Combined Use of Electrocardiography and Ultrasound to Detect Cardiac and Pulmonary Involvement after Recovery from COVID-19 Pneumonia: A Case Series
Abstract
:1. Introduction
2. Materials and Methods
3. Results
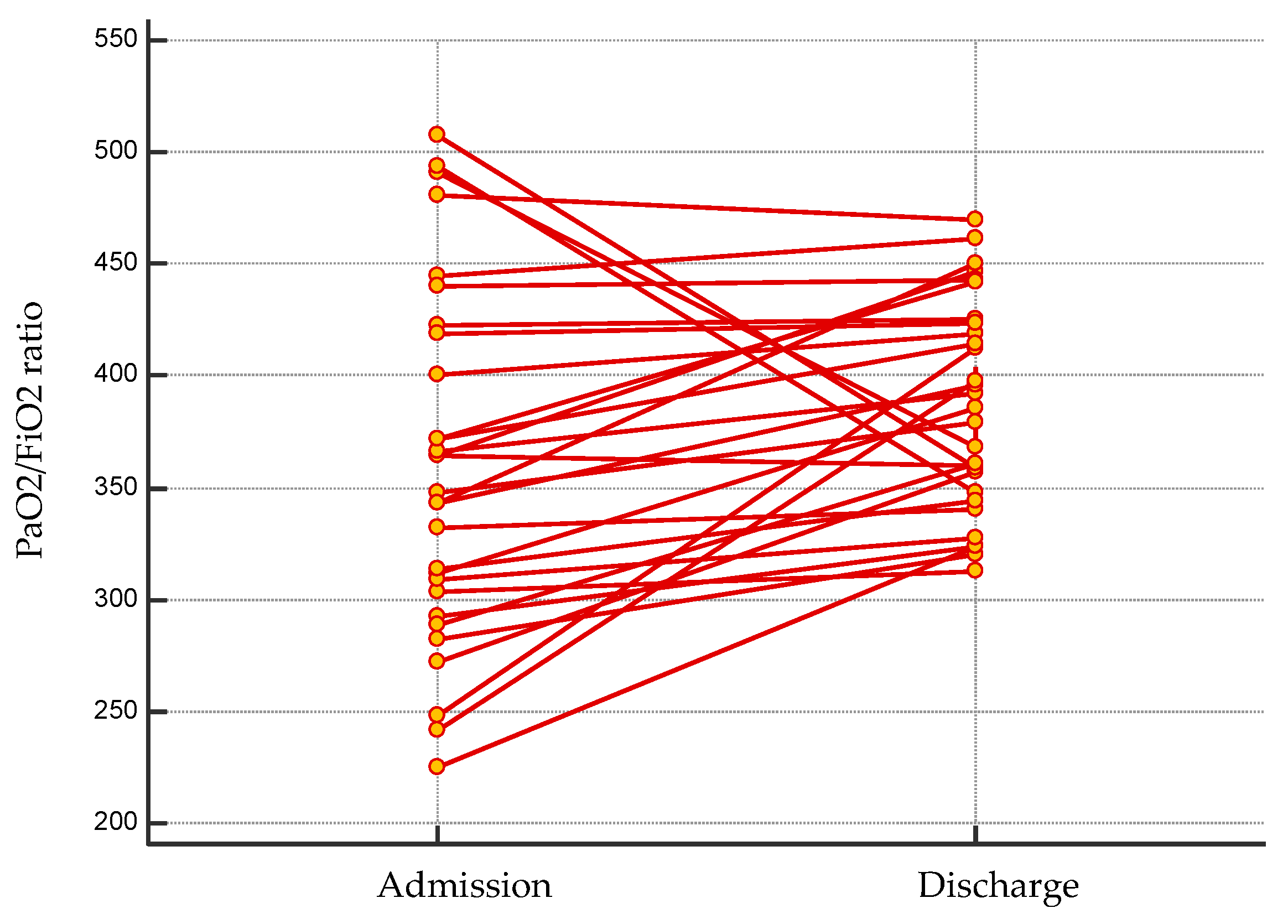
3.1. Patient Population
3.2. Electrocardiographic Findings
3.3. Transthoracic Echocardiogram
3.4. Compression Ultrasonography, Lung Ultrasound and Thoracic CT Scan Findings
4. Discussion
5. Conclusions
Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, L.Q.; Huang, T.; Wang, Y.Q.; Wang, Z.P.; Liang, Y.; Huang, T.B.; Zhang, H.Y.; Sun, W.; Wang, Y. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J. Med. Virol. 2020, 92, 577–583. [Google Scholar] [CrossRef]
- Angeli, F.; Reboldi, G.; Verdecchia, P. Ageing, ACE2 deficiency and bad outcome in COVID-19. Clin. Chem. Lab. Med. 2021, 59, 1607–1609. [Google Scholar] [CrossRef]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020, 76, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. COVID-19: ACE2centric Infective Disease? Hypertension 2020, 76, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Angeli, F.; Zappa, M.; Reboldi, G.; Trapasso, M.; Cavallini, C.; Spanevello, A.; Verdecchia, P. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection: One year later. Eur. J. Intern. Med. 2021, S0953-6205(21)00307-1. [Google Scholar] [CrossRef]
- Mandal, S.; Barnett, J.; Brill, S.E.; Brown, J.S.; Denneny, E.K.; Hare, S.S.; Heightman, M.; Hillman, T.E.; Jacob, J.; Jarvis, H.C.; et al. ‘Long-COVID’: A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021, 76, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.J.; Heyman, B.; Ko, J.P.; Condos, R.; Lynch, D.A. CT of Post-Acute Lung Complications of COVID-19. Radiology 2021, 211396. [Google Scholar] [CrossRef]
- Angeli, F.; Bachetti, T. Temporal changes in co-morbidities and mortality in patients hospitalized for COVID-19 in Italy. Eur. J. Intern. Med. 2020, 82, 123–125. [Google Scholar] [CrossRef]
- Angeli, F.; Marazzato, J.; Verdecchia, P.; Balestrino, A.; Bruschi, C.; Ceriana, P.; Chiovato, L.; Dalla Vecchia, L.A.; De Ponti, R.; Fanfulla, F.; et al. Joint effect of heart failure and coronary artery disease on the risk of death during hospitalization for COVID-19. Eur. J. Intern. Med. 2021, 89, 81–86. [Google Scholar] [CrossRef]
- Angeli, F.; Spanevello, A.; De Ponti, R.; Visca, D.; Marazzato, J.; Palmiotto, G.; Feci, D.; Reboldi, G.; Fabbri, L.M.; Verdecchia, P. Electrocardiographic features of patients with COVID-19 pneumonia. Eur. J. Intern. Med. 2020, 78, 101–106. [Google Scholar] [CrossRef]
