Cauterization as a Simple Method for Regeneration Studies in the Zebrafish Heart
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Surgery and Cautery Injury
2.3. Imaging and Measurements of Injury Size by Brightfield and Scanning Electron Microscopy
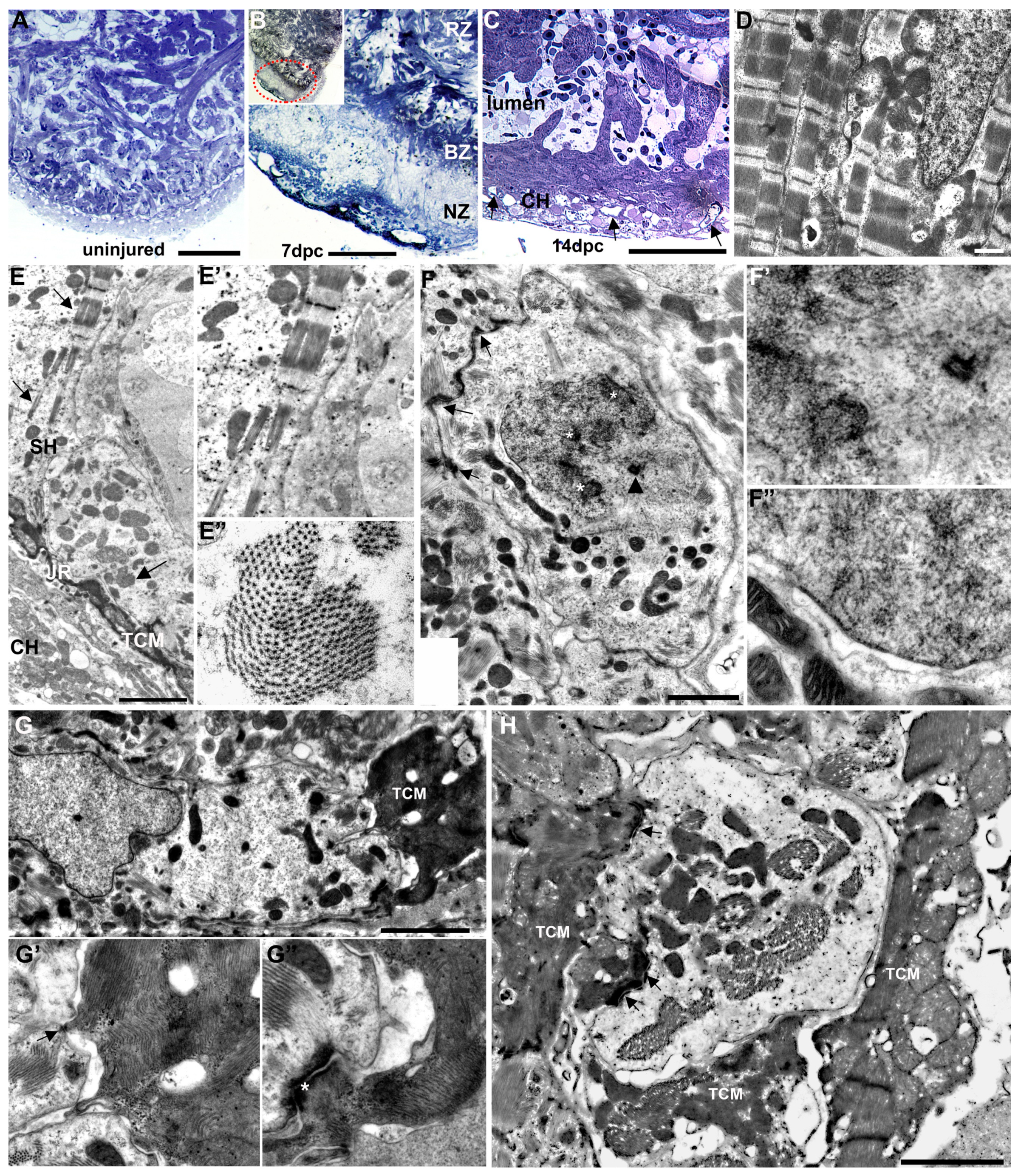
2.4. Characterization of Injury and Repair by Transmission Electron Microscopy (TEM)
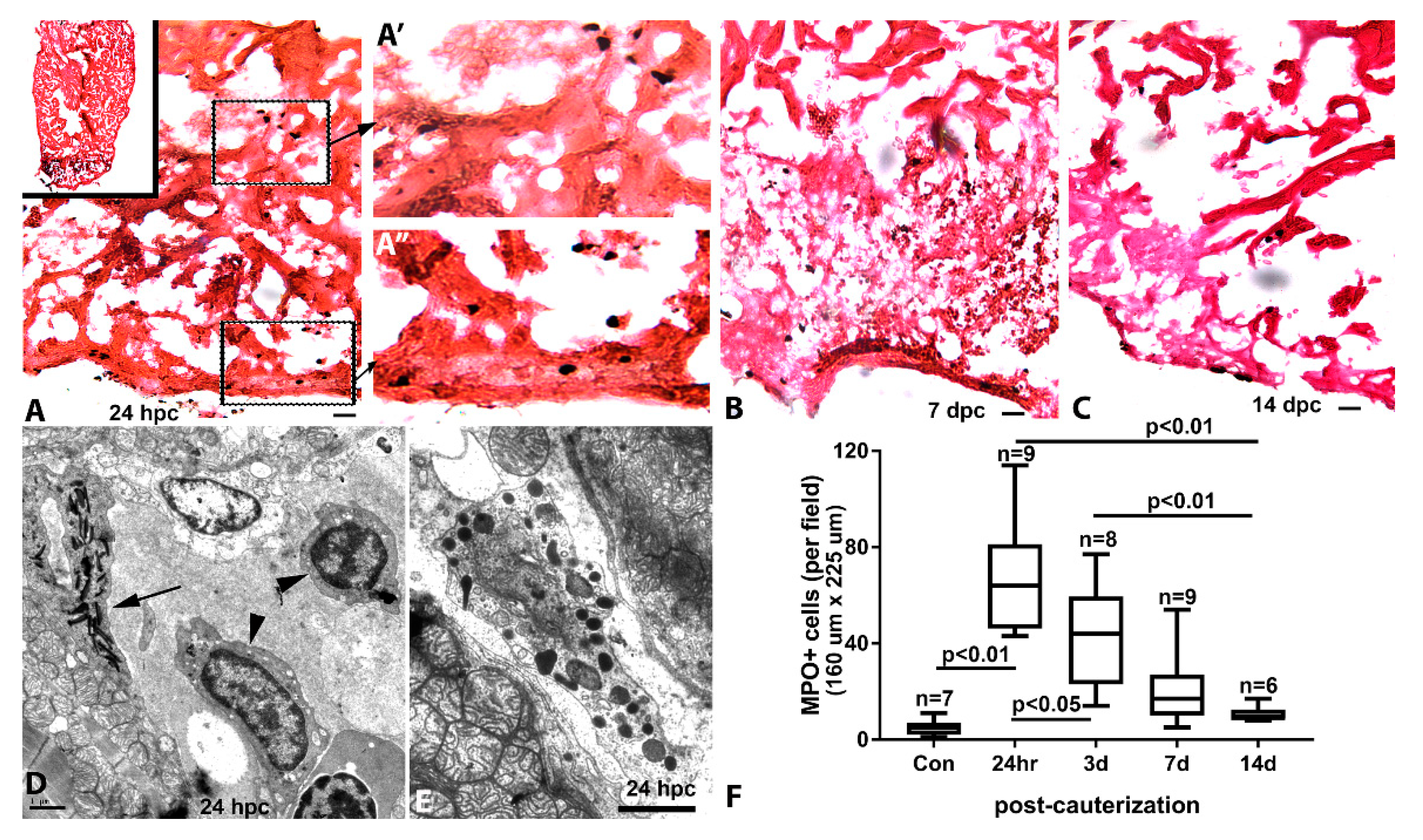
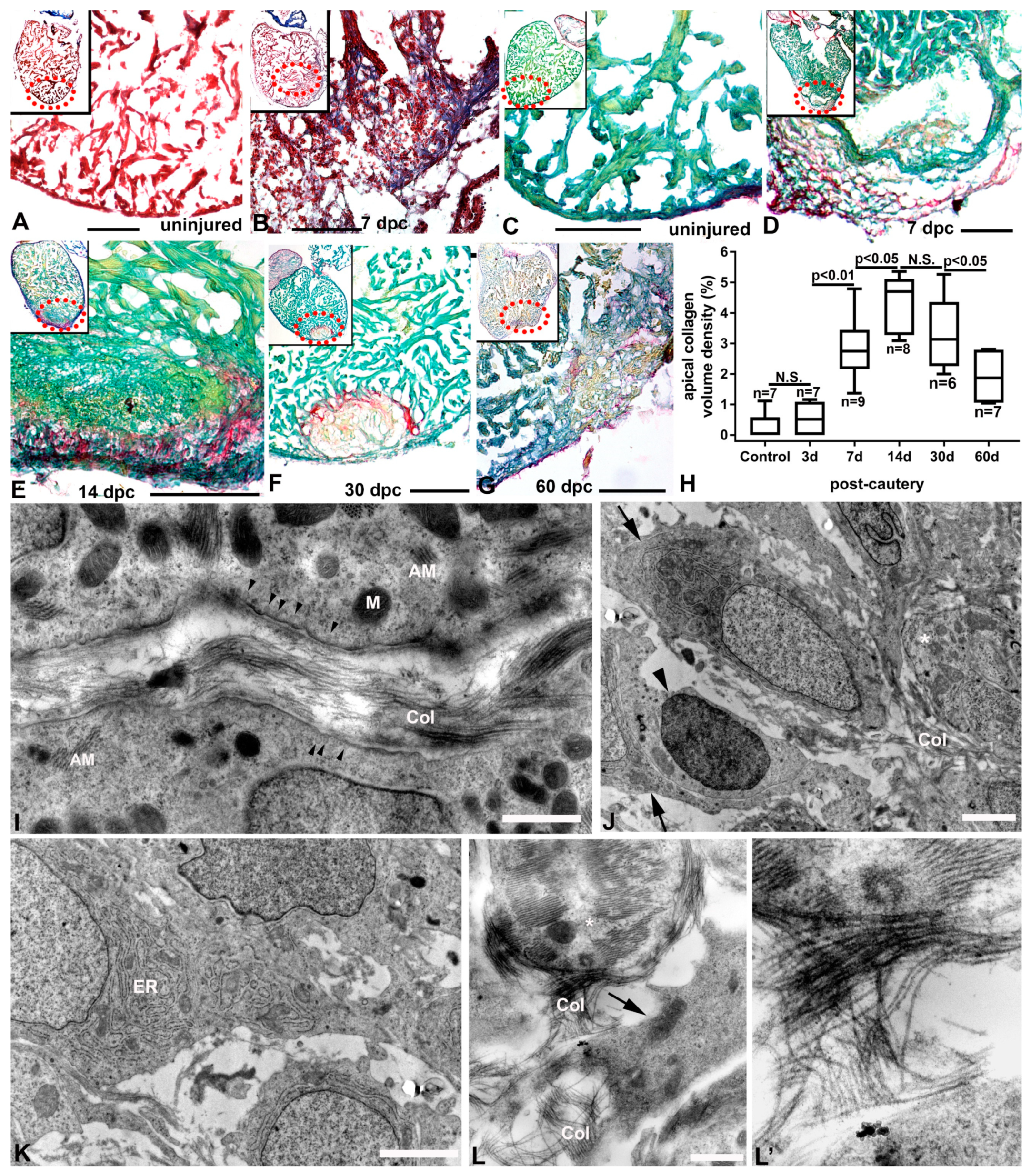
2.5. Characterization of Injury and Repair by Histochemistry (Collagen and Inflammatory Cells)
2.6. Nerves and Fluorescence Imaging
2.7. Statistical Analysis
3. Results
3.1. Gross Characterization of ZebrafishHeart Subjected to Cautery Injury
3.2. Characterization of ZebrafishCauterized Heart by SEM
3.3. Ultrastructural Characterization of the Injury Response by TEM
3.4. Inflammation and Remodeling in the Cauterized Zebrafish Ventricle
3.5. Re-Innervation of the Injured and Regenerating Zebrafish Heart
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ritchey, M.D.; Wall, H.K.; George, M.G.; Wright, J.S. US trends in premature heart disease mortality over the past 50 years: Where do we go from here? Trends Cardiovasc. Med. 2019. [Google Scholar] [CrossRef]
- Yusuf, S.; Joseph, P.; Rangarajan, S.; Islam, S.; Mente, A.; Hystad, P.; Brauer, M.; Kutty, V.R.; Gupta, R.; Wielgosz, A.; et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet 2020, 395, 795–808. [Google Scholar] [CrossRef] [Green Version]
- Rosengren, A.; Smyth, A.; Rangarajan, S.; Ramasundarahettige, C.; Bangdiwala, S.I.; AlHabib, K.F.; Avezum, A.; Boström, K.B.; Chifamba, J.; Gulec, S.; et al. Socioeconomic status and risk of cardiovascular disease in 20 low-income, middle-income, and high-income countries: The Prospective Urban Rural Epidemiologic (PURE) study. Lancet Glob. Health 2019, 7, e748–e760. [Google Scholar] [CrossRef] [Green Version]
- Elgendy, I.Y.; Mahtta, D.; Pepine, C.J. Medical Therapy for Heart Failure Caused by Ischemic Heart Disease. Circ. Res. 2019, 124, 1520–1535. [Google Scholar] [CrossRef] [PubMed]
- Soonpaa, M.H.; Field, L.J. Assessment of cardiomyocyte DNA synthesis in normal and injured adult mouse hearts. Am. J. Physiol. 1997, 272, H220–H226. [Google Scholar] [CrossRef] [PubMed]
