Cardiac Tissue Engineering for the Treatment of Myocardial Infarction
Abstract
:1. Introduction
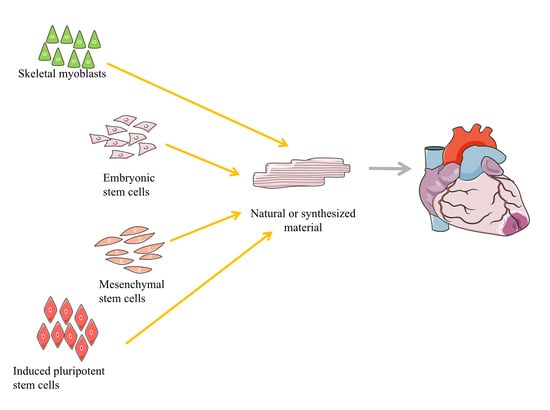
2. Types of Progenitor or Stem Cells Used for the Treatment of Myocardial Infarction
3. Application of Tissue Engineering in PSC Reprogramming, Differentiation, and Treatment of Myocardial Infarction
3.1. Tissue Engineering for the Regulation of PSC Reprogramming
3.2. Tissue Engineering for the Differentiation of PSCs into Cardiovascular Cells
3.3. Tissue Engineering Using PSC-Derived Cardiovascular Cells for the Treatment of MI
3.3.1. Natural Biomaterials
| Stem Cell Types | Model | Animal | Biomaterials | ROA | Reference |
|---|---|---|---|---|---|
| hESC-CMs | I/R | Rat | Matrigel + collagen | Applied on epicardium | [42] |
| hESC-CM or hIPSC-CM + fibroblasts | MI | Rat | Matrigel + collagen | Applied on epicardium | [43] |
| hESC-CMs | MI | Rat | Collagen | Applied on epicardium | [57] |
| hESC-CM, hiPSC-CM, HUVEC, MSC, and MEF | Uninjured heart | Rat | Collagen | Applied on epicardium | [58] |
| hiPSC-CM, hiPSC-ECs, and hiPSC-SMCs | MI | Rat | Collagen | Applied on epicardium | [59] |
| hESC-SSEA-1 + progenitor cells | MI | Rat | Fibrin/thrombin | Applied on epicardium | [82] |
| hiPSC-CMs | MI | Mouse | Cells, CHIR99021, and FGF1 loaded into fibrin/thrombin scaffold | Applied on epicardium | [83] |
| hiPSC-ECs and pericyte | MI | Rat | Fibrin/thrombin | Applied on epicardium | [60] |
| hiPSC-CMs and pericytes | MI | Rat | Fibrin/thrombin | Applied on epicardium | [61] |
| Ang-1 modified hiPSC-CMs | MI | Rat | Fibrin/thrombin | Applied on epicardium | [22] |
| hhiPSC-CMs | Cryo-injury | Guinea pig and pig | Fibrin/thrombin | Applied on epicardium | [62] |
| hiPSC-CMs and hiPSC-ECs | Cryo-injury | Guinea pig | Fibrin/thrombin | Applied on epicardium | [79] |
| hiPSC-CMs and hiPSC-ECs | Cryo-injury | Guinea pig | Fibrin/thrombin | Applied on epicardium | [63] |
| hiPSC-CMs, hiPSC-ECs, and hiPSC-SMCs | MI | Mouse | Gelatin | Applied on epicardium | [47] |
| Stem Cell Types | Model | Animal | Biomaterials | ROA | Reference |
|---|---|---|---|---|---|
| hiPSC-CM | Chronic ischemia | Pigs | hiPSC-CM sheet | Applied on epicardium | [84] |
| hiPSC-CM | Chronic ischemia | Pigs | Omentum flap | Intramyocardial injection of cells + omentum flap applied on epicardium | [85] |
| hiPSC-CM and hMSC | Chronic ischemia | Pigs | hiPSC-CM and hMSC sheet; Omentum flap | Applied on epicardium | [86] |
| hiPSC-EC, and hiPSC-SMC | I/R | Pigs | Fibrin/thrombin | Applied on epicardium | [87] |
| hiPSC-CM, hiPSC-EC, and hiPSC-SMC | I/R | Pigs | Fibrin/thrombin | Intramyocardial injection of cells + IGF-1 loaded fibrin/thrombin scaffold applied on epicardium | [21] |
| hiPSC-CM | MI | Pigs | Gelatin and fibrin/thrombin |
| [23] |
| hiPSC-CM | MI | Micro mini-pigs | Gelatin | Applied on epicardium | [88] |
| hESC-SSEA-1+ | I/R | Patients | Cells were cultured in fibrin/thrombin patch | Applied on epicardium in adjunction to CABG | [89,90] |
3.3.2. Synthetic Materials
3.4. 3D Printing in Cardiac Tissue Engineering Using PSC Derived Cardiovascular Cells
4. Challenges and Future Directions of Cardiac Tissue Engineering
- a.