- Noor, F.M.; Islam, M.M. Prevalence and associated risk factors of mortality among COVID-19 patients: A meta-analysis. J. Community Health 2020, 45, 1270–1282. [Google Scholar] [CrossRef]
- Prineas, R.J.; Crow, R.S.; Zhang, Z.-M. The Minnesota Code Manual of Electrocardiographic Findings; Springer: London, UK, 2010. [Google Scholar] [CrossRef]
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases. Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar] [CrossRef]
- A Borger, M.; Zamorano, J.L.; Gencer, B.; Bax, J.J.; Cuisset, T.; Bugiardini, R.; Rosemann, T.; Richter, D.; Roffi, M.; Steg, P.G.; et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2015, 37, 267–315. [Google Scholar]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardio-Thorac. Surg. 2017, 38, 2739–2786. [Google Scholar] [CrossRef] [PubMed]
- Grünig, E.; Henn, P.; D’Andrea, A.; Claussen, M.; Ehlken, N.; Maier, F.; Naeije, R.; Nagel, C.; Prange, F.; Weidenhammer, J.; et al. Reference Values for and Determinants of Right Atrial Area in Healthy Adults by 2-Dimensional Echocardiography. Circ. Cardiovasc. Imaging 2013, 6, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bossone, E.; D’Andrea, A.; D’Alto, M.; Citro, R.; Argiento, P.; Ferrara, F.; Cittadini, A.; Rubenfire, M.; Naeije, R. Echocardiography in Pulmonary Arterial Hypertension: From Diagnosis to Prognosis. J. Am. Soc. Echocardiogr. 2013, 26, 1–14. [Google Scholar] [CrossRef]
- Lee, F.C.Y. The Curtain Sign in Lung Ultrasound. J. Med. Ultrasound 2017, 25, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Picano, E.; Scali, M.C.; Ciampi, Q.; Lichtenstein, D. Lung Ultrasound for the Cardiologist. JACC Cardiovasc. Imaging 2018, 11, 1692–1705. [Google Scholar] [CrossRef]
- Boussuges, A.; Gole, Y.; Blanc, P. Diaphragmatic Motion Studied by M-Mode Ultrasonography. Chest 2009, 135, 391–400. [Google Scholar] [CrossRef]
- Spiesshoefer, J.; Herkenrath, S.; Henke, C.; Langenbruch, L.; Schneppe, M.; Randerath, W.; Young, P.; Brix, T.; Boentert, M. Evaluation of Respiratory Muscle Strength and Diaphragm Ultrasound: Normative Values, Theoretical Considerations, and Practical Recommendations. Respiration 2020, 99, 369–381. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F.; for the Gemelli against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients after Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Hayward, S.A.; Innes, S.M.; Miller, A.S.C. Point-of-care lung ultrasound in patients with COVID-19—A narrative review. Anaesthesia 2020, 75, 1096–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lala, A.; Johnson, K.W.; Januzzi, J.L.; Russak, A.J.; Paranjpe, I.; Richter, F.; Zhao, S.; Somani, S.; Van Vleck, T.; Vaid, A.; et al. Prevalence and Impact of Myocardial Injury in Pa-tients Hospitalized With COVID-19 Infection. J. Am. Coll. Cardiol. 