Radiation Dose and Image Quality of a High-Pitch Prospective Spiral First Approach in Coronary Computed Tomography Angiography (CCTA)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion/Exclusion
2.2. General Scan Protocol
2.3. Scan Methods and Acquisition Algorithm
2.4. Image Analysis
- Grade 0: Non-diagnostic scan, need for repetition
- Grade 1: Diagnostic scan, mild artefacts, good to satisfactory contrast
- Grade 2: Excellent scan, no artefacts, clear contrast in all coronary segments
2.5. Radiation Exposure
2.6. Statistical Analysis
3. Results
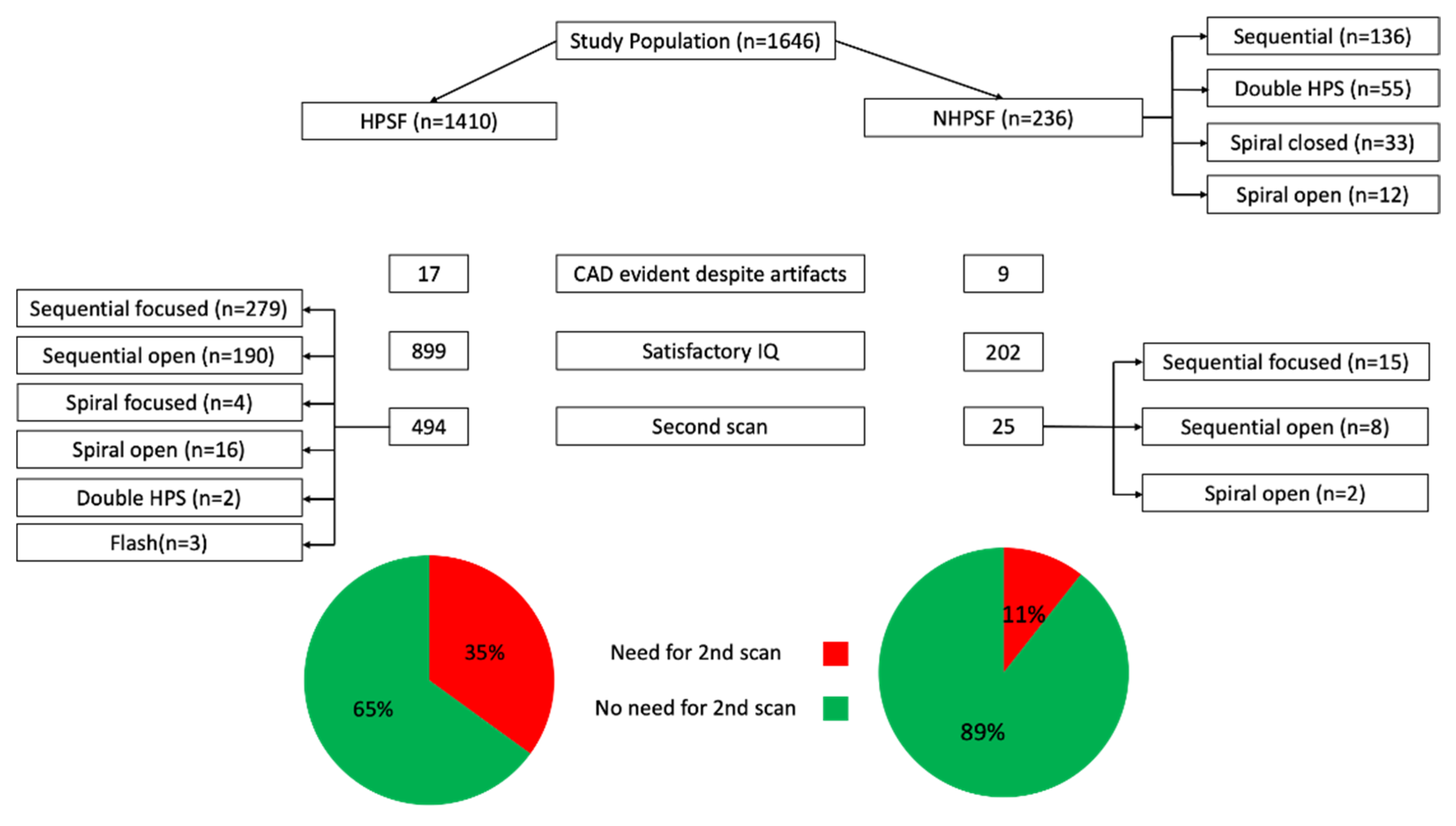
3.1. Patients
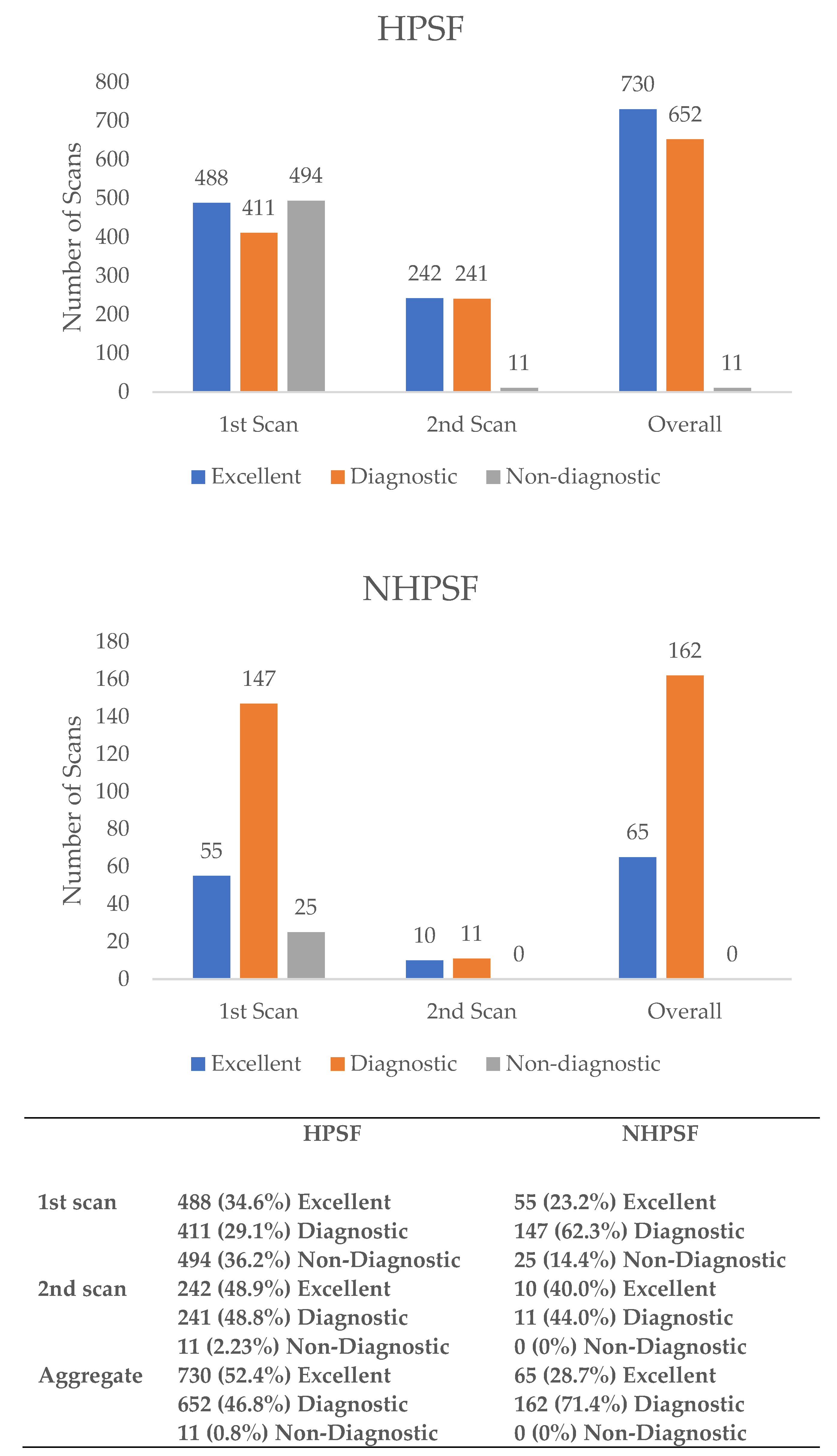
3.2. Image Quality
3.3. Artefact Distribution
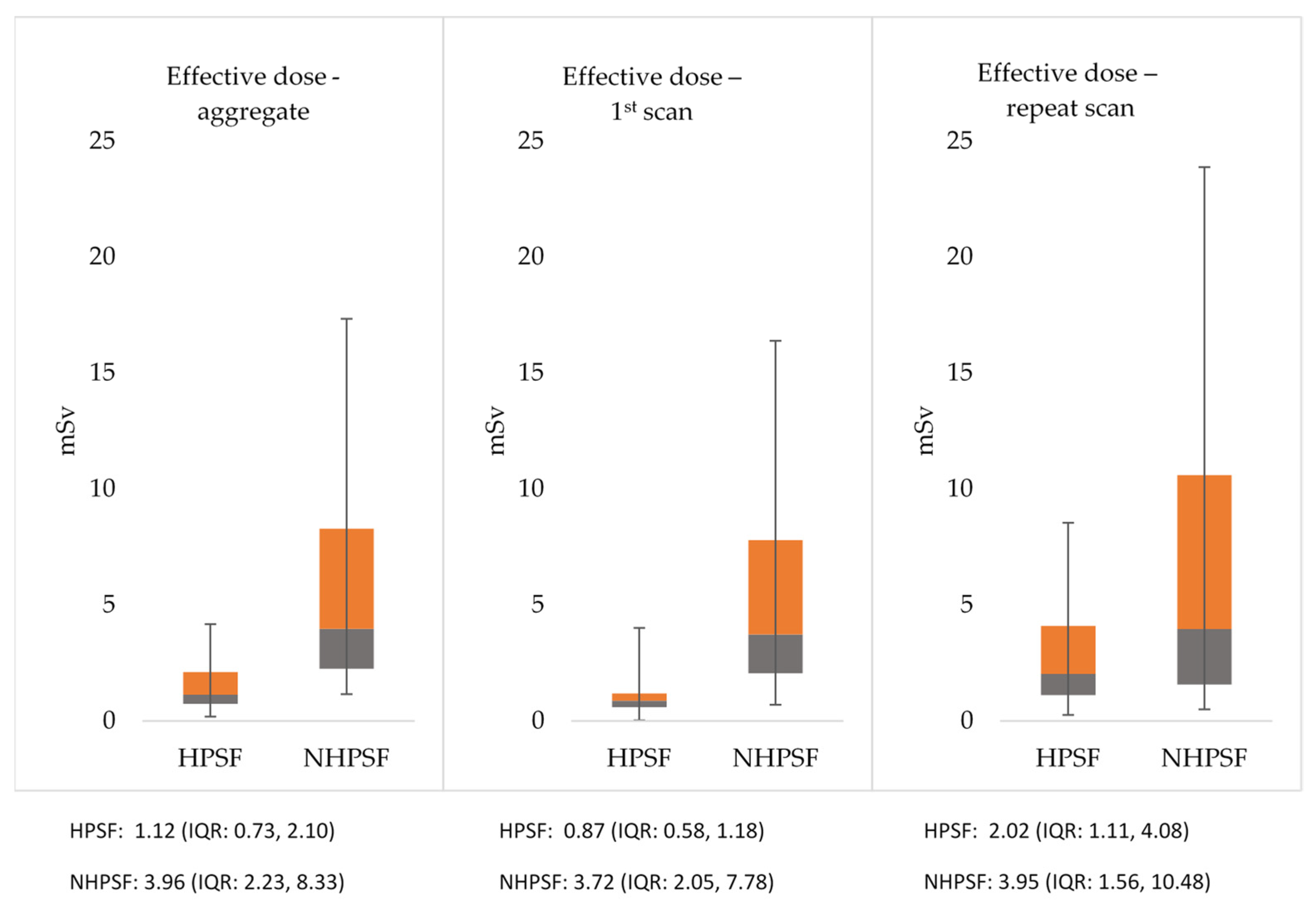
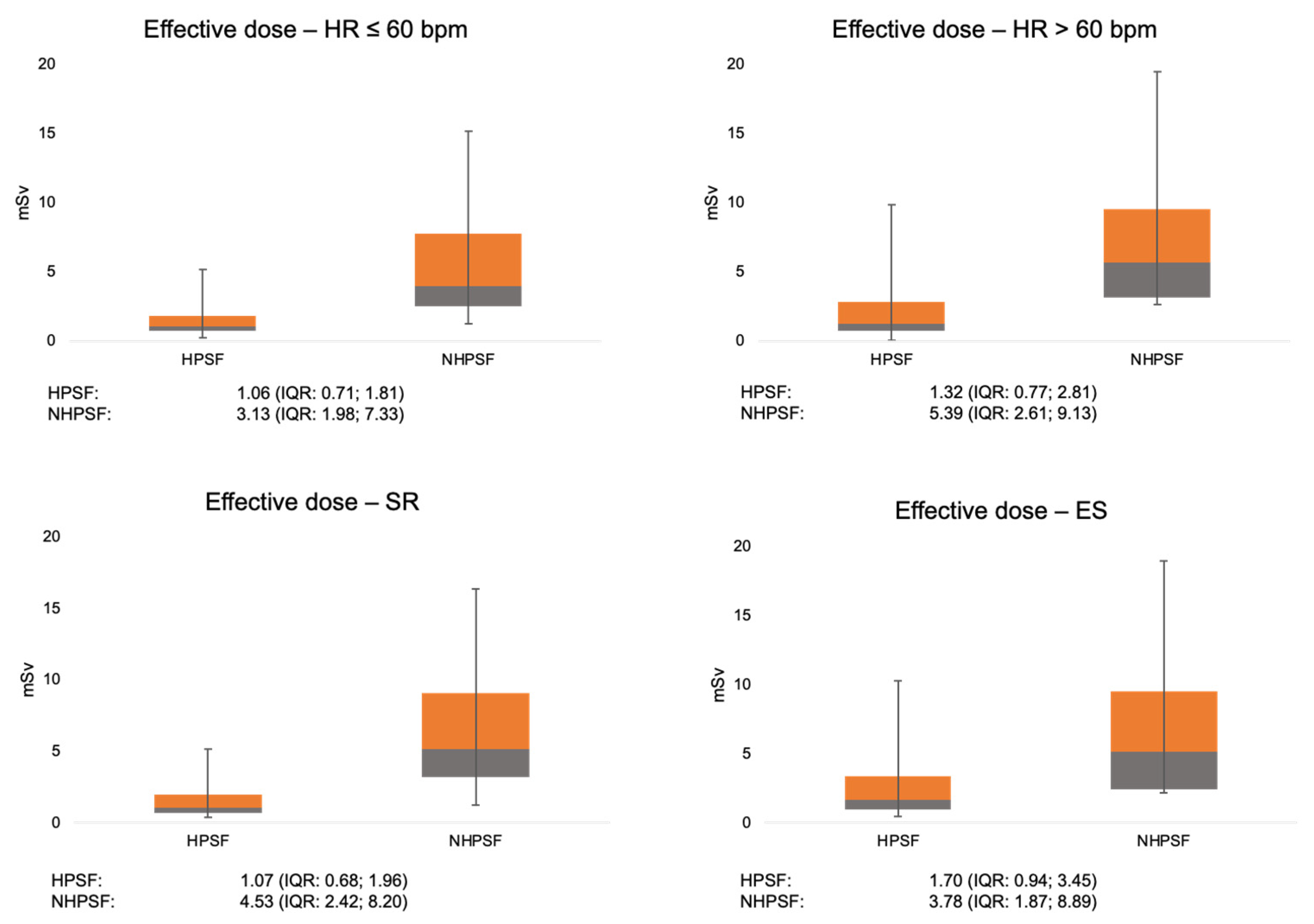
3.4. Radiation Exposure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Scheffel, H.; Alkadhi, H.; Leschka, S.; Plass, A.; Desbiolles, L.; Guber, I.; Krauss, T.; Gruenenfelder, J.; Genoni, M.; Luescher, T.F.; et al. Low-dose CT coronary angiography in the step-and-shoot mode: Diagnostic performance. Heart 2008, 94, 