Low Dose of Direct Oral Anticoagulants after Left Atrial Appendage Occlusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection and Follow-Up
2.2. Study Endpoints and Definitions
2.3. Statistical Analysis
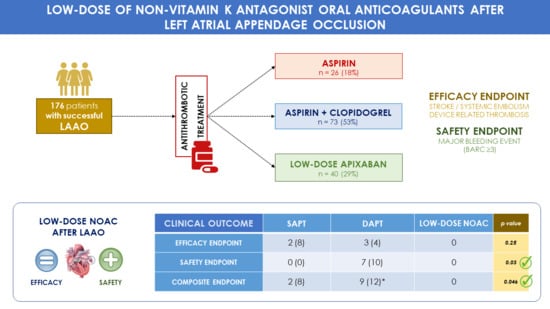
3. Results
4. Discussion
4.1. Low-Dose NOAC Showed a Better Safety Profile Compared to SAPT or DAPT
4.2. Low-Dose NOAC Showed a Similar Efficacy Profile Compared to SAPT or DAPT
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lippi, G.; Sanchis-Gomar, F.; Cervellin, G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int. J. Stroke 2021, 16, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Murphy, N.; Walker, A.; McGuire, A.; McMurray, J.J.V. Cost of an emerging epidemic: An economic analysis of atrial fibrillation in the UK. Heart 2004, 90, 286–292. [Google Scholar] [CrossRef] [Green Version]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, I.M.; Newton, N.; Welner, S.A.; Cowell, W.; Lip, G.Y.H. Underuse of Oral Anticoagulants in Atrial Fibrillation: A Systematic Review. Am. J. Med. 2010, 123, 638–645. [Google Scholar] [CrossRef]
- Tzikas, A.; Shakir, S.; Gafoor, S.; Omran, H.; Berti, S.; Santoro, G.; Kefer, J.; Landmesser, U.; Nielsen-Kudsk, J.E.; Cruz-Gonzalez, I.; et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: Multicentre experience with the AMPLATZER Cardiac Plug. EuroIntervention 2016, 11, 1170–1179. [Google Scholar] [CrossRef]
- Holmes, D.R.; Reddy, V.Y.; Turi, Z.G.; Doshi, S.K.; Sievert, H.; Buchbinder, M.; Mullin, C.M.; Sick, P. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: A randomised non-inferiority trial. Lancet 2009, 374, 534–542. [Google Scholar] [CrossRef]
- Saw, J.; Nielsen-Kudsk, J.E.; Bergmann, M.; Daniels, M.J.; Tzikas, A.; Reisman, M.; Rana, B.S. Antithrombotic Therapy and Device-Related Thrombosis Following Endovascular Left Atrial Appendage Closure. JACC Cardiovasc. Interv. 2019, 12, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.J.; Eikelboom, J.; Joyner, C.; Diener, H.-C.; Hart, R.; Golitsyn, S.; Flaker, G.; Avezum, A.; Hohnloser, S.H.; Diaz, R.; et al. Apixaban in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 364, 806–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzikas, A.; Gafoor, S.; Meerkin, D.; Freixa, X.; Cruz-Gonzalez, I.; Lewalter, T.; Saw, J.; Berti, S.; Nielsen-Kudsk, J.E.; Ibrahim, R.; et al. Left atrial appendage occlusion with the AMPLATZER Amulet device: An expert consensus step-by-step approach. EuroIntervention 2016, 11, 1512–1521. [Google Scholar] [CrossRef] [Green Version]
- Tzikas, A.; Holmes, D.R.; Gafoor, S.; Ruiz, C.E.; Blomstrom-Lundqvist, C.; Diener, H.C.; Cappato, R.; Kar, S.; Lee, R.J.; Byrne, R.A.; et al. Percutaneous left atrial appendage occlusion: The Munich consensus document on definitions, endpoints, and data collection requirements for clinical studies. Europace 2017, 19, 4–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steg, P.G.; Huber, K.; Andreotti, F.; Arnesen, H.; Atar, D.; Badimon, L.; Bassand, J.P.; De Caterina, R.; Eikelboom, J.A.; Gulba, D.; et al. Bleeding in acute coronary syndromes and percutaneous coronary interventions: Position paper by the Working Group on Thrombosis of the European Society of Cardiology. Eur. Heart J. 2011, 32, 1854–1864. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Tondo, C.; Camm, J.; Diener, H.C.; Paul, V.; Schmidt, B.; Settergren, M.; Teiger, E.; Nielsen-Kudsk, J.E.; Hildick-Smith, D. Left atrial appendage occlusion with the AMPLATZER Amulet device: One-year follow-up from the prospective global Amulet observational registry. EuroIntervention 2018, 14, e590–e597. [Google Scholar] [CrossRef] [PubMed]