- Natural Biomaterials vs. Synthetic Biomaterials
- b.
- Choice of Cells
- c.
- Progenitor Cells vs. Terminally Differentiated Cardiovascular Cells
- d.
- Alignment vs. Non-Alignment
Funding
Acknowledgments
Conflicts of Interest
References
- Dai, H.; Zhang, Q.; Much, A.A.; Maor, E.; Segev, A.; Beinart, R.; Adawi, S.; Lu, Y.; Bragazzi, N.L.; Wu, J. Global, regional, and national prevalence, incidence, mortality, and risk factors for atrial fibrillation, 1990–2017: Results from the Global Burden of Disease Study 2017. Eur. Heart J. Qual. Care Clin. Outcomes 2021, 7, 574–582. [Google Scholar] [CrossRef]
- Chiong, M.; Wang, Z.V.; Pedrozo, Z.; Cao, D.J.; Troncoso, R.; Ibacache, M.; Criollo, A.; Nemchenko, A.; Hill, J.A.; Lavandero, S. Cardiomyocyte death: Mechanisms and translational implications. Cell Death Dis 2011, 2, e244. [Google Scholar] [CrossRef]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; Dos Remedios, C.; et al. Dynamics of Cell Generation and Turnover in the Human Heart. Cell 2015, 161, 1566–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerber, Y.; Weston, S.A.; Enriquez-Sarano, M.; Berardi, C.; Chamberlain, A.M.; Manemann, S.M.; Jiang, R.; Dunlay, S.M.; Roger, V.L. Mortality Associated With Heart Failure After Myocardial Infarction: A Contemporary Community Perspective. Circ. Heart Fail. 2016, 9, e002460. [Google Scholar] [CrossRef] [Green Version]
- Muller, P.; Lemcke, H.; David, R. Stem Cell Therapy in Heart Diseases—Cell Types, Mechanisms and Improvement Strategies. Cell Physiol. Biochem. 2018, 48, 2607–2655. [Google Scholar] [CrossRef]
- Katarzyna, R. Adult Stem Cell Therapy for Cardiac Repair in Patients After Acute Myocardial Infarction Leading to Ischemic Heart Failure: An Overview of Evidence from the Recent Clinical Trials. Curr. Cardiol. Rev. 2017, 13, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Mirotsou, M.; Jayawardena, T.M.; Schmeckpeper, J.; Gnecchi, M.; Dzau, V.J. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J. Mol. Cell Cardiol. 2011, 50, 280–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, S.G.; Kwon, Y.W.; Lee, T.W.; Park, G.T.; Kim, J.H. Recent advances in stem cell therapeutics and tissue engineering strategies. Biomater. Res. 2018, 22, 36. [Google Scholar] [CrossRef] [Green Version]
- Berry, J.L.; Zhu, W.; Tang, Y.L.; Krishnamurthy, P.; Ge, Y.; Cooke, J.P.; Chen, Y.; Garry, D.J.; Yang, H.-T.; Rajasekaran, N.S.; et al. Convergences of Life Sciences and Engineering in Understanding and Treating Heart Failure. Circ. Res. 2019, 124, 161–169. [Google Scholar] [CrossRef]
- Nguyen, A.H.; Marsh, P.; Schmiess-Heine, L.; Burke, P.J.; Lee, A.; Lee, J.; Cao, H. Cardiac tissue engineering: State-of-the-art methods and outlook. J. Biol. Eng. 2019, 13, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, I.C.P.; Kaasi, A.; Maciel Filho, R.; Jardini, A.L.; Gabriel, L.P. Cardiac tissue engineering: Current state-of-the-art materials, cells and tissue formation. Einstein (Sao Paulo) 2018, 16, eRB4538. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzynski, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Haider, H.; Tan, R.; Toh, W.; Law, P.K.; Tan, W.; Su, L.; Zhang, W.; Ge, R.; Zhang, Y.; et al. Transplantation of nanoparticle transfected skeletal myoblasts overexpressing vascular endothelial growth factor-165 for cardiac repair. Circulation 2007, 116, I113–I120. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Haider, H.; Tan, R.; Su, L.; Law, P.K.; Zhang, W.; Sim, E.K. Angiomyogenesis using liposome based vascular endothelial growth factor-165 transfection with skeletal myoblast for cardiac repair. Biomaterials 2008, 29, 2125–2137. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Haider, H.; Jiang, S.; Tan, R.S.; Ge, R.; Law, P.K.; Sim, E.K. Improved angiogenic response in pig heart following ischaemic injury using human skeletal myoblast simultaneously expressing VEGF165 and angiopoietin-1. Eur. J. Heart Fail. 2007, 9, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Sid-Otmane, C.; Perrault, L.P.; Ly, H.Q. Mesenchymal stem cell mediates cardiac repair through autocrine, paracrine and endocrine axes. J. Transl. Med. 2020, 18, 336. