Abstract
(1) Background: This study aimed to evaluate the etiologies and clinical outcomes of patients with pericardial effusion (PE) treated with echo-guided percutaneous pericardiocentesis. (2) Methods: Between July 2010 and December 2020, a total of 502 patients underwent echo-guided percutaneous pericardiocentesis for PE at our hospital. The reasons for PE were malignancy (N = 277), and non-malignancy (N = 225). The comorbidities, complications, and all-cause mortality were compared between the malignancy and non-malignancy groups. (3) Results: In multivariable Cox regression analyses for 1-year mortality, malignancy related PE, nasopharyngeal and oropharyngeal cancer, and metastatic status were positive predictors. A higher incidence of in-hospital and 1-year mortality were observed in patients with malignancy-related PE than with non-malignancy-related PE. In patients with malignancy-related PE, the Kaplan-Meier curve of 1-year all-cause mortality significantly differed between patients with or without metastasis; however, PE with or without malignant cells did not influence the prognosis. (4) Conclusions: In the patients with large PE requiring percutaneous pericardiocentesis, malignancy-related PE, nasopharyngeal and oropharyngeal cancer, and metastatic status were positive predictors of 1-year mortality. In patients with malignancy, a higher incidence of all-cause mortality was noted in patients with metastasis but did not differ between the groups with and without malignant cells in PE.
1. Introduction
For patients with a large, symptomatic pericardial effusion (PE), percutaneous needle pericardiocentesis has been the most useful therapeutic procedure for the early management of cardiac tamponade, and it is also used as a diagnostic procedure in certain patients who required cytologic proof, even though the diagnostic yield is low [1,2,3,4]. In patients with cardiac tamponade, echocardiography is the main method for the diagnosis and evaluation of hemodynamic status. Echo-guided percutaneous needle pericardiocentesis can be performed after the selection of the most appropriate anatomical approach among the apical, subcostal, and parasternal approaches [5]. The major risks associated with percutaneous pericardiocentesis are chamber or coronary artery laceration requiring surgery, an injury to an intercostal vessel necessitating surgery, and pneumothorax which requires chest tube placement [6]. Moreover, the causes of PE have changed, and the prognosis of PE may differ over time due to an aging society [7,8].
PE commonly occurs in patients with malignancy and has been reported in up to 21% of patients, and lung cancer is the most common primary malignancy associated with PE, followed by breast cancer and lymphoma [9,10]. Malignancy is a common cause of PE and is a marker of poor prognosis. PE associated with malignancy may lead to cardiac tamponade, which is a life-threatening condition. Pericardial fluid drainage is typically performed in symptomatic patients and may play a role in its diagnosis and staging. Surgical drainage can be used for its management; however, it is associated with a high rate of morbidity [11]. In cancer patients with a poor prognosis, surgery may not be suitable as it involves high risk. Therefore, a less invasive strategy of echo-guided percutaneous needle pericardiocentesis presents the potential of the safe and effective management of these patients with cancer. However, the long-term outcomes of pericardiocentesis are less defined in the East Asian population.
For these reasons, this study aimed to evaluate the etiologies, clinical outcomes, and complications of patients with a large, symptomatic PE treated with echo-guided pericardiocentesis.
2. Methods
2.1. Patient Population
Between July 2010 and December 2020, a total of 502 patients underwent percutaneous pericardiocentesis for massive or symptomatic PE at our hospital. Patients were recruited if they underwent primary percutaneous pericardiocentesis and were excluded if they underwent pericardial window surgery. The reasons for PE include malignancy (N = 277; 55.2%), infection (N = 42; 8.4%), pericarditis (N = 21; 4.2%), uremia (N = 16; 3.2%), thyroid dysfunction (N = 4; 0.8%), connective tissue disease (N = 5; 1.0%), procedure-related (N = 51; 10.2%), trauma (N = 3; 0.6%), unknown (N = 50; 10.0%), post-myocardial infarction (N = 9; 1.8%), heart failure (N = 18; 3.6%), liver cirrhosis (N = 1; 0.2%), and aortic dissection (N = 5; 1.0%) (Figure 1).