2020, 76, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Angeli, F.; Reboldi, G.; Spanevello, A.; De Ponti, R.; Visca, D.; Marazzato, J.; Zappa, M.; Trapasso, M.; Masnaghetti, S.; Fabbri, L.M.; et al. Electrocardiographic Features of Patients with COVID-19: One Year of Unexpected Manifestations. Eur. J. Int. Med. 2021. [Google Scholar] [CrossRef]
- Angeli, F.; Masnaghetti, S.; Visca, D.; Rossoni, A.; Taddeo, S.; Biagini, F.; Verdecchia, P. Severity of COVID-19: The importance of being hypertensive. Monaldi Arch. Chest Dis. 2020, 90. [Google Scholar] [CrossRef] [PubMed]
- Angeli, F.; Reboldi, G.; Verdecchia, P. SARS-CoV-2 infection and ACE2 inhibition. J. Hypertens. 2021, 39, 1555–1558. [Google Scholar] [CrossRef]
- Verdecchia, P.; Reboldi, G.; Cavallini, C.; Mazzotta, G.; Angeli, F. [ACE-inhibitors, angiotensin receptor blockers and severe acute respiratory syndrome caused by coronavirus]. G. Ital. Cardiol. (Rome) 2020, 21, 321–327. [Google Scholar]
- Verdecchia, P.; Angeli, F.; Reboldi, G. Angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers and coronavirus. J. Hypertens. 2020, 38, 1190–1191. [Google Scholar] [CrossRef]
- Sauer, F.; Dagrenat, C.; Couppie, P.; Jochum, G.; Leddet, P.; D’Amario, D.; Asher, E.; Rudzínski, P.N.; Camm, C.F.; Thomson, R. Pericardial effusion in patients with COVID-19: Case series. Eur. Heart J.-Case Rep. 2020, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, R.; Sakamoto, A.; Kawai, K.; Gianatti, A.; Pellegrini, D.; Nasr, A.; Kutys, B.; Guo, L.; Cornelissen, A.; Mori, M.; et al. Pathological Evidence for SARS-CoV-2 as a Cause of Myocarditis: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Beun, R.; Kusadasi, N.; Sikma, M.; Westerink, J.; Huisman, A. Thromboembolic events and apparent hep-arin resistance in patients infected with SARS-CoV-2. Int. J. Lab. Hematol. 2020, 42, 19–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Günay, N.; Demiröz, Ö.; Kahyaoğlu, M.; Başlılar, Ş.; Aydın, M.; Özer, M.Ç.; Ileri, Ç.; Keskin, M.; Bayam, E.; Uyan, C. The effect of moderate and severe COVID-19 pneumonia on short-term right ventricular functions: A prospective observational single pandemic center analysis. Int. J. Cardiovasc. Imaging 2021, 37, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Helmy, M.A.; Milad, L.M.; Osman, S.H.; Ali, M.A.; Hasanin, A. Diaphragmatic excursion: A possible key player for predicting successful weaning in patients with severe COVID-19. Anaesth. Crit. Care Pain Med. 2021, 40, 100875. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, M.; Higashimoto, Y.; Sugiya, R.; Mizusawa, H.; Takeda, Y.; Fujita, S.; Nishiyama, O.; Kudo, S.; Kimura, T.; Chiba, Y.; et al. Diaphragmatic excursion correlates with exercise capacity and dynamic hyperinflation in COPD patients. ERJ Open Res. 2020, 6, 00589-02020. [Google Scholar] [CrossRef]