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, L.A.; Al-Massri, K.F. Directions for Enhancement of the Therapeutic Efficacy of Mesenchymal Stem Cells in Different Neurodegenerative and Cardiovascular Diseases: Current Status and Future Perspectives. Curr. Stem Cell Res. Ther. 2021, 16, 858–876. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, P.; Duval, S.; Su, L.; Xiong, Q.; Zhang, J. Thymosin beta4 increases the potency of transplanted mesenchymal stem cells for myocardial repair. Circulation 2013, 128, S32–S41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Q.; Ye, L.; Zhang, P.; Lepley, M.; Swingen, C.; Zhang, L.; Kaufman, D.S.; Zhang, J. Bioenergetic and functional consequences of cellular therapy: Activation of endogenous cardiovascular progenitor cells. Circ. Res. 2012, 111, 455–468. [Google Scholar] [CrossRef] [Green Version]
- Yap, L.; Wang, J.W.; Moreno-Moral, A.; Chong, L.Y.; Sun, Y.; Harmston, N.; Wang, X.; Chong, S.Y.; Vanezis, K.; Ohman, M.K.; et al. In Vivo Generation of Post-infarct Human Cardiac Muscle by Laminin-Promoted Cardiovascular Progenitors. Cell Rep. 2020, 31, 107714. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Chang, Y.H.; Xiong, Q.; Zhang, P.; Zhang, L.; Somasundaram, P.; Lepley, M.; Swingen, C.; Su, L.; Wendel, J.S.; et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell 2014, 15, 750–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, Z.; Loo, S.; Su, L.; Tan, S.; Tee, G.; Gan, S.U.; Zhang, J.; Chen, X.; Ye, L. Angiopoietin-1 enhanced myocyte mitosis, engraftment, and the reparability of hiPSC-CMs for treatment of myocardial infarction. Cardiovasc. Res. 2021, 117, 1578–1591. [Google Scholar] [CrossRef]
- Tan, S.H.; Loo, S.J.; Gao, Y.; Tao, Z.H.; Su, L.P.; Wang, C.X.; Zhang, S.L.; Mu, Y.H.; Cui, Y.H.; Abdurrachim, D.; et al. Thymosin beta4 increases cardiac cell proliferation, cell engraftment, and the reparative potency of human induced-pluripotent stem cell-derived cardiomyocytes in a porcine model of acute myocardial infarction. Theranostics 2021, 11, 7879–7895. [Google Scholar] [CrossRef]
- Weinberger, L.; Ayyash, M.; Novershtern, N.; Hanna, J.H. Dynamic stem cell states: Naive to primed pluripotency in rodents and humans. Nat. Rev. Mol. Cell Biol. 2016, 17, 155–169. [Google Scholar] [CrossRef]
- Nichols, J.; Smith, A. Naive and primed pluripotent states. Cell Stem Cell 2009, 4, 487–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downing, T.L.; Soto, J.; Morez, C.; Houssin, T.; Fritz, A.; Yuan, F.; Chu, J.; Patel, S.; Schaffer, D.V.; Li, S. Biophysical regulation of epigenetic state and cell reprogramming. Nat. Mater. 2013, 12, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Tajik, A.; Chen, J.; Jia, Q.; Chowdhury, F.; Wang, L.; Chen, J.; Zhang, S.; Hong, Y.; Yi, H.; et al. Matrix softness regulates plasticity of tumour-repopulating cells via H3K9 demethylation and Sox2 expression. Nat. Commun. 2014, 5, 4619. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, H.; Liu, J.; Qi, J.; Wei, B.; Yang, J.; Liang, H.; Chen, Y.; Chen, J.; Wu, Y.; et al. H3K9 methylation is a barrier during somatic cell reprogramming into iPSCs. Nat. Genet. 2013, 45, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Jaenisch, R.; Young, R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell 2008, 132, 567–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwart, D.; Paquet, D.; Teo, S.; Tessier-Lavigne, M. Precise and efficient scarless genome editing in stem cells using CORRECT. Nat. Protoc. 2017, 12, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Hockemeyer, D.; Jaenisch, R. Induced Pluripotent Stem Cells Meet Genome Editing. Cell Stem Cell 2016, 18, 573–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weltner, J.; Balboa, D.; Katayama, S.; Bespalov, M.; Krjutskov, K.; Jouhilahti, E.M.; Trokovic, R.; Kere, J.; Otonkoski, T. Human pluripotent reprogramming with CRISPR activators. Nat. Commun. 2018, 9, 2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howden, S.E.; Thomson, J.A.; Little, M.H. Simultaneous reprogramming and gene editing of human fibroblasts. Nat. Protoc. 2018, 13, 875–898. [Google Scholar] [CrossRef]