1132–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koplay, M.; Erdogan, H.; Avci, A.; Sivri, M.; Demir, K.; Guler, I.; Demir, L.S.; Paksoy, Y. Radiation dose and diagnostic accuracy of high-pitch dual-source coronary angiography in the evaluation of coronary artery stenoses. Diagn. Interv. Imaging 2016, 97, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Deseive, S.; Pugliese, F.; Meave, A.; Alexanderson, E.; Martinoff, S.; Hadamitzky, M.; Massberg, S.; Hausleiter, J. Image quality and radiation dose of a prospectively electrocardiography-triggered high-pitch data acquisition strategy for coronary CT angiography: The multicenter, randomized PROTECTION IV study. J. Cardiovasc. Comput. Tomogr. 2015, 9, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Leber, A.W.; Knez, A.; Becker, A.; Becker, C.; von Ziegler, F.; Nikolaou, K.; Rist, C.; Reiser, M.; White, C.; Steinbeck, G.; et al. Accuracy of multidetector spiral computed tomography in identifying and differentiating the composition of coronary atherosclerotic plaques: A comparative study with intracoronary ultrasound. J. Am. Coll. Cardiol. 2004, 43, 1241–1247. [Google Scholar] [CrossRef] [Green Version]
- Austen, W.G.; Edwards, J.E.; Frye, R.L.; Gensini, G.G.; Gott, V.L.; Griffith, L.S.; McGoon, D.C.; Murphy, M.L.; Roe, B.B. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975, 51, 5–40. [Google Scholar] [CrossRef] [Green Version]
- Jessen, K.A.; Shrimpton, P.C.; Geleijns, J.; Panzer, W.; Tosi, G. Dosimetry for optimisation of patient protection in computed tomography. Appl. Radiat. Isot. 1999, 50, 165–172. [Google Scholar] [CrossRef]
- Team RDC. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://wwwR-projectorg (accessed on 10 November 2019).