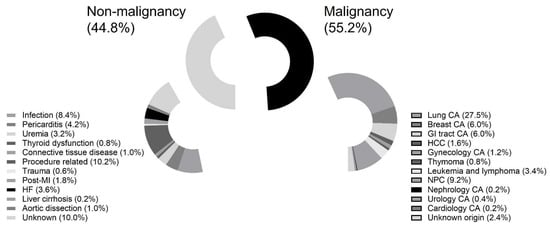
Figure 1.
Patient eligibility. Abbreviations: CA: cancer; GI: gastrointestinal; HCC: hepatological cancer; NPC: nasopharyngeal cancer.
The comorbidities, laboratory data, prior cancer therapy (including chemotherapy, or radiation therapy), clinical symptoms (dyspnea, tachycardia, and shock), echocardiographic findings, color and laboratory data of PE, complications, and 1-year all-cause mortality were compared between the malignancy and non-malignancy groups. The effusion pathology and microbiological results obtained at the time of pericardiocentesis were reviewed. The 1-year all-cause mortality was compared between subgroups of the malignancy group, including those with and without metastasis, with or without malignant cells in PE, with or without recurrent malignancy status, and between different cancers.
2.2. Percutaneous Pericardiocentesis Procedures
Percutaneous pericardiocentesis was performed under echocardiographic guidance at the shortest distance to the pericardial cavity from the apical, subcostal, and parasternal sites. Both the needle and catheter positions were confirmed using a saline contrast injection via echocardiography. After accessing the pericardial space, the needle was exchanged over a wire to a dilator, followed by a multiple side-hole pigtail catheter. The catheter was sutured and fixed to the chest wall. A PE sample (50 mL) of aspirated fluid was sent for pathology, chemistry, and microbiological testing. Post-procedure chest radiographs were performed regularly to assess the catheter position and any immediate complications. The catheter was removed at an earlier stage if fluid drainage fell below 50 mL per day and if no residual effusion was observed during follow-up echocardiography.
2.3. Definition
Cardiac tamponade was diagnosed by observing the compression of the right ventricle in diastole using echocardiography, in the presence of tachycardia (heart rate > 100 beats per min) or pulsus paradoxus (>10 mmHg decreases in the systolic blood pressure on inspiration) [12]. A large PE was diagnosed after the detection of the distance of the echo-free space of >2 cm on echocardiography [13]. All-cause mortality was defined as death resultant of any cause.
2.4. Study Endpoint
The study endpoint was all-cause mortality at a 1-year follow-up.
2.5. Statistical Analysis
Data are presented as mean ± standard deviation, or median ± quartile deviation if they are non-normally distributed parameters or numbers (percentages). The characteristics of the study groups were compared using the t-test for continuous variables and the chi-square test for categorical variables. Univariable and multivariable Cox regression analyses for one-year mortality were both performed to identify significant determinants. The multivariable Cox regression analysis included a hazard ratio (HR) <0.100 for one-year mortality in the univariable Cox regression analyses. Kaplan–Meier curves were created to illustrate the 1-year all-cause mortality data for each group. A statistical analysis was performed using statistical software (SPSS for Windows, version 22, IBM. Corp., Armonk, NY, USA), and a two-sided p-value of < 0.05 was defined as statistically significant.
3. Results
3.1. Baseline Characteristics of the Study Patients
The baseline characteristics of the study population are presented in Table 1. All patients underwent a percutaneous pericardiocentesis of a large PE with or without cardiac tamponade. The mean age was 63 ± 13.4 years, and most patients were males (61.6%). The participants in the malignancy group were younger than those in the non-malignancy group (59 ± 12.3 years vs. 67 ± 15.3 years; p < 0.001). In the non-malignancy group, a higher prevalence of diabetes mellitus (32.9% vs. 18.8%; p < 0.001), hypertension (32.9% vs. 18.8%; p < 0.001), coronary artery disease (32.9% vs. 5.1%; p < 0.001), and end-stage renal disease (16.4% vs. 4.0%; p < 0.001) were observed than in the malignancy group. A prior history of pericardiocentesis was higher in the malignancy group than in the non-malignancy group (9.7% vs. 4.0%; p = 0.014).