- Aminian, A.; De Backer, O.; Nielsen-Kudsk, J.E.; Mazzone, P.; Berti, S.; Fischer, S.; Lund, J.; Montorfano, M.; Lam, S.C.C.; Freixa, X.; et al. Incidence and Clinical Impact of Major Bleeding Following Left Atrial Appendage Occlusion: Insights from the AmplatzerTM AmuletTM LAA Occluder Observational Study. EuroIntervention 2021, 17, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Glikson, M.; Wolff, R.; Hindricks, G.; Mandrola, J.; Camm, A.; Lip, G.; Fauchier, L.; Betts, T.; Lewalter, T.; Saw, J.; et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion—An update. EuroIntervention 2020, 15, 1133–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, V.Y.; Sievert, H.; Halperin, J.; Doshi, S.K.; Buchbinder, M.; Neuzil, P.; Huber, K.; Whisenant, B.; Kar, S.; Swarup, V.; et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation a randomized clinical trial. JAMA J. Am. Med. Assoc. 2014, 312, 1988–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, D.R.; Kar, S.; Price, M.J.; Whisenant, B.; Sievert, H.; Doshi, S.K.; Huber, K.; Reddy, V.Y. Prospective randomized evaluation of the watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: The PREVAIL trial. J. Am. Coll. Cardiol. 2014, 64, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osman, M.; Busu, T.; Osman, K.; Khan, S.U.; Daniels, M.; Holmes, D.R.; Alkhouli, M. Short-Term Antiplatelet Versus Anticoagulant Therapy After Left Atrial Appendage Occlusion: A Systematic Review and Meta-Analysis. JACC Clin. Electrophysiol. 2020, 6, 494–506. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Möbius-Winkler, S.; Miller, M.A.; Neuzil, P.; Schuler, G.; Wiebe, J.; Sick, P.; Sievert, H. Left atrial appendage closure with the watchman device in patients with a contraindication for oral anticoagulation: The ASAP study (ASA plavix feasibility study with watchman left atrial appendage closure technology). J. Am. Coll. Cardiol. 2013, 61, 2551–2556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faroux, L.; Cruz-González, I.; Arzamendi, D.; Freixa, X.; Nombela-Franco, L.; Peral, V.; Caneiro-Queija, B.; Mangieri, A.; Trejo-Velasco, B.; Asmarats, L.; et al. Short-term direct oral anticoagulation or dual antiplatelet therapy following left atrial appendage closure in patients with relative contraindications to chronic anticoagulation therapy. Int. J. Cardiol. 2021, 333, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Matusik, P.T.; Heleniak, Z.; Papuga-Szela, E.; Plens, K.; Lelakowski, J.; Undas, A. Chronic Kidney Disease and Its Impact on a Prothrombotic State in Patients with Atrial Fibrillation. J. Clin. Med. 2020, 9, 2476. [Google Scholar] [CrossRef] [PubMed]
- Tapias, J.B.; Flores-Umanzor, E.; Cepas-Guillén, P.L.; Regueiro, A.; Sanchís, L.; Broseta, J.J.; Cases, A.; Freixa, X. Prognostic impact of the presence of chronic kidney disease on percutaneous left trial appendage closure for atrial fibrillation: A single center experience. Nefrologia 2021. [Google Scholar] [CrossRef]
- Alkhouli, M.; Busu, T.; Shah, K.; Osman, M.; Alqahtani, F.; Raybuck, B. Incidence and Clinical Impact of Device-Related Thrombus Following Percutaneous Left Atrial Appendage Occlusion: A Meta-Analysis. JACC Clin. Electrophysiol. 2018, 4, 1629–1637. [Google Scholar] [CrossRef]