- Talman, V.; Ruskoaho, H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 2016, 365, 563–581. [Google Scholar] [CrossRef] [Green Version]
- Katz, A.M. The cardiomyopathy of overload: An unnatural growth response in the hypertrophied heart. Ann. Intern. Med. 1994, 121, 363–371. [Google Scholar] [CrossRef]
- Ahuja, P.; Sdek, P.; MacLellan, W.R. Cardiac myocyte cell cycle control in development, disease, and regeneration. Physiol. Rev. 2007, 87, 521–544. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, P.; Perriard, E.; Pedrazzini, T.; Satoh, S.; Perriard, J.C.; Ehler, E. Re-expression of proteins involved in cytokinesis during cardiac hypertrophy. Exp. Cell Res. 2007, 313, 1270–1283. [Google Scholar] [CrossRef]
- Sadek, H.; Olson, E.N. Toward the Goal of Human Heart Regeneration. Cell Stem Cell 2020, 26, 7–16. [Google Scholar] [CrossRef]
- Whittaker, P. Collagen and ventricular remodeling after acute myocardial infarction: Concepts and hypotheses. Basic Res. Cardiol. 1997, 92, 79–81. [Google Scholar] [CrossRef]
- Christia, P.; Bujak, M.; Gonzalez-Quesada, C.; Chen, W.; Dobaczewski, M.; Reddy, A.; Frangogiannis, N.G. Systematic characterization of myocardial inflammation, repair, and remodeling in a mouse model of reperfused myocardial infarction. J. Histochem. Cytochem. 2013, 61, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Hill, J.A.; Richardson, J.A.; Olson, E.N.; Sadek, H.A. Transient regenerative potential of the neonatal mouse heart. Science 2011, 331, 1078–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryant, D.M.; O’Meara, C.C.; Ho, N.N.; Gannon, J.; Cai, L.; Lee, R.T. A systematic analysis of neonatal mouse heart regeneration after apical resection. J. Mol. Cell Cardiol. 2015, 79, 315–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, D.C.; Ganesalingam, S.; Jensen, C.H.; Sheikh, S.P. Do neonatal mouse hearts regenerate following heart apex resection? Stem Cell Rep. 2014, 2, 406–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, C.D.; Jensen, H.C.; Baun, C.; Hvidsten, S.; Zebrowski, C.D.; Engel, B.F.; Sheikh, P.S. Persistent scarring and dilated cardiomyopathy suggest incomplete regeneration of the apex resected neonatal mouse myocardium—A 180 days follow up study. J. Mol. Cell. Cardiol. 