- Melo, U.S.; de Souza Leite, F.; Costa, S.; Rosenberg, C.; Zatz, M. A fast method to reprogram and CRISPR/Cas9 gene editing from erythroblasts. Stem Cell Res. 2018, 31, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Poh, Y.C.; Chen, J.; Hong, Y.; Yi, H.; Zhang, S.; Chen, J.; Wu, D.C.; Wang, L.; Jia, Q.; Singh, R.; et al. Generation of organized germ layers from a single mouse embryonic stem cell. Nat. Commun. 2014, 5, 4000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Dutton, J.R.; Su, L.; Zhang, J.; Ye, L. The influence of a spatiotemporal 3D environment on endothelial cell differentiation of human induced pluripotent stem cells. Biomaterials 2014, 35, 3786–3793. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Kong, X.; Lim, S.; Loo, S.; Tan, S.; Poh, K.; Dutton, J.; Stewart, C.; Cook, S.; Su, X.; et al. The prostaglandin H2 analog U-46619 improves the differentiation efficiency of human induced pluripotent stem cells into endothelial cells by activating both p38MAPK and ERK1/2 signaling pathways. Stem Cell Res. Ther. 2018, 9, 313. [Google Scholar] [CrossRef]
- Zanotelli, M.R.; Ardalani, H.; Zhang, J.; Hou, Z.; Nguyen, E.H.; Swanson, S.; Nguyen, B.K.; Bolin, J.; Elwell, A.; Bischel, L.L.; et al. Stable engineered vascular networks from human induced pluripotent stem cell-derived endothelial cells cultured in synthetic hydrogels. Acta Biomater. 2016, 35, 32–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ting, S.; Chen, A.; Reuveny, S.; Oh, S. An intermittent rocking platform for integrated expansion and differentiation of human pluripotent stem cells to cardiomyocytes in suspended microcarrier cultures. Stem Cell Res. 2014, 13, 202–213. [Google Scholar] [CrossRef] [Green Version]
- Prabhakaran, M.P.; Mobarakeh, L.G.; Kai, D.; Karbalaie, K.; Nasr-Esfahani, M.H.; Ramakrishna, S. Differentiation of embryonic stem cells to cardiomyocytes on electrospun nanofibrous substrates. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Singh, S.P.; Panicker, M.M.; Gupta, P.K. Amniotic membrane as novel scaffold for human iPSC-derived cardiomyogenesis. In Vitro Cell Dev. Biol. Anim. 2019, 55, 272–284. [Google Scholar] [CrossRef]
- Riegler, J.; Tiburcy, M.; Ebert, A.; Tzatzalos, E.; Raaz, U.; Abilez, O.J.; Shen, Q.; Kooreman, N.G.; Neofytou, E.; Chen, V.C.; et al. Human Engineered Heart Muscles Engraft and Survive Long Term in a Rodent Myocardial Infarction Model. Circ. Res. 2015, 117, 720–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiburcy, M.; Hudson, J.E.; Balfanz, P.; Schlick, S.; Meyer, T.; Chang Liao, M.L.; Levent, E.; Raad, F.; Zeidler, S.; Wingender, E.; et al. Defined Engineered Human Myocardium With Advanced Maturation for Applications in Heart Failure Modeling and Repair. Circulation 2017, 135, 1832–1847. [Google Scholar] [CrossRef] [PubMed]
- Bakunts, K.; Gillum, N.; Karabekian, Z.; Sarvazyan, N. Formation of cardiac fibers in Matrigel matrix. Biotechniques 2008, 44, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jha, A.K.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Hyaluronic Acid-Based Hydrogels: From a Natural Polysaccharide to Complex Networks. Soft Matter. 2012, 8, 3280–3294. [Google Scholar] [CrossRef] [Green Version]
- Borzacchiello, A.; Russo, L.; Malle, B.M.; Schwach-Abdellaoui, K.; Ambrosio, L. Hyaluronic Acid Based Hydrogels for Regenerative Medicine Applications. Biomed. Res. Int. 2015, 2015, 871218. [Google Scholar] [CrossRef]
- Gao, L.; Kupfer, M.E.; Jung, J.P.; Yang, L.; Zhang, P.; Da Sie, Y.; Tran, Q.; Ajeti, V.; Freeman, B.T.; Fast, V.G.; et al. Myocardial Tissue Engineering With Cells Derived From Human-Induced Pluripotent Stem Cells and a Native-Like, High-Resolution, 3-Dimensionally Printed Scaffold. Circ. Res. 2017, 120, 1318–1325. [Google Scholar] [CrossRef] [Green Version]