- Feng, R.; Mao, J.; Liu, X.; Zhao, Y.; Tong, J.; Zhang, L. High-Pitch Coronary Computed Tomographic Angiography Using the Third-Generation Dual-Source Computed Tomography: Initial Experience in Patients With High Heart Rate. J. Comput. Assist. Tomogr. 2018, 42, 248–255. [Google Scholar] [CrossRef]
- Bischoff, B.; Meinel, F.G.; Del Prete, A.; Reiser, M.F.; Becker, H.C. High-pitch coronary CT angiography in dual-source CT during free breathing vs. breath holding in patients with low heart rates. Eur. J. Radiol. 2013, 82, 2217–2221. [Google Scholar] [CrossRef]
- Zhang, L.J.; Qi, L.; De Cecco, C.N.; Zhou, C.S.; Spearman, J.V.; Schoepf, U.J.; Lu, G.M. High-pitch coronary CT angiography at 70 kVp with low contrast medium volume: Comparison of 80 and 100 kVp high-pitch protocols. Medicine (Baltimore) 2014, 93, e92. [Google Scholar] [CrossRef]
- Matsubara, K.; Sakuda, K.; Nunome, H.; Takata, T.; Koshida, K.; Gabata, T. 128-slice dual-source CT coronary angiography with prospectively electrocardiography-triggered high-pitch spiral mode: Radiation dose, image quality, and diagnostic acceptability. Acta Radiol. 2016, 57, 25–32. [Google Scholar] [CrossRef]
- Kropil, P.; Rojas, C.A.; Ghoshhajra, B.; Lanzman, R.S.; Miese, F.R.; Scherer, A.; Kalra, M.; Abbara, S. Prospectively ECG-triggered high-pitch spiral acquisition for cardiac CT angiography in routine clinical practice: Initial results. J. Thorac. Imaging 2012, 27, 194–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srichai, M.B.; Lim, R.P.; Donnino, R.; Mannelli, L.; Hiralal, R.; Avery, R.; Ho, C.; Babb, J.S.; Jacobs, J.E. Low-dose, prospective triggered high-pitch spiral coronary computed tomography angiography: Comparison with retrospective spiral technique. Acad. Radiol. 2012, 19, 554–561. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, J.; He, B.; Zhou, Y.; Yang, J.J.; Hou, X.L.; Yang, X.B.; Chen, J.H.; Chen, Y.D. Double prospectively ECG-triggered high-pitch spiral acquisition for CT coronary angiography: Initial experience. Clin. Radiol. 2013, 68, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Chaosuwannakit, N.; Makarawate, P. Reduction of radiation dose for coronary computed tomography angiography using prospective electrocardiography-triggered high-pitch acquisition in clinical routine. Pol. J. Radiol. 2018, 83, e260–e267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordic, S.; Husarik, D.B.; Desbiolles, L.; Leschka, S.; Frauenfelder, T.; Alkadhi, H. High-pitch coronary CT angiography with third generation dual-source CT: Limits of heart rate. Int. J. Cardiovasc. Imaging 2014, 30, 1173–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, W.H.; Lu, B.; Hou, Z.H.; Li, N.; Han, L.; Wu, Y.J.; Niu, H.X.; Silverman, J.R.; Nicola De Cecco, C.; Schoepf, U.J. Detection of coronary artery stenosis with sub-milliSievert radiation dose by prospectively ECG-triggered high-pitch spiral CT angiography and iterative reconstruction. Eur. Radiol. 2013, 23, 2927–2933. [Google Scholar] [CrossRef] [PubMed]