2016, 90, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Jewhurst, K.; McLaughlin, K.A. Beyond the Mammalian Heart: Fish and Amphibians as a Model for Cardiac Repair and Regeneration. J. Dev. Biol. 2016, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Borchardt, T.; Braun, T. Cardiovascular regeneration in non-mammalian model systems: What are the differences between newts and man? Thromb. Haemost. 2007, 98, 311–318. [Google Scholar]
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart regeneration in zebrafish. Science 2002, 298, 2188–2190. [Google Scholar] [CrossRef]
- Gonzalez-Rosa, J.M.; Burns, C.E.; Burns, C.G. Zebrafish heart regeneration: 15 years of discoveries. Regeneration 2017, 4, 105–123. [Google Scholar] [CrossRef]
- Michael, L.H.; Ballantyne, C.M.; Zachariah, J.P.; Gould, K.E.; Pocius, J.S.; Taffet, G.E.; Hartley, C.J.; Pham, T.T.; Daniel, S.L.; Funk, E.; et al. Myocardial infarction and remodeling in mice: Effect of reperfusion. Am. J. Physiol. 1999, 277, H660–H668. [Google Scholar] [CrossRef] [PubMed]
- Nossuli, T.O.; Frangogiannis, N.G.; Knuefermann, P.; Lakshminarayanan, V.; Dewald, O.; Evans, A.J.; Peschon, J.; Mann, D.L.; Michael, L.H.; Entman, M.L. Brief murine myocardial I/R induces chemokines in a TNF-alpha-independent manner: Role of oxygen radicals. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H2549–H2558. [Google Scholar] [CrossRef] [PubMed]
- Brooks, W.W.; Garibaldi, B.A.; Conrad, C.H. Myocardial injury in the mouse induced by transthoracic cauterization. Lab. Anim Sci 1998, 48, 374–378. [Google Scholar] [PubMed]
- van den Bos, E.J.; Mees, B.M.; de Waard, M.C.; de Crom, R.; Duncker, D.J. A novel model of cryoinjury-induced myocardial infarction in the mouse: A comparison with coronary artery ligation. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1291–H1300. [Google Scholar] [CrossRef] [Green Version]
- Dewald, O.; Frangogiannis, N.G.; Zoerlein, M.P.; Duerr, G.D.; Taffet, G.; Michael, L.H.; Welz, A.; Entman, M.L. A murine model of ischemic cardiomyopathy induced by repetitive ischemia and reperfusion. Thorac. Cardiovasc. Surg. 2004, 52, 305–311. [Google Scholar] [CrossRef]
- Nossuli, T.O.; Lakshminarayanan, V.; Baumgarten, G.; Taffet, G.E.; Ballantyne, C.M.; Michael, L.H.; Entman, M.A. A chronic mouse model of myocardial ischemia-reperfusion: Essential in cytokine studies. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H1049–H1055. [Google Scholar] [CrossRef]