- McCain, M.L.; Agarwal, A.; Nesmith, H.W.; Nesmith, A.P.; Parker, K.K. Micromolded gelatin hydrogels for extended culture of engineered cardiac tissues. Biomaterials 2014, 35, 5462–5471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Zhang, N.; Meng, H.X.; Liu, H.X.; Lu, Y.Q.; Liu, C.M.; Zhang, Z.M.; Qu, K.Y.; Huang, N.P. Easy Applied Gelatin-Based Hydrogel System for Long-Term Functional Cardiomyocyte Culture and Myocardium Formation. ACS Biomater. Sci. Eng. 2019, 5, 3022–3031. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar] [PubMed]
- Shariatinia, Z.; Jalali, A.M. Chitosan-based hydrogels: Preparation, properties and applications. Int. J. Biol. Macromol. 2018, 115, 194–220. [Google Scholar] [CrossRef]
- Neves, M.I.; Moroni, L.; Barrias, C.C. Modulating Alginate Hydrogels for Improved Biological Performance as Cellular 3D Microenvironments. Front. Bioeng. Biotechnol. 2020, 8, 665. [Google Scholar] [CrossRef] [PubMed]
- Santana, B.P.; Nedel, F.; Piva, E.; de Carvalho, R.V.; Demarco, F.F.; Carreno, N.L. Preparation, modification, and characterization of alginate hydrogel with nano-/microfibers: A new perspective for tissue engineering. Biomed. Res. Int. 2013, 2013, 307602. [Google Scholar] [CrossRef] [PubMed]
- Liberski, A.; Latif, N.; Raynaud, C.; Bollensdorff, C.; Yacoub, M. Alginate for cardiac regeneration: From seaweed to clinical trials. Glob. Cardiol. Sci. Pract. 2016, 2016, e201604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, N.J.; Kant, R.J.; Minor, A.J.; Coulombe, K.L.K. Optimizing Blended Collagen-Fibrin Hydrogels for Cardiac Tissue Engineering with Human iPSC-derived Cardiomyocytes. ACS Biomater. Sci. Eng. 2019, 5, 887–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edalat, S.G.; Jang, Y.; Kim, J.; Park, Y. Collagen Type I Containing Hybrid Hydrogel Enhances Cardiomyocyte Maturation in a 3D Cardiac Model. Polymers 2019, 11, 687. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Riegler, J.; Tiburcy, M.; Zhao, X.; Chour, T.; Ndoye, B.; Nguyen, M.; Adams, J.; Ameen, M.; Denney, T.S., Jr.; et al. Magnetic Resonance Imaging of Cardiac Strain Pattern Following Transplantation of Human Tissue Engineered Heart Muscles. Circ. Cardiovasc. Imaging 2016, 9, e004731. [Google Scholar] [CrossRef] [Green Version]
- Tulloch, N.L.; Muskheli, V.; Razumova, M.V.; Korte, F.S.; Regnier, M.; Hauch, K.D.; Pabon, L.; Reinecke, H.; Murry, C.E. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ. Res. 2011, 109, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Nakane, T.; Masumoto, H.; Tinney, J.P.; Yuan, F.; Kowalski, W.J.; Ye, F.; LeBlanc, A.J.; Sakata, R.; Yamashita, J.K.; Keller, B.B. Impact of Cell Composition and Geometry on Human Induced Pluripotent Stem Cells-Derived Engineered Cardiac Tissue. Sci. Rep. 2017, 7, 45641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riemenschneider, S.B.; Mattia, D.J.; Wendel, J.S.; Schaefer, J.A.; Ye, L.; Guzman, P.A.; Tranquillo, R.T. Inosculation and perfusion of pre-vascularized tissue patches containing aligned human microvessels after myocardial infarction. Biomaterials 2016, 97, 51–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendel, J.S.; Ye, L.; Tao, R.; Zhang, J.; Zhang, J.; Kamp, T.J.; Tranquillo, R.T. Functional Effects of a Tissue-Engineered Cardiac Patch From Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes in a Rat Infarct Model. Stem Cells Transl. Med. 2015, 4, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Querdel, E.; Reinsch, M.; Castro, L.; Kose, D.; Bahr, A.; Reich, S.; Geertz, B.; Ulmer, B.; Schulze, M.; Lemoine, M.D.; et al. Human Engineered Heart Tissue Patches Remuscularize the Injured Heart in a Dose-Dependent Manner. Circulation 2021, 143, 1991–2006. [Google Scholar] [CrossRef]