- Frija-Masson, J.; Debray, M.-P.; Boussouar, S.; Khalil, A.; Bancal, C.; Motiejunaite, J.; Galarza-Jimenez, M.A.; Benzaquen, H.; Penaud, D.; Laveneziana, P.; et al. Residual ground glass opacities three months after Covid-19 pneumonia correlate to alteration of respiratory function: The post Covid M3 study. Respir. Med. 2021, 184, 106435. [Google Scholar] [CrossRef]
- Lewis, K.L.; Helgeson, S.A.; Tatari, M.M.; Mallea, J.M.; Baig, H.Z.; Patel, N.M. COVID-19 and the effects on pulmonary function following infection: A retrospective analysis. EClinicalMedicine 2021, 39, 101079. [Google Scholar] [CrossRef]
- Bertini, M.; Ferrari, R.; Guardigli, G.; Malagu, M.; Vitali, F.; Zucchetti, O.; D’Aniello, E.; Volta, C.A.; Cimaglia, P.; Piovaccari, G.; et al. Electrocardiographic features of 431 consecutive, critically ill COVID-19 patients: An insight into the mechanisms of cardiac involvement. Europace 2020, 22, 1848–1854. [Google Scholar] [CrossRef]

| Comorbidities | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pt. | Sex | Age (Years) | Obesity a | COPD | CAD | CVD | HF | AF | DVT | Acute Pericarditis |
| 1 | M | 57 | - | - | - | - | - | - | - | - |
| 2 | M | 74 | - | - | - | - | - | - | - | - |
| 3 | M | 83 | Yes | Yes | - | - | - | - | - | - |
| 4 | F | 78 | Yes | - | - | - | - | - | - | - |
| 5 | M | 71 | - | - | - | - | - | - | - | Yes |
| 6 | F | 85 | - | - | - | - | - | Yes | - | - |
| 7 | M | 58 | Yes | - | - | - | - | - | - | - |
| 8 | M | 82 | - | - | - | Yes | - | Yes | - | - |
| 9 | F | 74 | - | - | - | - | - | - | Yes | - |
| 10 | F | 79 | Yes | - | Yes | Yes | Yes | - | - | - |
| 11 | M | 73 | - | - | - | - | - | - | - | - |
| 12 | M | 62 | - | Yes | - | - | - | - | - | - |
| 13 | M | 59 | - | - | - | - | - | - | - | - |
| 14 | F | 84 | - | - | - | - | - | - | - | - |
| 15 | M | 58 | Yes | - | - | - | - | - | - | - |
| 16 | M | 59 | - | - | Yes | - | - | - | - | - |
| 17 | F | 78 | - | - | - | - | - | - | - | - |
| 18 | M | 71 | - | - | Yes | - | - | - | - | - |
| 19 | M | 54 | - | - | - | - | - | - | - | - |
| 20 | M | 65 | - | - | - | - | - | - | - | - |
| 21 | M | 75 | - | - | - | - | - | Yes | - | - |
| 22 | M | 75 | Yes | Yes | - | - | - | Yes | - | - |
| 23 | M | 68 | - | - | - | - | - | - | - | - |
| 24 | F | 79 | - | - | - | - | - | - | - | - |
| 25 | M | 52 | Yes | - | - | - | - | - | - | - |
| 26 | F | 50 | Yes | - | - | - | - | - | - | - |
| 27 | F | 81 | - | - | - | - | - | Yes | - | - |