- Flink, I.L. Cell cycle reentry of ventricular and atrial cardiomyocytes and cells within the epicardium following amputation of the ventricular apex in the axolotl, Amblystoma mexicanum: Confocal microscopic immunofluorescent image analysis of bromodeoxyuridine-labeled nuclei. Anat. Embryol. 2002, 205, 235–244. [Google Scholar] [CrossRef]
- Oberpriller, J.; Oberpriller, J.C. Mitosis in adult newt ventricle. J. Cell Biol. 1971, 49, 560–563. [Google Scholar] [CrossRef] [Green Version]
- Bader, D.; Oberpriller, J.O. Repair and reorganization of minced cardiac muscle in the adult newt (Notophthalmus viridescens). J. Morphol. 1978, 155, 349–357. [Google Scholar] [CrossRef]
- Raya, Á.; Koth, C.M.; Büscher, D.; Kawakami, Y.; Itoh, T.; Raya, R.M.; Sternik, G.; Tsai, H.-J.; Rodríguez-Esteban, C.; Izpisúa-Belmonte, J.C. Activation of Notch signaling pathway precedes heart regeneration in zebrafish. Proc. Natl. Acad. Sci. USA 2003, 100 (Suppl. 1), 11889–11895. [Google Scholar] [CrossRef] [Green Version]
- Itou, J.; Akiyama, R.; Pehoski, S.; Yu, X.; Kawakami, H.; Kawakami, Y. Regenerative responses after mild heart injuries for cardiomyocyte proliferation in zebrafish. Dev. Dyn. 2014, 243, 1477–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, V.; Gemberling, M.; Karra, R.; Rosenfeld, G.E.; Evans, T.; Poss, K.D. An injury-responsive gata4 program shapes the zebrafish cardiac ventricle. Curr. Biol. 2013, 23, 1221–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikuchi, K.; Holdway, J.E.; Major, R.J.; Blum, N.; Dahn, R.D.; Begemann, G.; Poss, K.D. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev. Cell 2011, 20, 397–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Panáková, D.; Kikuchi, K.; Holdway, J.E.; Gemberling, M.; Burris, J.S.; Singh, S.P.; Dickson, A.L.; Lin, Y.F.; Sabeh, M.K.; et al. The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development 2011, 138, 3421–3430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Han, P.; Yang, H.; Ouyang, K.; Lee, D.; Lin, Y.F.; Ocorr, K.; Kang, G.; Chen, J.; Stainier, D.Y.; et al. In vivo cardiac reprogramming contributes to zebrafish heart regeneration. Nature 2013, 498, 497–501. [Google Scholar] [CrossRef]
