 | 0.003 | 0.171 | >45 | 78.47 | 38.67 | 1.28 | 0.56 |
| pSBP (mmHg) | 0.58 | 0.53–0.61 | 0.005 | 0.145 | >128 | 47.22 | 67.28 | 1.44 | 0.78 |
| HR (b.p.m.) | 0.57 | 0.52–0.60 | 0.015 | 0.109 | <63 | 47.55 | 63.39 | 1.30 | 0.83 |
| BMI (Kg/m2) | 0.56 | 0.51–0.59 | 0.040 | 0.129 | >25.80 | 63.83 | 49.06 | 1.25 | 0.74 |
| Atherosclerotic burden (Nº of plaques, 1 or >1) (n = 144) | |||||||||
| Age (y.). | 0.66 | 0.58–0.74 | <0.001 | 0.319 | >58.18 | 85.00 | 46.88 | 1.60 | 0.32 |
| 10-y./ASSIGN Risk Score (%) | 0.61 | 0.52–0.69 | 0.026 | 0.205 | >9.30 | 80.82 | 39.68 | 1.34 | 0.48 |
| cPP (mmHg) | 0.58 | 0.49–0.66 | 0.080 | 0.159 | >34 | 73.75 | 42.19 | 1.28 | 0.62 |
| Atherosclerotic burden (Nº of arterial territories, 1 or >1) (n = 144) | |||||||||
| 10-y./FRS-CVD DEATH (%) | 0.70 | 0.58–0.79 | 0.004 | 0.336 | >5.23 | 52.38 | 81.25 | 2.79 | 0.59 |
| 10-y./FRS-Stroke (%) | 0.70 | 0.58–0.79 | 0.004 | 0.354 | >2.33 | 66.67 | 68.75 | 2.13 | 0.48 |
| 10-y./FRS-CVD (%) | 0.69 | 0.58–0.78 | 0.003 | 0.355 | >14.88 | 71.43 | 64.06 | 1.99 | 0.45 |
| 10-y./BNF Risk Score (%) | 0.67 | 0.555–0.765 | 0.011 | 0.250 | >7.148 | 100.00 | 25.00 | 1.33 | 0.00 |
| 10-y./FRS-CHD DEATH (%) | 0.66 | 0.547–0.757 | 0.023 | 0.257 | >2.362 | 47.62 | 78.12 | 2.18 | 0.67 |
| Glycemia (mg/dL) | 0.65 | 0.528–0.765 | 0.065 | 0.331 | >95 | 61.11 | 72.00 | 2.18 | 0.54 |
| 10-y./ASSIGN Risk Score (%) | 0.64 | 0.55–0.71 | 0.009 | 0.243 | >8.31 | 92.68 | 31.58 | 1.35 | 0.23 |
| Age (y.) | 0.63 | 0.54–0.71 | 0.006 | 0.305 | >61.94 | 68.89 | 61.62 | 1.79 | 0.50 |
| AUC | AUC 95% CI | AUC p | Youden Index(J) | Associated Criterion | SE | SP | +LR | -LR | |
|---|---|---|---|---|---|---|---|---|---|
| Surface area (mm2) (<p75th or ≥p75th) (n = 206) | |||||||||
| 10-y./FRS-Stroke (%) | 0.73 | 0.63–0.81 | <0.001 | 0.386 | >3.302 | 64.29 | 74.36 | 2.51 | 0.48 |
| 10-y./FRS-CVD DEATH (%) | 0.72 | 0.62–0.80 | <0.001 | 0.321 | >5.960 | 50.00 | 82.05 | 2.79 | 0.61 |
| 10-y./FRS-CVD (%) | 0.71 | 0.61–0.79 | <0.001 | 0.370 | >13.231 | 85.71 | 51.28 | 1.76 | 0.28 |
| 10-y./FRS-CHD DEATH (%) | 0.71 | 0.61–0.79 | <0.001 | 0.365 | >0.988 | 92.86 | 43.59 | 1.65 | 0.16 |
| 10-y./BNF Risk Score (%) | 0.71 | 0.61–0.79 | <0.001 | 0.370 | >10.310 | 85.71 | 51.28 | 1.76 | 0.28 |
| Age (y.) | 0.69 | 0.62–0.75 | <0.001 | 0.349 | >63.705 | 75.00 | 59.86 | 1.87 | 0.42 |