| 28 | M | 70 | Yes | - | Yes | - | - | - | - | - |
| 29 | M | 77 | - | - | Yes | - | - | - | - | - |
| COVID-19-Related Clinical Presentation and Management | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pt. | Sex | Age (Years) | Pneumonia | Pulmonary Embolism | NIV/ETI | ARV | HCQ | Steroids | Tocilizumab |
| 1 | M | 57 | Yes | - | - | Yes | Yes | Yes | - |
| 2 | M | 74 | Yes | - | - | - | Yes | - | - |
| 3 | M | 83 | Yes | - | Yes | Yes | Yes | Yes | - |
| 4 | F | 78 | Yes | - | - | - | Yes | - | - |
| 5 | M | 71 | Yes | - | Yes | Yes | Yes | Yes | Yes |
| 6 | F | 85 | Yes | - | - | - | - | - | - |
| 7 | M | 58 | Yes | - | Yes | Yes | Yes | Yes | - |
| 8 | M | 82 | Yes | - | - | Yes | Yes | - | - |
| 9 | F | 74 | Yes | - | - | Yes | Yes | - | - |
| 10 | F | 79 | Yes | Yes | - | Yes | Yes | - | - |
| 11 | M | 73 | Yes | - | - | Yes | Yes | - | - |
| 12 | M | 62 | Yes | - | Yes | - | Yes | - | - |
| 13 | M | 59 | Yes | - | Yes | Yes | Yes | - | - |
| 14 | F | 84 | Yes | - | - | Yes | Yes | - | - |
| 15 | M | 58 | Yes | - | Yes | Yes | Yes | - | - |
| 16 | M | 59 | Yes | - | Yes | Yes | Yes | - | - |
| 17 | F | 78 | Yes | - | - | - | Yes | - | - |
| 18 | M | 71 | Yes | - | Yes | - | Yes | Yes | - |
| 19 | M | 54 | Yes | - | - | - | - | Yes | Yes |
| 20 | M | 65 | Yes | - | Yes | - | - | - | - |
| 21 | M | 75 | Yes | - | - | - | - | - | - |
| 22 | M | 75 | Yes | - | Yes | Yes | Yes | Yes | - |
| 23 | M | 68 | Yes | - | - | Yes | Yes | - | - |
| 24 | F | 79 | Yes | - | - | - | Yes | Yes | - |
| 25 | M | 52 | Yes | - | Yes | Yes | Yes | Yes | Yes |
| 26 | F | 50 | Yes | - | Yes | Yes | Yes | - | - |
| 27 | F | 81 | Yes | - | Yes | - | Yes | - | - |
| 28 | M | 70 | Yes | - | - | Yes | Yes | Yes | - |
| 29 | M | 77 | Yes | - | Yes | Yes | - | Yes | - |
| Abnormal ECG Features | Transthoracic Echocardiogram | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pt. | Recognised at Admission | Changes from the Acute Phase | RAA (cm2) | RVEDD (mm) | TAPSE (mm) | TR (grade) | IVC (mm) | SPAP (mmHg) | Pericardial Effusion | Pericardial Thickening |
| 1 | ST, A-STT | Persistence | 20 | 31 | 22 | - | 19 | - | - | Yes |
| 2 | ST, A-STT | Persistence | 16 | 29 | 22 | Mild | 18 | 38 | Yes [5] | Yes |
| 3 | A-STT, LVs | New | 21 | 32 | 21 | Mild | 17 | 37 | Yes [6] | Yes |
| 4 | ST, A-STT | Persistence | 16 | 28 | 25 | - | 16 | - | - | - |
| 5 | ST, A-STT | New | 10 | 37 | 20 | Mild | 19 | N/A | Yes [3] | Yes |
| 6 | AF, A-STT | Persistence | 20 | 34 | 29 | Severe | 20 | 40 | Yes [4] | Yes |
| 7 | No | No | 15 | 35 | 26 | Mild | 18 | N/A | Yes [1] | - |
| 8 | ST, A-STT | Persistence | 10 | 32 | 24 | Mild | 15 | 33 | Yes [5] | - |