Table 1.
Demographics and clinical characteristics.
A higher white blood cell count (WBC) (9.6 ± 4.0 × 103/μL vs. 10.8 ± 6.8 × 103/μL; p = 0.010) and higher levels of lactate dehydrogenase (242 ± 246 mg/dL vs. 284 ± 288 mg/dL; p = 0.016) were observed in the malignancy group. A higher prevalence of asymptomatic participants in the non-malignancy group was documented (18.2% vs. 10.1%; p = 0.009). Furthermore, a higher prevalence of combined pleural effusion (78.7% vs. 60.4%; p < 0.001), cardiac tamponade (88.8% vs. 75.6%; p < 0.001), and low voltage on electrocardiography (42.0% vs. 25.4%; p < 0.001) were observed in the malignancy group. The need for subsequent pericardial peritoneal window surgery did not differ between the two groups. The incidence of complications related to pericardiocentesis was similar between the two groups. The complications included right ventricular laceration (1), left ventricular laceration (1), pneumothorax (1), and mixed pleural space insertion (2).
A higher incidence of in-hospital mortality (46.9% vs. 14.7%; p < 0.001) and all-cause mortality (56.3% vs. 18.7%; p < 0.001) were observed in the malignancy group. A shorter median of the follow-up duration (106 ± 107 days vs. 630 ± 636 days; p < 0.001) was observed in the malignancy group.
3.2. The Type of Malignancy and Associated Mortality Rate
Most of the original sites in the malignancy group were lung cancer (49.8%), nasopharyngeal and oropharyngeal cancer (16.6%), breast cancer (10.8%), and gastrointestinal tract cancer (10.8%) (Table 2). Of the total participants in the malignancy group, 6.5% had double cancer. A total of 76.2% of participants had metastasis, and 16.6% had recurrent status. Over half of the participants (59.2%) received prior chemotherapy and/or radiotherapy. A total of 35.4% of the participants’ PE samples contained malignant cells.

Table 2.
Type of malignancy.
In our study, patients with nasopharyngeal and oropharyngeal cancer had the highest in-hospital mortality rate (Figure 2A). Patients with gastrointestinal tract cancer and nasopharyngeal and oropharyngeal cancer had the highest incidence of 1-year mortality (Figure 2B).
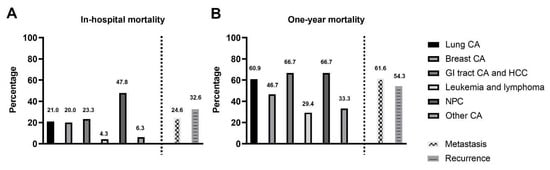
Figure 2.
(A): The incidence of in-hospital mortality in different cancers: The patients with nasopharyngeal and oropharyngeal cancer had the highest in-hospital mortality rate. (B). The incidence of 1-year all-cause mortality in different cancers: The patients with gastrointestinal tract cancer and nasopharyngeal and oropharyngeal cancer had the highest 1-year mortality rate. Abbreviations: CA: cancer; GI: gastrointestinal; HCC: hepatological cancer; NPC: nasopharyngeal cancer.
3.3. Univariable and Multivariable Cox Regression Analyses of Predictors of One-Year Mortality
Hypertension, liver cirrhosis, coronary artery disease, end-stage renal disease, procedure related malignancy, lung cancer, gastrointestinal tract cancer and hepatological cancer, nasopharyngeal cancer, metastatic and recurrent status, malignant cells in PE, symptoms including dyspnea, tachycardia, and pleural effusion were included for the multivariable Cox regression analyses of one-year mortality incidence (Table 3). Malignancy (HR: 2.084; 95% confidence interval (CI): 1.246–3.488; p = 0.005), nasopharyngeal cancer (HR: 1.801; 95% CI: 1.194–2.717; p = 0.005), and the metastatic status (HR: 2.088; 95% CI: 1.352−3.223; p = 0.001) were independent predictors of one-year mortality (Table 3).

Table 3.
Univariable and multivariable Cox regression analyses of predictors of one-year mortality.