| 10-y./ASSIGN Risk Score (%) | 0.68 | 0.60–0.74 | <0.001 | 0.292 | >9.750 | 93.48 | 35.71 | 1.45 | 0.18 |
| 10-y./FRS-CHD (%) | 0.67 | 0.57–0.75 | 0.006 | 0.283 | >7.141 | 82.14 | 46.15 | 1.53 | 0.39 |
| cPP (mmHg) | 0.66 | 0.58–0.72 | <0.001 | 0.328 | >39 | 72.92 | 59.86 | 1.82 | 0.45 |
| 10-y./FRS-MI (%) | 0.65 | 0.54–0.73 | 0.021 | 0.242 | >8.293 | 35.71 | 88.46 | 3.10 | 0.73 |
| pPP (mmHg) | 0.63 | 0.56–0.70 | 0.003 | 0.213 | >62 | 39.58 | 81.69 | 2.16 | 0.74 |
| cSBP (mmHg) | 0.62 | 0.54–0.68 | 0.016 | 0.211 | >122 | 52.08 | 69.01 | 1.68 | 0.69 |
| pSBP (mmHg) | 0.59 | 0.51–0.660 | 0.060 | 0.172 | >144 | 31.25 | 85.92 | 2.22 | 0.80 |
| LDL/HDL | 0.56 | 0.45–0.65 | 0.442 | 0.194 | >2.970 | 46.15 | 73.24 | 1.72 | 0.74 |
| AI (TC/HDL) | 0.55 | 0.45–0.65 | 0.413 | 0.200 | >3.936 | 75.86 | 44.16 | 1.36 | 0.55 |
| Plaque composition (0: fibrous, 1: lipidic + fibrolipidic) (n = 206) | |||||||||
| PAI (LogTG/HDL) | 0.58 | 0.50–0.65 | 0.067 | 0.207 | >0.301 | 53.03 | 67.68 | 1.64 | 0.69 |
| LDL/HDL | 0.55 | 0.47–0.62 | 0.275 | 0.146 | >2.590 | 54.55 | 60.00 | 1.36 | 0.76 |
| AI (TC/HDL) | 0.53 | 0.44–0.60 | 0.584 | 0.102 | >4.300 | 39.13 | 71.03 | 1.35 | 0.86 |
| Plaque composition (GSM ≤105 or >105) (n = 206) | |||||||||
| LDL/HDL | 0.61 | 0.51–0.70 | 0.044 | 0.244 | >2.461 | 72.41 | 52.00 | 1.51 | 0.53 |
| PAI (LogTG/HDL) | 0.61 | 0.50–0.71 | 0.052 | 0.220 | >0.135 | 87.27 | 34.78 | 1.34 | 0.37 |
| AI (TC/HDL) | 0.58 | 0.47- 0.67 | 0.177 | 0.186 | >3.432 | 90.00 | 28.57 | 1.26 | 0.35 |
| Plaque composition (Media grayscale ≤110 or >110) (n = 206) | |||||||||
| LDL/HDL | 0.61 | 0.51–071 | 0.040 | 0.252 | >2.462 | 73.21 | 52.00 | 1.53 | 0.52 |
| PAI (LogTG/HDL) | 0.60 | 0.50–0.69 | 0.085 | 0.218 | >0.135 | 87.04 | 34.78 | 1.33 | 0.37 |
| AI (TC/HDL) | 0.56 | 0.48- 0.67 | 0.182 | 0.200 | >3.433 | 91.38 | 28.57 | 1.28 | 0.3 |
| Plaque composition (Media grayscale 1mm ≤110 or >110) (n =206) | |||||||||
| PAI (LogTG/HDL) | 0.64 | 0.53–0.73 | 0.016 | 0.237 | >0.216 | 67.92 | 55.81 | 1.54 | 0.57 |
| LDL/HDL | 0.63 | 0.53–0.72 | 0.018 | 0.224 | >2.461 | 71.43 | 51.06 | 1.46 | 0.56 |
| AI (TC/HDL) | 0.58 | 0.48–0.67 | 0.142 | 0.182 | >4.3 | 46.55 | 71.74 | 1.65 | 0.75 |
| Surface Area (mm2) | Width (mm) | Height (mm) | GS Median (GSM) | GS Mean | Heterog. (%) | Lipidic (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | p | R | p | R | p | R | p | R | p | R | p | R | p | |