- Rodés-Cabau, J.; O’Hara, G.; Paradis, J.M.; Bernier, M.; Rodriguez-Gabella, T.; Regueiro, A.; O’Connor, K.; Beaudoin, J.; Puri, R.; Côté, M.; et al. Changes in Coagulation and Platelet Activation Markers Following Transcatheter Left Atrial Appendage Closure. Am. J. Cardiol. 2017, 120, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Flores-Umanzor, E.; Cepas-Guillen, P.; Sanchis, L.; Regueiro, A.; Navarro, R.; Brugaletta, S.; Vidal, B.; Sitges, M.; Sabaté, M.; Freixa, X. Device related thrombosis after left atrial appendage occlusion: Does thrombus location always predicts its origin? J. Interv. Card. Electrophysiol. 2021, 60, 347–348. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, J.W.; Connolly, S.J.; Brueckmann, M.; Granger, C.B.; Kappetein, A.P.; Mack, M.J.; Blatchford, J.; Devenny, K.; Friedman, J.; Guiver, K.; et al. Dabigatran versus Warfarin in Patients with Mechanical Heart Valves. N. Engl. J. Med. 2013, 369, 1206–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bovin, A.; Vase, H.; Nielsen-Kudsk, J.E.; Grove, E.L. Direct Oral Anticoagulants after Percutaneous Patent Foramen Ovale (PFO) Closure: A Call for Caution. Am. J. Case Rep. 2020, 21, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Flores-Umanzor, E.J.; Cepas-Guillen, P.L.; Arzamendi, D.; Cruz-González, I.; Regueiro, A.; Freixa, X. Rationale and design of a randomized clinical trial to compare two antithrombotic strategies after left atrial appendage occlusion: Double antiplatelet therapy vs. apixaban (ADALA study). J. Interv. Card. Electrophysiol. 2020, 59, 471–477. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Total (n = 139) | Single Antiplatelet (n = 26) | Dual Antiplatelet (n = 73) | Apixaban 2.5 mg/12 h (n = 40) | p Value |
|---|---|---|---|---|---|
| Age (years) | 73.1 ± 9 | 75.6 ± 8 | 71.7 ± 10 | 74.1 ± 7 | 0.09 |
| Male gender | 89 (65) | 18 (69) | 47 (65) | 24 (60) | 0.82 |
| Type of AF | 0.82 | ||||
| 58 (41) | 13 (50) | 29 (40) | 16 (39) | |
| 3 (2) | 0 | 2 (3) | 1 (2) | |
| 78 (57) | 13 (50) | 42 (57) | 23 (59) | |
| Hypertension | 125 (91) | 21 (81) | 68 (93) | 36 (90) | 0.16 |
| Previous stroke | 49 (36) | 11 (42) | 18 (25) | 20 (50) | 0.01 |
| Previous TIA | 9 (7) | 4 (15) | 3 (4) | 2 (5) | 0.18 |
| Previous major bleed event | 106 (77) | 23 (89) | 53 (73) | 30 (72) | 0.27 |
| 49 (46) 44 (42) | 13 (57) 9 (39) | 27 (51) 17 (32) | 9 (30) 18 (18) | |
| 3 (3) | 0 | 2 (4) | 1 (3) | |
| 5 (5) | 1 (4) | 2 (4) | 2 (7) | |
| 2 (2) | 0 | 2 (4) | 0 | |
| 3 (6) | 0 | 3 (4) | 0 | |
| Previous PCI or CABG | 34 (25) | 7 (27) | 20 (27) | 7 (18) | 0.52 |
| CHA2DS2-VASc Score | 4.3 ± 1.5 | 4.4 ± 1.4 | 4.1 ± 1.5 | 4.5 ± 1.4 | 0.42 |
| CHA2DS2-VASc Score ≥ 4 | 97 (70) | 21 (81) | 45 (62) | 31 (78) | 0.06 |
| Baseline Stroke Risk | 5.9 ± 2.8 | 6.1 ± 2.7 | 5.6 ± 2.9 | 6.3 ± 2.8 | 0.42 |
| HAS-BLED Score | 3.6 ± 1.0 | 3.7 ± 0.8 | 3.5 ± 1 | 3.7 ± 0.9 | 0.39 |
| HAS-BLED Score ≥ 3 | 125 (91) | 25 (96) | 64 (88) | 36 (90) | 0.41 |
| Previous AT | <0.001 | ||||
| 32 (23) | 11 (42) | 13 (18) | 8 (21) | |
| 45 (33) | 8 (31) | 32 (44) | 5 (13) | |
| 9 (7) | 1 (4) | 7 (10) | 1 (3) | |
| 24 (17) | 4 (15) | 13 (17) | 7 (18) | |
| 28 (20) | 2 (8) | 8 (11) | 18 (45) | |
| 14 (10) | 1 (4) | 5 (7) | 8 (20) | |
| Absolute CI to OAC | 61 (44) | 13 (50) | 30 (41) | 18 (45) | 0.71 |