3.4. Kaplan–Meier Curves Showing 1-Year All-Cause Mortality Data of the Two Groups and Subgroups of the Malignancy Population
Six patients in the non-malignancy group and seven patients in the malignancy group died within a day of pericardiocentesis. Figure 3 shows a Kaplan–Meier curve that illustrates the difference in 1-year all-cause mortality between the non-malignancy and malignancy groups (log-rank p < 0.001).
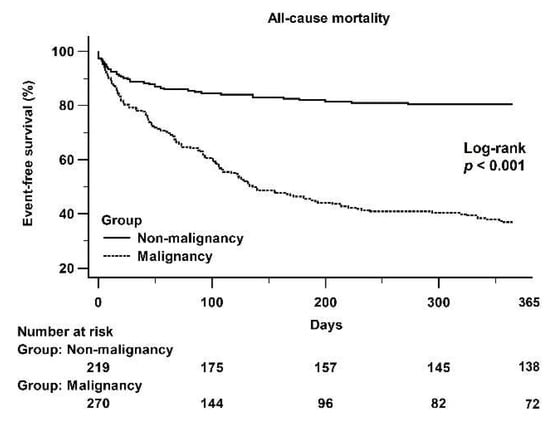
Figure 3.
A Kaplan–Meier curve of the 1-year all-cause mortality between the non-malignancy and malignancy groups: There was a significant difference between the non-malignancy and malignancy groups (log-rank p < 0.001).
In the malignancy group, there was a significant difference in 1-year all-cause mortality between the subgroups, both with and without metastasis (log-rank p = 0.003) (Figure 4A). A significant difference in the 1-year all-cause mortality between the recurrent and non-recurrent (Figure 4B) was not observed, and with or without the presence of malignant cells in PE (Figure 4C). Figure 4D shows that there was not a significant difference in the 1-year all-cause mortality between lung and breast cancer and other cancers.
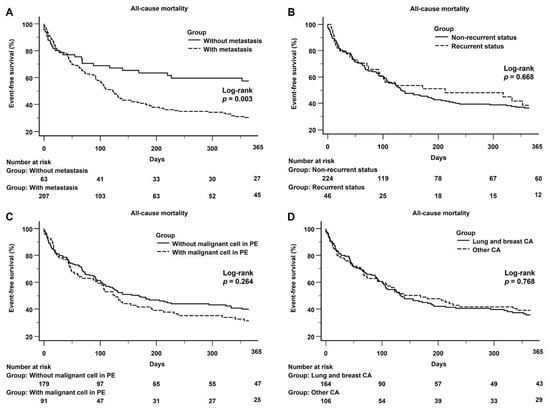
Figure 4.
(A): Kaplan–Meier curve of the 1-year all-cause mortality between the subgroups with and without metastasis: There was a significant difference between the subgroups with and without metastasis (log-rank p = 0.003). (B): Kaplan–Meier curve of the 1-year all-cause mortality between the subgroups with recurrent and non-recurrent status: There was no significant difference between the subgroups with and without metastasis (log-rank p = 0.668). (C): Kaplan–Meier curve of the 1-year all-cause mortality between the subgroups with and without malignancy in PE: There was no significant difference between the subgroups with and without malignancy in PE (log-rank p = 0.264). (D): Kaplan–Meier curve of the 1-year all-cause mortality between lung and breast cancer and other cancers, no significant difference between lung and breast cancer and other cancers was observed (log-rank p = 0.768).
4. Discussion
In the present study, a low incidence of complications (1.0%) was noted for the entire study population; however, two patients required surgery for cardiac ventricular laceration. The incidence of all-cause mortality was significantly higher in the malignancy group than in the non-malignancy group. Most of the malignancies were associated with lung cancer (49.8%), nasopharyngeal and oropharyngeal cancer (16.6%), breast cancer (10.8%), and gastrointestinal tract cancer (10.8%). Of all the participants, 76.2% were of a metastatic status, and 59.2% had received chemotherapy and/or radiotherapy. Participants with metastatic malignancy also presented poorer outcomes than those without metastatic malignancy. The prognosis did not differ between direct invasion (lung and breast cancer) and non-direct invasion (other types of malignancy). In our study, cytology, with or without malignant cells in the PE sample, did not influence the prognosis. Patients with nasopharyngeal and oropharyngeal cancer had the highest in-hospital mortality rate. Patients with gastrointestinal tract cancer and nasopharyngeal and oropharyngeal cancer had the highest 1-year mortality rate. Malignancy related PE, nasopharyngeal and oropharyngeal cancer, and metastatic status were positive predictors of one-year mortality.