- Hell, M.M.; Bittner, D.; Schuhbaeck, A.; Muschiol, G.; Brand, M.; Lell, M.; Uder, M.; Achenbach, S.; Marwan, M. Prospectively ECG-triggered high-pitch coronary angiography with third-generation dual-source CT at 70 kVp tube voltage: Feasibility, image quality, radiation dose, and effect of iterative reconstruction. J. Cardiovasc. Comput. Tomogr. 2014, 8, 418–425. [Google Scholar] [CrossRef]
- Stolzmann, P.; Goetti, R.P.; Maurovich-Horvat, P.; Hoffmann, U.; Flohr, T.G.; Leschka, S.; Alkadhi, H. Predictors of image quality in high-pitch coronary CT angiography. AJR Am. J. Roentgenol. 2011, 197, 851–858. [Google Scholar] [CrossRef]
- Livingstone, R.S.; Dinakaran, P.M.; Cherian, R.S.; Eapen, A. Comparison of radiation doses using weight-based protocol and dose modulation techniques for patients undergoing biphasic abdominal computed tomography examinations. J. Med. Phys. 2009, 34, 217–222. [Google Scholar] [CrossRef]
- Smettei, O.A.; Sayed, S.; Al Habib, A.M.; Alharbi, F.; Abazid, R.M. Ultra-fast, low dose high-pitch (FLASH) versus prospectively-gated coronary computed tomography angiography: Comparison of image quality and patient radiation exposure. J. Saudi Heart Assoc. 2018, 30, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Forte, E.; Monti, S.; Parente, C.A.; Beyer, L.; De Rosa, R.; Infante, T.; Cavaliere, C.; Cademartiri, F.; Salvatore, M.; Stroszczynski, C.; et al. Image Quality and Dose Reduction by Dual Source Computed Tomography Coronary Angiography: Protocol Comparison. Dose Response 2018, 16, 1559325818805838. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.M.; Kim, N.R.; Kim, D.H.; Song, M.G.; Kim, J.H. Assessment of image quality and radiation dose in prospective ECG-triggered coronary CT angiography compared with retrospective ECG-gated coronary CT angiography. Int. J. Cardiovasc. Imaging 2010, 26 (Suppl. S1), 93–101. [Google Scholar] [CrossRef] [PubMed]
- Plank, F.; Friedrich, G.; Dichtl, W.; Klauser, A.; Jaschke, W.; Franz, W.M.; Feuchtner, G. The diagnostic and prognostic value of coronary CT angiography in asymptomatic high-risk patients: A cohort study. Open Heart 2014, 1, e000096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravipati, G.; Aronow, W.S.; Lai, H.; Shao, J.; DeLuca, A.J.; Weiss, M.B.; Pucillo, A.L.; Kalapatapu, K.; Monsen, C.E.; Belkin, R.N. Comparison of sensitivity, specificity, positive predictive value, and negative predictive value of stress testing versus 64-multislice coronary computed tomography angiography in predicting obstructive coronary artery disease diagnosed by coronary angiography. Am. J. Cardiol. 2008, 101, 774–775. [Google Scholar] [CrossRef] [PubMed]



| HPSF (n = 1410) | NHPSF (n = 236) | p | |
|---|---|---|---|
| HR (bpm) | 58 ± 12 (n = 1273) | 63 ± 13 (n = 203) | <0.001 |
| Male (%) | 64.6 | 71.6 | 0.036 |
| BMI | 25.8 ± 3.4 (n = 1258) | 27.2 ± 4.8 (n = 208) | <0.001 |
| SR | 943 (88.6%) (n = 1064) | 120 (68.6%) (n = 175) | <0.001 |
| ES | 121 (11.4%) (n = 1064) | 55 (31.4%) (n = 175) | <0.001 |
| Calcium score (Agatston units) | 73 ± 159 | 326 ± 331 | <0.001 |
| Contrast agent aggregate (cc) | 78 ± 34 | 77 ± 27 | 0.67 |
| Metoprolol dose (mg) | 5 ± 6 | 6 ± 6 | 0.018 |
| Calcified plaques | 3 ± 5 | 9 ± 8 | <0.001 |
| Scan length (mm) | 126.4 ± 15 | 122.4 ± 15 | <0.001 |
| Tube voltage (kVp) | 91.6 ± 13 | 101.6 ± 16 | <0.001 |