| 12 (19) | 3 (18) | 9 (30) | 0 | 0.27 |
| 45 (75) | 10 (82) | 17 (57) | 18 (100) | |
| 1 (2) | 0 | 1 (3) | 0 | |
| 1 (2) | 0 | 1 (3) | 0 | |
| 2 (4) | 0 | 2 (7) | 0 | |
| Indication for LAAO | 0.11 | ||||
| 131 (94) | 26 (100) | 68 (93) | 37 (93) | |
| 4 (3) | 0 | 2 (3) | 2 (5) | |
| 4 (3) | 0 | 3 (4) | 1 (2) |
| Total (n = 139) | Single Antiplatelet (n = 26) | Dual Antiplatelet (n = 73) | Apixaban 2.5 mg/12 h (n = 40) | p Value | |
|---|---|---|---|---|---|
| Fluoroscopic duration (minutes) | 17 ± 9.4 | 16.5 ± 7 | 18.1 ± 10 | 15.1 ± 9 | 0.27 |
| Contrast (mL) | 76 ± 44 | 79.8 ± 47 | 80.2 ± 48 | 65.7 ± 32 | 0.26 |
| Device type | <0.001 | ||||
| - ACP/Amulet | 111 (81) | 17 (65) | 68 (93) | 26 (67) | |
| - Watchman | 3 (2) | 2 (8) | 1 (1) | 0 | |
| - Lambre | 24 (17) | 7 (27) | 4 (6) | 13 (33) | |
| Patients with procedure- or device-related SAEs ≤7 days | 4 (4) | 0 | 4 (6) | 0 | 0.16 |
| - Device embolization | 0 | 0 | 0 | 0 | NA |
| - Ischemic stroke | 0 | 0 | 0 | 0 | NA |
| - Cardiac Tamponade | 0 | 0 | 0 | 0 | NA |
| - Vascular access complication | 2 (2) | 0 | 1 (1) | 0 | 1.00 |
| - Major bleeding (BARC ≥3) | 3 (3) | 0 | 3 (4) | 0 | 0.58 |
| - Death | 0 | 0 | 0 | 0 | NA |
| Clinical Outcome | Total (n = 139) | Single Antiplatelet (n = 26) | Dual Antiplatelet (n = 73) | Apixaban 2.5mg/12 h (n = 40) | p Value |
|---|---|---|---|---|---|
| Efficacy Endpoint (Stroke + SE + DRT) | 5 (4) | 2 (8) | 3 (4) | 0 | 0.25 |
| Safety Endpoint [Major bleeding (BARC ≥ 3)] | 7 (5) | 0 | 7 (10) | 0 | 0.03 |
| Composite Endpoint [Efficacy + Safety Endpoints] | 11 (8) | 2 (8) | 9 (12) * | 0 | 0.046 |
| Secondary Endpoints | |||||
| Ischemic stroke | 1 (1) | 0 | 1 (1) | 0 | 1.00 |
| Systemic Embolization | 0 | 0 | 0 | 0 | NA |
| Device related thrombus | 5 (4) | 2 (8) | 3 (4) | 0 | 0.25 |
| Any Bleeding (major + minor) | 13 (10) | 1 (4) | 11 (16) | 1 (3) | 0.06 |
| Mortality | 5 (4) | 1 (4) | 2 (3) | 2 (6) | 0.84 |
| CV or unknown cause | 3 (3) | 0 | 1 (2) | 2 (6) | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cepas-Guillen, P.L.; Flores-Umanzor, E.; Regueiro, A.; Brugaletta, S.; Ibañez, C.; Sanchis, L.; Sitges, M.; Rodés-Cabau, J.; Sabaté, M.; Freixa, X. Low Dose of Direct Oral Anticoagulants after Left Atrial Appendage Occlusion. J. Cardiovasc. Dev. Dis. 2021, 8, 142. https://doi.org/10.3390/jcdd8110142
Cepas-Guillen PL, Flores-Umanzor E, Regueiro A, Brugaletta S, Ibañez C, Sanchis L, Sitges M, Rodés-Cabau J, Sabaté M, Freixa X. Low Dose of Direct Oral Anticoagulants after Left Atrial Appendage Occlusion. Journal of Cardiovascular Development and Disease. 2021; 8(11):142. https://doi.org/10.3390/jcdd8110142
Chicago/Turabian StyleCepas-Guillen, Pedro Luis, Eduardo Flores-Umanzor, Ander Regueiro, Salvatore Brugaletta, Cristina Ibañez, Laura Sanchis, Marta Sitges, Josep Rodés-Cabau, Manel Sabaté, and Xavier Freixa. 2021. "Low Dose of Direct Oral Anticoagulants after Left Atrial Appendage Occlusion" Journal of Cardiovascular Development and Disease 8, no. 11: 142. https://doi.org/10.3390/jcdd8110142
APA StyleCepas-Guillen, P. L., Flores-Umanzor, E., Regueiro, A., Brugaletta, S., Ibañez, C., Sanchis, L., Sitges, M., Rodés-Cabau, J., Sabaté, M., & Freixa, X. (2021). Low Dose of Direct Oral Anticoagulants after Left Atrial Appendage Occlusion. Journal of Cardiovascular Development and Disease, 8(11), 142. https://doi.org/10.3390/jcdd8110142