4.1. Poor Prognosis in Malignant PE
Over time, the causes of large PE, with or without cardiac tamponade, changed from iatrogenic problems to malignancy, and patients with malignant PE had poorer outcomes [4,8]. In our study, the median survival duration was significantly shorter in the malignancy population than in the non-malignancy population (106 ± 107 days vs. 630 ± 636 days; p < 0.001). Currently, the prolonged survival rate of patients with cancer may lead to additional problems, such as malignant PE. Echo-guided percutaneous pericardiocentesis with drainage is a safe and effective treatment for patients with a large and symptomatic PE and can improve symptoms and quality of life but does not prolong life [14]. Therefore, a conservative strategy or hospice care may be considered for patients with malignant PE who have a shorter survival duration, especially for patients with nasopharyngeal and oropharyngeal cancer, and metastatic status.
4.2. The Predictors of Mortality in the Cancer Population with Malignant PE
In our study, patients with a metastatic status had a poor prognosis; however, the occurrence of malignant cells in PE did not differ between the groups. Malignant cells in PE presented a significant in univariate analysis but did not constitute a positive predictor in the multivariate Cox regression analyses. Previous reports have stated that the presence of pericardial malignant cytology does not appear to significantly affect outcomes [8]. However, another study reported that pericardial malignant cytology can be used to predict poor clinical outcomes in patients with malignant PE [15]. Lekhakul reported that patients with either lymphoma or chronic leukemia presented better survival than those with carcinoma or sarcoma [14]. El Haddad found that malignant PE significantly shortens the survival outcome of patients with lung cancer, but not of patients with breast cancer [10]. Therefore, about malignant cytology in PE remains controversial, regarding whether it results in poor outcomes and which cancer has a worse prognosis in cancer patients with malignant PE. In our study, the prognosis did not differ between different types of cancer; however, cancer patients with a metastatic status had a poorer prognosis.
4.3. Study Limitations
One limitation of this study is its retrospective nature, and that it included data from only one medical center. As a retrospective chart review, decisions regarding the method, entry site and placement of the catheter for extended drainage were all dependent on the operator and the patient’s general status (possible selection bias). Our patient population’s initial performance status could not be obtained from the data collected. The benefits of symptom relief or of improved short-term quality of life after pericardiocentesis cannot be measured, especially in patients with terminal stage disease. However, we have provided important information with regard to a comparison between patients with malignant and non-malignant PE, and we reported the poor prognosis of cancer patients with malignant PE in the East-Asian population, especially in patients with nasopharyngeal and oropharyngeal cancer.
5. Conclusions
In the patients with a large PE requiring percutaneous pericardiocentesis, malignancy related PE, nasopharyngeal and oropharyngeal cancer, and metastatic status were positive predictors of one-year mortality. A higher incidence of all-cause mortality was noted in patients with metastasis, although no differences in mortality were observed between the groups with and without malignant cells in PE.
Author Contributions
Conceptualization, W.-C.L.; methodology, W.-C.L.; software, W.-C.L.; validation, C.-T.S. and W.-C.L.; formal analysis, W.-C.L.; investigation, W.-C.L.; resources, W.-C.L., H.-Y.F., P.-J.W., Y.-N.F. and S.-Z.C.; data curation, C.-T.S.; writing—original draft preparation, C.-T.S. and W.-C.L.; writing—review and editing, W.-C.L. and H.-Y.F.; visualization, W.-C.L.; supervision, W.-C.L.; project administration, W.-C.L.; funding acquisition, W.-C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Kaohsiung Chang Gung Memorial Hospital (number: 202101592B0; date of approval: 16 September 2021).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study.