- Pecha, S.; Yorgan, K.; Rohl, M.; Geertz, B.; Hansen, A.; Weinberger, F.; Sehner, S.; Ehmke, H.; Reichenspurner, H.; Eschenhagen, T.; et al. Human iPS cell-derived engineered heart tissue does not affect ventricular arrhythmias in a guinea pig cryo-injury model. Sci. Rep. 2019, 9, 9831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contessotto, P.; Orbanic, D.; Da Costa, M.; Jin, C.; Owens, P.; Chantepie, S.; Chinello, C.; Newell, J.; Magni, F.; Papy-Garcia, D.; et al. Elastin-like recombinamers-based hydrogel modulates post-ischemic remodeling in a non-transmural myocardial infarction in sheep. Sci. Transl. Med. 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Esser, T.U.; Trossmann, V.T.; Lentz, S.; Engel, F.B.; Scheibel, T. Designing of spider silk proteins for human induced pluripotent stem cell-based cardiac tissue engineering. Mater. Today Biol. 2021, 11, 100114. [Google Scholar] [CrossRef] [PubMed]
- Dobner, S.; Bezuidenhout, D.; Govender, P.; Zilla, P.; Davies, N. A synthetic non-degradable polyethylene glycol hydrogel retards adverse post-infarct left ventricular remodeling. J. Card. Fail. 2009, 15, 629–636. [Google Scholar] [CrossRef]
- Chow, A.; Stuckey, D.J.; Kidher, E.; Rocco, M.; Jabbour, R.J.; Mansfield, C.A.; Darzi, A.; Harding, S.E.; Stevens, M.M.; Athanasiou, T. Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Encapsulating Bioactive Hydrogels Improve Rat Heart Function Post Myocardial Infarction. Stem Cell Rep. 2017, 9, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Wang, J.; Shen, B.; Chan, C.W.; Wang, C.; Zhao, Y.; Chan, H.N.; Tian, Q.; Chen, Y.; Yao, C.; et al. Engineering a freestanding biomimetic cardiac patch using biodegradable poly(lactic-co-glycolic acid) (PLGA) and human embryonic stem cell-derived ventricular cardiomyocytes (hESC-VCMs). Macromol. Biosci. 2015, 15, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Song, S.Y.; Kim, H.; Yoo, J.; Kwon, S.P.; Park, B.W.; Kim, J.J.; Ban, K.; Char, K.; Park, H.J.; Kim, B.S. Prevascularized, multiple-layered cell sheets of direct cardiac reprogrammed cells for cardiac repair. Biomater. Sci. 2020, 8, 4508–4520. [Google Scholar] [CrossRef] [PubMed]
- Daliri, K.; Pfannkuche, K.; Garipcan, B. Effects of physicochemical properties of polyacrylamide (PAA) and (polydimethylsiloxane) PDMS on cardiac cell behavior. Soft Matter 2021, 17, 1156–1172. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Jiang, X.; Li, Z.; Wen, Y.; Chen, D.; Li, X.; Zhang, X.; Zhuo, R.; Chu, H. Physical properties of poly(N-isopropylacrylamide) hydrogel promote its effects on cardiac protection after myocardial infarction. J. Int. Med. Res. 2012, 40, 2167–2182. [Google Scholar] [CrossRef] [PubMed]
- Wanjare, M.; Hou, L.; Nakayama, K.H.; Kim, J.J.; Mezak, N.P.; Abilez, O.J.; Tzatzalos, E.; Wu, J.C.; Huang, N.F. Anisotropic microfibrous scaffolds enhance the organization and function of cardiomyocytes derived from induced pluripotent stem cells. Biomater. Sci. 2017, 5, 1567–1578. [Google Scholar] [CrossRef]
- Chiono, V.; Mozetic, P.; Boffito, M.; Sartori, S.; Gioffredi, E.; Silvestri, A.; Rainer, A.; Giannitelli, S.M.; Trombetta, M.; Nurzynska, D.; et al. Polyurethane-based scaffolds for myocardial tissue engineering. Interface Focus 2014, 4, 20130045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef] [Green Version]
- Navaei, A.; Truong, D.; Heffernan, J.; Cutts, J.; Brafman, D.; Sirianni, R.W.; Vernon, B.; Nikkhah, M. PNIPAAm-based biohybrid injectable hydrogel for cardiac tissue engineering. Acta Biomater. 2016, 32, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Kadner, K.; Dobner, S.; Franz, T.; Bezuidenhout, D.; Sirry, M.S.; Zilla, P.; Davies, N.H. The beneficial effects of deferred delivery on the efficiency of hydrogel therapy post myocardial infarction. Biomaterials 2012, 33, 2060–2066. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, T.; Zhu, Y.; Jiang, H.; D’Amore, A.; Sakaguchi, H.; Tchao, J.; Tobita, K.; Wagner, W.R. Timing effect of intramyocardial hydrogel injection for positively impacting left ventricular remodeling after myocardial infarction. Biomaterials 2016, 83, 182–193. [Google Scholar] [CrossRef] [Green Version]