| 9 | AV block I | New | 10 | 25 | 23 | Mild | 15 | N/A | Yes [14] | Yes |
| 10 | No | Persistence | 18 | 30 | 27 | Mild | 15 | N/A | - | - |
| 11 | ST, LVs | Persistence | 11 | 32 | 23 | Mild | - | 37 | Yes [5] | Yes |
| 12 | ST, AV block I | New | 12 | 32 | 19 | Mild | 16 | 33 | - | - |
| 13 | ST, A-STT | Persistence | 11 | 35 | 24 | Mild | 18 | 40 | Yes [4] | Yes |
| 14 | ST, A-STT | Persistence | 10 | 30 | 19 | Mild | 17 | 28 | Yes [1] | Yes |
| 15 | ST, A-STT, LVs | Persistence | 19 | 38 | 28 | Mild | 17 | 37 | Yes [1] | Yes |
| 16 | ST, A-STT, LVs | Persistence | 18 | 30 | 20 | Mild | 19 | 33 | Yes [3] | Yes |
| 17 | ST, A-STT | Persistence | 19 | 35 | 25 | Severe | 16 | 35 | Yes [1] | - |
| 18 | ST, A-STT, LVs | Persistence | 16 | 36 | 26 | Mild | 18 | 38 | Yes [4] | Yes |
| 19 | ST, A-STT, LVs | Persistence | 17 | 26 | 25 | Mild | 17 | N/A | Yes [5] | Yes |
| 20 | ST, A-STT, LVs | Persistence | 15 | 29 | 23 | Mild | 17 | 29 | Yes [8] | - |
| 21 | A-STT | New | 19 | 38 | 27 | Mild | 20 | 32 | - | Yes |
| 22 | AF, A-STT, LVs | Persistence | 18 | 30 | 18 | Mild | 16 | 28 | Yes [8] | Yes |
| 23 | A-STT | Persistence | 12 | 30 | 22 | - | 16 | - | - | Yes |
| 24 | A-STT, LVs | Persistence | 16 | 30 | 15 | Mild | 15 | 40 | - | - |
| 25 | A-STT | Persistence | 14 | 32 | 27 | Mild | - | 35 | Yes [3] | Yes |
| 26 | ST, A-STT, LVs | New | 11 | 30 | 23 | Mild | 17 | N/A | Yes [1] | Yes |
| 27 | AV block I | New | 17 | 30 | 23 | Severe | 17 | 38 | - | - |
| 28 | ST, A-STT | Persistence | 18 | 29 | 25 | Mild | 16 | N/A | Yes [6] | - |
| 29 | A-STT | Persistence | 14 | 32 | 25 | Mild | 13 | 32 | - | Yes |
| Lung Ultrasound | Fibrosis Bronchiectasis Emphysema (CT) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pt. | Sliding | B-Lines | Pleural Effusion | Pleural Thickening | Curtain Sign | Right DT Ratio | Left DT Ratio | Right DE (mm) | Left DE (mm) | |
| 1 | Yes | - | - | Yes | Yes | 1.5 | 1.7 | 40 | 35 | - |
| 2 | Yes | - | - | Yes | Yes | 1.7 | 2.3 | 37 | 39 | Yes |
| 3 | Yes | - | - | Yes | Yes | 2.0 | 2.0 | 39 | 38 | Yes |
| 4 | Yes | - | - | - | Yes | 2.0 | 1.5 | 46 | 40 | - |
| 5 | Yes | - | Yes | Yes | Yes | 1.4 | 1.1 | 25 | 27 | - |
| 6 | Yes | - | Yes | Yes | - | 2.3 | 2.1 | 55 | 47 | Yes |
| 7 | Yes | - | Yes | Yes | Yes | 1.8 | 2.6 | N/A | N/A | Yes |
| 8 | Yes | - | - | - | Yes | 1.9 | 1.9 | 52 | 40 | Yes |
| 9 | Yes | - | - | - | Yes | 1.6 | 1.5 | 35 | 43 | Yes |
| 10 | Yes | - | - | - | Yes | 1.7 | 1.7 | 49 | 32 | Yes |
| 11 | Yes | - | Yes | Yes | Yes | 2.1 | 1.6 | 40 | 37 | - |
| 12 | Yes | - | - | - | Yes | 1.1 | 1.1 | 30 | 34 | Yes |
| 13 | Yes | - | Yes | Yes | Yes | 1.6 | 1.5 | 44 | 43 | Yes |