Data Availability Statement
The data of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tsang, T.S.; Enriquez-Sarano, M.; Freeman, W.K.; Barnes, M.E.; Sinak, L.J.; Gersh, B.J.; Bailey, K.R.; Seward, J.B. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: Clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin. Proc. 2002, 77, 429–436. [Google Scholar] [CrossRef]
- Imazio, M.; Adler, Y. Management of pericardial effusion. Eur. Heart J. 2013, 34, 1186–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercé, J.; Sagristà-Sauleda, J.; Permanyer-Miralda, G.; Soler-Soler, J. Should pericardial drainage be performed routinely in patients who have a large pericardial effusion without tamponade? Am. J. Med. 1998, 105, 106–109. [Google Scholar] [CrossRef]
- Kil, U.H.; Jung, H.O.; Koh, Y.S.; Park, H.J.; Park, C.S.; Kim, P.J.; Baek, S.H.; Seung, K.B.; Choi, K.B. Prognosis of large, symptomatic pericardial effusion treated by echo-guided percutaneous pericardiocentesis. Clin. Cardiol. 2008, 31, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Flint, N.; Siegel, R.J. Echo-Guided Pericardiocentesis: When and How Should It Be Performed? Curr. Cardiol. Rep. 2020, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sinha, A.; Lin, M.J.; Uchino, R.; Butryn, T.; O’Mara, M.S.; Nanda, S.; Shirani, J.; Stawicki, S.P. Complications of pericardiocentesis: A clinical synopsis. Int. J. Crit. Illn. Inj. Sci. 2015, 5, 206–212. [Google Scholar] [PubMed] [Green Version]
- Kabukcu, M.; Demircioglu, F.; Yanik, E.; Basarici, I.; Ersel, F. Pericardial tamponade and large pericardial effusions: Causal factors and efficacy of percutaneous catheter drainage in 50 patients. Tex. Heart Inst. J. 2004, 31, 398–403. [Google Scholar] [PubMed]
- Strobbe, A.; Adriaenssens, T.; Bennett, J.; Dubois, C.; Desmet, W.; McCutcheon, K.; Van Cleemput, J.; Sinnaeve, P.R. Etiology and Long-Term Outcome of Patients Undergoing Pericardiocentesis. J. Am. Heart Assoc. 2017, 6, e007598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jama, G.M.; Scarci, M.; Bowden, J.; Marciniak, S.J. Palliative treatment for symptomatic malignant pericardial effusion . Interact. Cardiovasc. Thorac. Surg. 2014, 19, 1019−1026. [Google Scholar] [CrossRef] [PubMed]
- El Haddad, D.; Iliescu, C.; Yusuf, S.W.; William, W.N., Jr.; Khair, T.H.; Song, J.; Mouhayar, E.N. Outcomes of Cancer Patients Undergoing Percutaneous Pericardiocentesis for Pericardial Effusion. J. Am. Coll. Cardiol. 2015, 66, 1119–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltzman, A.J.; Paz, Y.E.; Rene, A.G.; Green, P.; Hassanin, A.; Argenziano, M.G.; Rabbani, L.; Dangas, G. Comparison of surgical pericardial drainage with percutaneous catheter drainage for pericardial effusion. J. Invasive Cardiol. 2012, 24, 590–593. [Google Scholar] [PubMed]
- Fowler, N.O. Cardiac tamponade. A clinical or an echocardiographic diagnosis? Circulation 1993, 87, 1738–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, P.J.; Schuck, J. Echocardiographic Assessment of Pericardial Effusion: A Brief Review. J. Diagn. Med. Sonography 2007, 23, 189–197. [Google Scholar] [CrossRef]
- Lekhakul, A.; Assawakawintip, C.; Fenstad, E.R.; Pislaru, S.V.; Thaden, J.J.; Sinak, L.J.; Kane, G.C. Safety and Outcome of Percutaneous Drainage of Pericardial Effusions in Patients with Cancer. Am. J. Cardiol. 2018, 122, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.W.; Cho, D.G.; Park, J.K.; Hyun, K.Y.; Choi, S.Y.; Suh, J.H.; Kim, Y.D. Prognostic factors affecting survival of patients with cancer-related pericardial effusion managed by surgery. World J. Surg. Oncol. 2014, 12, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).