- González-Rosa, J.M.; Mercader, N. Cryoinjury as a myocardial infarction model for the study of cardiac regeneration in the zebrafish. Nat. Protoc. 2012, 7, 782–788. [Google Scholar] [CrossRef]
- Chablais, F.; Veit, J.; Rainer, G.; Jaźwińska, A. The zebrafish heart regenerates after cryoinjury-induced myocardial infarction. BMC Dev. Biol. 2011, 11, 21. [Google Scholar] [CrossRef] [Green Version]
- Schnabel, K.; Wu, C.C.; Kurth, T.; Weidinger, G. Regeneration of cryoinjury induced necrotic heart lesions in zebrafish is associated with epicardial activation and cardiomyocyte proliferation. PLoS ONE 2011, 6, e18503. [Google Scholar] [CrossRef]
- Lafontant, P.J.; Burns, A.R.; Grivas, J.A.; Lesch, M.A.; Lala, T.D.; Reuter, S.P.; Field, L.J.; Frounfelter, T.D. The giant danio (D. aequipinnatus) as a model of cardiac remodeling and regeneration. Anat. Rec. 2012, 295, 234–248. [Google Scholar] [CrossRef] [Green Version]
- Grivas, J.; Haag, M.; Johnson, A.; Manalo, T.; Roell, J.; Das, T.L.; Brown, E.; Burns, A.R.; Lafontant, P.J. Cardiac repair and regenerative potential in the goldfish (Carassius auratus) heart. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014, 163, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Ito, K.; Morioka, M.; Kimura, S.; Tasaki, M.; Inohaya, K.; Kudo, A. Differential reparative phenotypes between zebrafish and medaka after cardiac injury. Dev. Dyn. 2014, 243, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Stockdale, W.T.; Lemieux, M.E.; Killen, A.C.; Zhao, J.; Hu, Z.; Riepsaame, J.; Hamilton, N.; Kudoh, T.; Riley, P.R.; van Aerle, R.; et al. Heart Regeneration in the Mexican Cavefish. Cell Rep. 2018, 25, 1997–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, S.L.; Marín-Juez, R.; Moura, P.L.; Kuenne, C.; Lai, J.K.H.; Tsedeke, A.T.; Guenther, S.; Looso, M.; Stainier, D.Y. Reciprocal analyses in zebrafish and medaka reveal that harnessing the immune response promotes cardiac regeneration. Elife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Jopling, C.; Sleep, E.; Raya, M.; Martí, M.; Raya, A.; Belmonte, J.C.I. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 2010, 464, 606–609. [Google Scholar] [CrossRef]