- Ye, L.; Basu, J.; Zhang, J. Fabrication of a myocardial patch with cells differentiated from human-induced pluripotent stem cells. Methods Mol. Biol. 2015, 1299, 103–114. [Google Scholar] [CrossRef]
- Weinberger, F.; Breckwoldt, K.; Pecha, S.; Kelly, A.; Geertz, B.; Starbatty, J.; Yorgan, T.; Cheng, K.H.; Lessmann, K.; Stolen, T.; et al. Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci. Transl. Med. 2016, 8, 363ra148. [Google Scholar] [CrossRef] [PubMed]
- Case Records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 29-1997. A 54-year-old diabetic woman with pain and swelling of the leg. N. Engl. J. Med. 1997, 337, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Gregorich, Z.R.; Zhu, W.; Mattapally, S.; Oduk, Y.; Lou, X.; Kannappan, R.; Borovjagin, A.V.; Walcott, G.P.; Pollard, A.E.; et al. Large Cardiac Muscle Patches Engineered From Human Induced-Pluripotent Stem Cell-Derived Cardiac Cells Improve Recovery From Myocardial Infarction in Swine. Circulation 2018, 137, 1712–1730. [Google Scholar] [CrossRef]
- Menasche, P.; Vanneaux, V.; Fabreguettes, J.R.; Bel, A.; Tosca, L.; Garcia, S.; Bellamy, V.; Farouz, Y.; Pouly, J.; Damour, O.; et al. Towards a clinical use of human embryonic stem cell-derived cardiac progenitors: A translational experience. Eur. Heart J. 2015, 36, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Tang, Y.; Zhao, M.; Lou, X.; Pretorius, D.; Menasche, P.; Zhu, W.; Zhang, J. CHIR99021 and fibroblast growth factor 1 enhance the regenerative potency of human cardiac muscle patch after myocardial infarction in mice. J. Mol. Cell Cardiol. 2020, 141, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Miyagawa, S.; Miki, K.; Saito, A.; Fukushima, S.; Higuchi, T.; Kawamura, T.; Kuratani, T.; Daimon, T.; Shimizu, T.; et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 2012, 126, S29–S37. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, M.; Miyagawa, S.; Fukushima, S.; Saito, A.; Miki, K.; Ito, E.; Sougawa, N.; Kawamura, T.; Daimon, T.; Shimizu, T.; et al. Enhanced survival of transplanted human induced pluripotent stem cell-derived cardiomyocytes by the combination of cell sheets with the pedicled omental flap technique in a porcine heart. Circulation 2013, 128, S87–S94. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, M.; Miyagawa, S.; Fukushima, S.; Saito, A.; Miki, K.; Funakoshi, S.; Yoshida, Y.; Yamanaka, S.; Shimizu, T.; Okano, T.; et al. Enhanced Therapeutic Effects of Human iPS Cell Derived-Cardiomyocyte by Combined Cell-Sheets with Omental Flap Technique in Porcine Ischemic Cardiomyopathy Model. Sci. Rep. 2017, 7, 8824. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Ye, L.; Zhang, P.; Lepley, M.; Tian, J.; Li, J.; Zhang, L.; Swingen, C.; Vaughan, J.T.; Kaufman, D.S.; et al. Functional consequences of human induced pluripotent stem cell therapy: Myocardial ATP turnover rate in the in vivo swine heart with postinfarction remodeling. Circulation 2013, 127, 997–1008. [Google Scholar] [CrossRef] [Green Version]
- Ishigami, M.; Masumoto, H.; Ikuno, T.; Aoki, T.; Kawatou, M.; Minakata, K.; Ikeda, T.; Sakata, R.; Yamashita, J.K.; Minatoya, K. Human iPS cell-derived cardiac tissue sheets for functional restoration of infarcted porcine hearts. PLoS ONE 2018, 13, e0201650. [Google Scholar] [CrossRef] [Green Version]
- Menasche, P.; Vanneaux, V.; Hagege, A.; Bel, A.; Cholley, B.; Cacciapuoti, I.; Parouchev, A.; Benhamouda, N.; Tachdjian, G.; Tosca, L.; et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: First clinical case report. Eur. Heart J. 2015, 36, 2011–2017. [Google Scholar] [CrossRef] [Green Version]
- Menasche, P.; Vanneaux, V.; Hagege, A.; Bel, A.; Cholley, B.; Parouchev, A.; Cacciapuoti, I.; Al-Daccak, R.; Benhamouda, N.; Blons, H.; et al. Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitors for Severe Ischemic Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2018, 71, 429–438. [Google Scholar] [CrossRef]