| 14 | Yes | - | - | - | Yes | 1.5 | 1.5 | 50 | 50 | Yes |
| 15 |  R+L R+L | - | Yes | - | Yes | 1.5 | 1.5 | 36 | 41 | Yes |
| 16 | Yes | - | Yes | Yes | Yes | 2.4 | 2.1 | 49 | 32 | Yes |
| 17 | Yes | - | Yes | Yes | Yes | 1.3 | 1.2 | 32 | 42 | Yes |
| 18 | Yes | - | - | Yes | Yes | 1.8 | 1.6 | 29 | 38 | Yes |
| 19 |  L L | - | - | Yes | Yes | 1.6 | 1.6 | 41 | 36 | Yes |
| 20 | Yes | - | - | - | Yes | 1.5 | 1.5 | 40 | 41 | - |
| 21 | Yes | - | - | Yes | Yes | 1.7 | 1.8 | 40 | 41 | Yes |
| 22 | Yes | - | Yes | Yes | Yes | 1.6 | 2.5 | 51 | 37 | Yes |
| 23 | Yes | - | - | Yes | Yes | 1.6 | 1.9 | 47 | 41 | N/A |
| 24 | Yes | - | - | - | Yes | 1.6 | 1.8 | 38 | 38 | Yes |
| 25 | Yes | - | Yes | Yes | Yes | 1.7 | 1.7 | 47 | 41 | N/A |
| 26 | Yes | - | Yes | Yes | Yes | 1.7 | 1.7 | 40 | 47 | Yes |
| 27 | Yes | - | - | - | Yes | 2.1 | 2.1 | N/A | N/A | Yes |
| 28 | Yes | - | Yes | - | Yes | 1.9 | 2.0 | 40 | 30 | Yes |
| 29 | Yes | - | - | - | Yes | 1.9 | 2.0 | 37 | 38 | Yes |
 , reduction.
, reduction.Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marazzato, J.; De Ponti, R.; Verdecchia, P.; Masnaghetti, S.; Visca, D.; Spanevello, A.; Trapasso, M.; Zappa, M.; Mancinelli, A.; Angeli, F. Combined Use of Electrocardiography and Ultrasound to Detect Cardiac and Pulmonary Involvement after Recovery from COVID-19 Pneumonia: A Case Series. J. Cardiovasc. Dev. Dis. 2021, 8, 133. https://doi.org/10.3390/jcdd8100133
Marazzato J, De Ponti R, Verdecchia P, Masnaghetti S, Visca D, Spanevello A, Trapasso M, Zappa M, Mancinelli A, Angeli F. Combined Use of Electrocardiography and Ultrasound to Detect Cardiac and Pulmonary Involvement after Recovery from COVID-19 Pneumonia: A Case Series. Journal of Cardiovascular Development and Disease. 2021; 8(10):133. https://doi.org/10.3390/jcdd8100133
Chicago/Turabian StyleMarazzato, Jacopo, Roberto De Ponti, Paolo Verdecchia, Sergio Masnaghetti, Dina Visca, Antonio Spanevello, Monica Trapasso, Martina Zappa, Antonella Mancinelli, and Fabio Angeli. 2021. "Combined Use of Electrocardiography and Ultrasound to Detect Cardiac and Pulmonary Involvement after Recovery from COVID-19 Pneumonia: A Case Series" Journal of Cardiovascular Development and Disease 8, no. 10: 133. https://doi.org/10.3390/jcdd8100133
APA StyleMarazzato, J., De Ponti, R., Verdecchia, P., Masnaghetti, S., Visca, D., Spanevello, A., Trapasso, M., Zappa, M., Mancinelli, A., & Angeli, F. (2021). Combined Use of Electrocardiography and Ultrasound to Detect Cardiac and Pulmonary Involvement after Recovery from COVID-19 Pneumonia: A Case Series. Journal of Cardiovascular Development and Disease, 8(10), 133. https://doi.org/10.3390/jcdd8100133