- Foglia, M.J.; Poss, K.D. Building and re-building the heart by cardiomyocyte proliferation. Development 2016, 143, 729–740. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, K.; Holdway, J.E.; Werdich, A.A.; Anderson, R.M.; Fang, Y.; Egnaczyk, G.F.; Evans, T.; MacRae, C.A.; Stainier, D.Y.; Poss, K.D. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature 2010, 464, 601–605. [Google Scholar] [CrossRef]
- Honkoop, H.; de Bakker, D.E.; Aharonov, A.; Kruse, F.; Shakked, A.; Nguyen, P.D.; de Heus, C.; Garric, L.; Muraro, M.J.; Shoffner, A.; et al. Single-cell analysis uncovers that metabolic reprogramming by ErbB2 signaling is essential for cardiomyocyte proliferation in the regenerating heart. Elife 2019, 8, e50163. [Google Scholar] [CrossRef]
- González-Rosa, J.M.; Sharpe, M.; Field, D.; Soonpaa, M.H.; Field, L.J.; Burns, C.E.; Burns, C.G. Myocardial Polyploidization Creates a Barrier to Heart Regeneration in Zebrafish. Dev. Cell 2018, 44, 433–446. [Google Scholar] [CrossRef] [Green Version]
- Lafontant, P.J.; Behzad, A.R.; Brown, E.; Landry, P.; Hu, N.; Burns, A.R. Cardiac myocyte diversity and a fibroblast network in the junctional region of the zebrafish heart revealed by transmission and serial block-face scanning electron microscopy. PLoS ONE 2013, 8, e72388. [Google Scholar] [CrossRef] [Green Version]
- Pfefferli, C.; Jaźwińska, A. The careg element reveals a common regulation of regeneration in the zebrafish myocardium and fin. Nat. Commun. 2017, 8, 15151. [Google Scholar] [CrossRef]
- Yu, F.; Li, R.; Parks, E.; Takabe, W.; Hsiai, T.K. Electrocardiogram signals to assess zebrafish heart regeneration: Implication of long QT intervals. Ann. Biomed. Eng. 2010, 38, 2346–2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verberne, M.E.; Gittenberger-De Groot, A.C.; van Iperen, L.; Poelmann, R.E. Distribution of different regions of cardiac neural crest in the extrinsic and the intrinsic cardiac nervous system. Dev. Dyn. 2000, 217, 191–204. [Google Scholar] [CrossRef]
- Beaumont, E.; Salavatian, S.; Southerland, E.M.; Vinet, A.; Jacquemet, V.; Armour, J.A.; Ardell, J.L. Network interactions within the canine intrinsic cardiac nervous system: Implications for reflex control of regional cardiac function. J. Physiol. 2013, 591, 4515–4533. [Google Scholar] [CrossRef]