| Area (mm2) | 1.00 | 0.84 | <0.001 | 0.66 | <0.001 | −0.03 | 0.649 | −0.01 | 0.869 | 0.01 | 0.877 | 0.04 | 0.546 | |
| Width (mm) | 0.84 | <0.001 | 1.00 | 0.37 | <0.001 | −0.14 | 0.060 | −0.13 | 0.085 | −0.17 | 0.023 | 0.10 | 0.184 | |
| Height (mm) | 0.66 | <0.001 | 0.37 | <0.001 | 1.00 | 0.03 | 0.707 | 0.05 | 0.481 | 0.27 | <0.001 | 0.03 | 0.687 | |
| GS median | −0.03 | 0.649 | −0.14 | 0.060 | 0.03 | 0.707 | 1.00 | 0.98 | <0.001 | 0.46 | <0.001 | −0.85 | <0.001 | |
| GS mean | −0.01 | 0.869 | −0.13 | 0.085 | 0.05 | 0.481 | 0.98 | <0.001 | 1.00 | 0.56 | <0.001 | −0.88 | <0.001 | |
| Heterog. (%) | 0.01 | 0.877 | −0.17 | 0.023 | 0.27 | <0.001 | 0.46 | <0.001 | 0.56 | <0.001 | 1.00 | −0.40 | <0.001 | |
| Lipidic (%) | 0.04 | 0.546 | 0.10 | 0.184 | 0.03 | 0.687 | −0.85 | <0.001 | −0.88 | <0.001 | −0.40 | <0.001 | 1.00 | |
| Fib-Lip. (%) | −0.09 | 0.215 | 0.02 | 0.768 | −0.19 | 0.008 | −0.50 | <0.001 | −0.45 | <0.001 | −0.45 | <0.001 | 0.07 | 0.374 |
| Fibrous (%) | 0.00 | 0.973 | −0.10 | 0.165 | 0.06 | 0.412 | 0.95 | <0.001 | 0.97 | <0.001 | 0.55 | <0.001 | −0.88 | <0.001 |
| GS mean 1mm | −0.04 | 0.616 | −0.18 | 0.013 | 0.08 | 0.264 | 0.95 | <0.001 | 0.96 | <0.001 | 0.57 | <0.001 | −0.85 | <0.001 |
| Lipidic 1mm (%) | 0.04 | 0.565 | 0.11 | 0.144 | 0.01 | 0.905 | −0.78 | <0.001 | −0.81 | <0.001 | −0.39 | <0.001 | 0.92 | <0.001 |
| Fib-Lip. 1mm (%) | −0.07 | 0.347 | 0.06 | 0.423 | −0.18 | 0.013 | −0.41 | <0.001 | −0.37 | <0.001 | −0.46 | <0.001 | −0.03 | 0.676 |
| Fibrous 1mm (%) | −0.03 | 0.660 | −0.15 | 0.036 | 0.06 | 0.447 | 0.92 | <0.001 | 0.94 | <0.001 | 0.59 | <0.001 | −0.83 | <0.001 |
| Fibrolipidic (%) | Fibrous (%) | GS mean 1 mm | Lipidic 1 mm (%) | Fibrolipidic 1 mm (%) | Fibrous 1 mm (%) | |||||||||
| R | p | R | p | R | p | R | p | R | p | R | p | |||
| Area (mm2) | −0.09 | 0.215 | 0.00 | 0.973 | −0.04 | 0.616 | 0.04 | 0.565 | −0.07 | 0.347 | −0.03 | 0.660 | ||
| Width (mm) | 0.02 | 0.768 | −0.10 | 0.165 | −0.18 | 0.013 | 0.11 | 0.144 | 0.06 | 0.423 | −0.15 | 0.036 | ||
| Height (mm) | −0.19 | 0.008 | 0.06 | 0.412 | 0.08 | 0.264 | 0.01 | 0.905 | −0.18 | 0.013 | 0.06 | 0.447 | ||
| GS median | −0.50 | <0.001 | 0.95 | <0.001 | 0.95 | <0.001 | −0.78 | <0.001 | −0.41 | <0.001 | 0.92 | <0.001 | ||
| GS mean | −0.45 | <0.001 | 0.97 | <0.001 | 0.96 | <0.001 | −0.81 | <0.001 | −0.37 | <0.001 | 0.94 | <0.001 | ||
| Heterog, (%) | −0.45 | <0.001 | 0.55 | <0.001 | 0.57 | <0.001 | −0.39 | <0.001 | −0.46 | <0.001 | 0.59 | <0.001 | ||