| Primary Successful | HPSF | p | NHPSF | p |
|---|---|---|---|---|
| Successful 1st Scans | Successful 1st Scans | |||
| HR ≤ 60 bpm (n = 1003) | 612/900 = 68.0% | 0.003 | 93/103 = 90.3% | 0.34 |
| HR > 60 bpm (n = 437) | 221/373 = 59.2% | 86/100 = 86.0% | ||
| SR (n = 1063) | 629/943 = 66.7% | <0.001 | 112/120 = 93.3% | 0.004 |
| ES (n = 176) | 51/121 = 42.1% | 43/55 = 78.2% | ||
| Male (n = 1079) | 585/910 = 64.3% | 0.47 | 151/169 = 89.3% | 0.79 |
| Female (n = 567) | 331/500 = 66.2% | 59/67 = 88.1% | ||
| BMI < 25 (n = 645) | 387/573 = 67.5% | 0.017 | 62/72 = 86.1% | 0.44 |
| BMI ≥ 25 (n = 831) | 424/695 = 61.0% | 122/136 = 89.7% |
| HPSF (n = 1976) | NHPSF (n = 100) | p | LM | LAD | LCX | RCA | Total | |
|---|---|---|---|---|---|---|---|---|
| Motion | 441 (22.3%) | 39 (39.0%) | <0.001 | 34 | 108 | 104 | 234 | 480 |
| Calcification | 216 (10.9%) | 5 (5.0%) | 0.06 | 10 | 159 | 36 | 16 | 221 |
| Insufficient contrast | 16 (0.8%) | 0 | 0.37 | 0 | 6 | 6 | 5 | 17 |
| Image noise | 19 (1.0%) | 1 (1.0%) | >0.99 | 2 | 5 | 6 | 6 | 19 |
| Intramyocardial | 10 (0.5%) | 0 | 0.48 | 0 | 10 | 0 | 0 | 10 |
| Total | 702 (35.5%) | 45 (45.0%) | 0.054 | 46 | 288 | 152 | 261 | 747 |
| Radiation Exposure | HPSF | p | NHPSF | p |
|---|---|---|---|---|
| mSv | mSv | |||
| HR ≤ 60 bpm (n = 1003) | 1.06 (0.71; 1.81) | 0.001 | 3.13 (1.98; 7.33) | 0.015 |
| HR > 60 bpm (n = 437) | 1.32 (0.77; 2.81) | 5.39 (2.61; 9.13) | ||
| SR (n = 1063) | 1.07 (0.68; 1.96) | <0.001 | 4.53 (2.42; 8.20) | 0.55 |
| ES (n = 176) | 1.70 (0.94; 3.45) | 3.78 (1.87; 8.89) | ||
| Male (n = 1079) | 1.22 (0.83; 2.28) | <0.001 | 4.36 (2.33; 8.49) | 0.21 |
| Female (n = 567) | 0.95 (0.57; 1.81) | 3.37 (1.90; 8.00) | ||
| BMI < 25 (n = 645) | 0.78 (0.54; 1.42) | <0.001 | 2.37 (1.39; 4.34) | <0.001 |
| BMI ≥ 25 (n = 831) | 1.46 (0.96; 3.09) | 5.59 (2.84; 10.02) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finck, T.; Klambauer, K.; Hendrich, E.; Will, A.; Martinoff, S.; Hadamitzky, M. Radiation Dose and Image Quality of a High-Pitch Prospective Spiral First Approach in Coronary Computed Tomography Angiography (CCTA). J. Cardiovasc. Dev. Dis. 2021, 8, 119. https://doi.org/10.3390/jcdd8100119
Finck T, Klambauer K, Hendrich E, Will A, Martinoff S, Hadamitzky M. Radiation Dose and Image Quality of a High-Pitch Prospective Spiral First Approach in Coronary Computed Tomography Angiography (CCTA). Journal of Cardiovascular Development and Disease. 2021; 8(10):119. https://doi.org/10.3390/jcdd8100119
Chicago/Turabian StyleFinck, Tom, Konstantin Klambauer, Eva Hendrich, Albrecht Will, Stefan Martinoff, and Martin Hadamitzky. 2021. "Radiation Dose and Image Quality of a High-Pitch Prospective Spiral First Approach in Coronary Computed Tomography Angiography (CCTA)" Journal of Cardiovascular Development and Disease 8, no. 10: 119. https://doi.org/10.3390/jcdd8100119
APA StyleFinck, T., Klambauer, K., Hendrich, E., Will, A., Martinoff, S., & Hadamitzky, M. (2021). Radiation Dose and Image Quality of a High-Pitch Prospective Spiral First Approach in Coronary Computed Tomography Angiography (CCTA). Journal of Cardiovascular Development and Disease, 8(10), 119. https://doi.org/10.3390/jcdd8100119