- Newton, C.M.; Stoyek, M.R.; Croll, R.P.; Smith, F.M. Regional innervation of the heart in the goldfish, Carassius auratus: A confocal microscopy study. J. Comp. Neurol. 2014, 522, 456–478. [Google Scholar] [CrossRef] [PubMed]
- Stoyek, M.R.; Croll, R.P.; Smith, F.M. Intrinsic and extrinsic innervation of the heart in zebrafish (Danio rerio). J. Comp. Neurol. 2015, 523, 1683–1700. [Google Scholar] [CrossRef]
- Brockes, J.P. The nerve dependence of amphibian limb regeneration. J. Exp. Biol. 1987, 132, 79–91. [Google Scholar]
- Fekete, D.M.; Brockes, J.P. Evidence that the nerve controls molecular identity of progenitor cells for limb regeneration. Development 1988, 103, 567–573. [Google Scholar] [PubMed]
- Mahmoud, A.I.; O’Meara, C.C.; Gemberling, M.; Zhao, L.; Bryant, D.M.; Zheng, R.; Gannon, J.B.; Cai, L.; Choi, W.Y.; Egnaczyk, G.F.; et al. Nerves Regulate Cardiomyocyte Proliferation and Heart Regeneration. Dev. Cell 2015, 34, 387–399. [Google Scholar] [CrossRef] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyck, P.K.V.; Hockaden, N.; Nelson, E.C.; Koch, A.R.; Hester, K.L.; Pillai, N.; Coffing, G.C.; Burns, A.R.; Lafontant, P.J. Cauterization as a Simple Method for Regeneration Studies in the Zebrafish Heart. J. Cardiovasc. Dev. Dis. 2020, 7, 41. https://doi.org/10.3390/jcdd7040041
Dyck PKV, Hockaden N, Nelson EC, Koch AR, Hester KL, Pillai N, Coffing GC, Burns AR, Lafontant PJ. Cauterization as a Simple Method for Regeneration Studies in the Zebrafish Heart. Journal of Cardiovascular Development and Disease. 2020; 7(4):41. https://doi.org/10.3390/jcdd7040041
Chicago/Turabian StyleDyck, Papa K. Van, Natasha Hockaden, Emma C. Nelson, Alyssa R. Koch, Kamil L. Hester, Neil Pillai, Gabrielle C. Coffing, Alan R. Burns, and Pascal J. Lafontant. 2020. "Cauterization as a Simple Method for Regeneration Studies in the Zebrafish Heart" Journal of Cardiovascular Development and Disease 7, no. 4: 41. https://doi.org/10.3390/jcdd7040041
APA StyleDyck, P. K. V., Hockaden, N., Nelson, E. C., Koch, A. R., Hester, K. L., Pillai, N., Coffing, G. C., Burns, A. R., & Lafontant, P. J. (2020). Cauterization as a Simple Method for Regeneration Studies in the Zebrafish Heart. Journal of Cardiovascular Development and Disease, 7(4), 41. https://doi.org/10.3390/jcdd7040041