| Lipidic (%) | 0.07 | 0.374 | −0.88 | <0.001 | −0.85 | <0.001 | 0.92 | <0.001 | −0.03 | 0.676 | −0.83 | <0.001 | ||
| Fib-Lip. (%) | 1.00 | −0.54 | <0.001 | −0.47 | <0.001 | 0.05 | 0.531 | 0.93 | <0.001 | −0.53 | <0.001 | |||
| Fibrous (%) | −0.54 | <0.001 | 1.00 | 0.94 | <0.001 | −0.80 | <0.001 | −0.43 | <0.001 | 0.95 | <0.001 | |||
| GS mean 1mm | −0.47 | <0.001 | 0.94 | <0.001 | 1.00 | −0.83 | <0.001 | −0.43 | <0.001 | 0.97 | <0.001 | |||
| Lipidic 1mm (%) | 0.05 | 0.531 | −0.80 | <0.001 | −0.83 | <0.001 | 1.00 | −0.03 | 0.729 | −0.82 | <0.001 | |||
| Fib-Lip. 1mm (%) | 0.93 | <0.001 | −0.43 | <0.001 | −0.43 | <0.001 | −0.03 | 0.729 | 1.00 | −0.49 | <0.001 | |||
| Fibrous 1mm (%) | −0.53 | <0.001 | 0.95 | <0.001 | 0.97 | <0.001 | −0.82 | <0.001 | −0.49 | <0.001 | 1.00 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin, M.; Bia, D.; Zócalo, Y. Carotid and Femoral Atherosclerotic Plaques in Asymptomatic and Non-Treated Subjects: Cardiovascular Risk Factors, 10-Years Risk Scores, and Lipid Ratios’ Capability to Detect Plaque Presence, Burden, Fibro-Lipid Composition and Geometry. J. Cardiovasc. Dev. Dis. 2020, 7, 11. https://doi.org/10.3390/jcdd7010011
Marin M, Bia D, Zócalo Y. Carotid and Femoral Atherosclerotic Plaques in Asymptomatic and Non-Treated Subjects: Cardiovascular Risk Factors, 10-Years Risk Scores, and Lipid Ratios’ Capability to Detect Plaque Presence, Burden, Fibro-Lipid Composition and Geometry. Journal of Cardiovascular Development and Disease. 2020; 7(1):11. https://doi.org/10.3390/jcdd7010011
Chicago/Turabian StyleMarin, Mariana, Daniel Bia, and Yanina Zócalo. 2020. "Carotid and Femoral Atherosclerotic Plaques in Asymptomatic and Non-Treated Subjects: Cardiovascular Risk Factors, 10-Years Risk Scores, and Lipid Ratios’ Capability to Detect Plaque Presence, Burden, Fibro-Lipid Composition and Geometry" Journal of Cardiovascular Development and Disease 7, no. 1: 11. https://doi.org/10.3390/jcdd7010011
APA StyleMarin, M., Bia, D., & Zócalo, Y. (2020). Carotid and Femoral Atherosclerotic Plaques in Asymptomatic and Non-Treated Subjects: Cardiovascular Risk Factors, 10-Years Risk Scores, and Lipid Ratios’ Capability to Detect Plaque Presence, Burden, Fibro-Lipid Composition and Geometry. Journal of Cardiovascular Development and Disease, 7(1), 11. https://doi.org/10.3390/jcdd7010011