- Ban, K.; Park, H.J.; Kim, S.; Andukuri, A.; Cho, K.W.; Hwang, J.W.; Cha, H.J.; Kim, S.Y.; Kim, W.S.; Jun, H.W.; et al. Cell therapy with embryonic stem cell-derived cardiomyocytes encapsulated in injectable nanomatrix gel enhances cell engraftment and promotes cardiac repair. ACS Nano 2014, 8, 10815–10825. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, Y.; Fu, W.; Yao, M.; Ding, Z.; Xuan, J.; Li, D.; Wang, S.; Xia, Y.; Cao, M. Poly(N-isopropylacrylamide)-Based Thermoresponsive Composite Hydrogels for Biomedical Applications. Polymers 2020, 12, 580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaetani, R.; Doevendans, P.A.; Metz, C.H.; Alblas, J.; Messina, E.; Giacomello, A.; Sluijter, J.P. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials 2012, 33, 1782–1790. [Google Scholar] [CrossRef]
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivi, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.E.; Seliktar, D.; et al. A multi-cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC-derived cardiomyocytes. Sci. Rep. 2018, 8, 13532. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Tytgat, L.; Dobos, A.; Markovic, M.; Van Damme, L.; Van Hoorick, J.; Bray, F.; Thienpont, H.; Ottevaere, H.; Dubruel, P.; Ovsianikov, A.; et al. High-Resolution 3D Bioprinting of Photo-Cross-linkable Recombinant Collagen to Serve Tissue Engineering Applications. Biomacromolecules 2020, 21, 3997–4007. [Google Scholar] [CrossRef]
- AnilKumar, S.; Allen, S.C.; Tasnim, N.; Akter, T.; Park, S.; Kumar, A.; Chattopadhyay, M.; Ito, Y.; Suggs, L.J.; Joddar, B. The applicability of furfuryl-gelatin as a novel bioink for tissue engineering applications. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 314–323. [Google Scholar] [CrossRef]
- Gaetani, R.; Feyen, D.A.; Verhage, V.; Slaats, R.; Messina, E.; Christman, K.L.; Giacomello, A.; Doevendans, P.A.; Sluijter, J.P. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials 2015, 61, 339–348. [Google Scholar] [CrossRef]
- Koivisto, J.T.; Gering, C.; Karvinen, J.; Maria Cherian, R.; Belay, B.; Hyttinen, J.; Aalto-Setala, K.; Kellomaki, M.; Parraga, J. Mechanically Biomimetic Gelatin-Gellan Gum Hydrogels for 3D Culture of Beating Human Cardiomyocytes. ACS Appl. Mater. Interfaces 2019, 11, 20589–20602. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Jiang, X.; Li, L.; Chen, Z.N.; Gao, G.; Yao, R.; Sun, W. 3D printing human induced pluripotent stem cells with novel hydroxypropyl chitin bioink: Scalable expansion and uniform aggregation. Biofabrication 2018, 10, 044101. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Yang, Q.; Liao, Y.; Zhu, B.; Zhao, G.; Shen, R.; Lu, X.; Qu, S. A novel thixotropic magnesium phosphate-based bioink with excellent printability for application in 3D printing. J. Mater. Chem. B 2018, 6, 4502–4513. [Google Scholar] [CrossRef] [PubMed]
- Pati, F.; Jang, J.; Ha, D.H.; Won Kim, S.; Rhie, J.W.; Shim, J.H.; Kim, D.H.; Cho, D.W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014, 5, 3935. [Google Scholar] [CrossRef] [Green Version]
- Kc, P.; Hong, Y.; Zhang, G. Cardiac tissue-derived extracellular matrix scaffolds for myocardial repair: Advantages and challenges. Regen. Biomater. 2019, 6, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Basara, G.; Ozcebe, S.G.; Ellis, B.W.; Zorlutuna, P. Tunable Human Myocardium Derived Decellularized Extracellular Matrix for 3D Bioprinting and Cardiac Tissue Engineering. Gels 2021, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef] [Green Version]
- Su, L.; Kong, X.; Loo, S.J.; Gao, Y.; Kovalik, J.P.; Su, X.; Ma, J.; Ye, L. Diabetic Endothelial Cells Differentiated From Patient iPSCs Show Dysregulated Glycine Homeostasis and Senescence Associated Phenotypes. Front. Cell Dev. Biol. 2021, 9, 667252. [Google Scholar] [CrossRef]
- Tan, S.H.; Ye, L. Maturation of Pluripotent Stem Cell-Derived Cardiomyocytes: A Critical Step for Drug Development and Cell Therapy. J. Cardiovasc. Transl. Res. 2018, 11, 375–392. [Google Scholar] [CrossRef]
- Anderson, R.H.; Ho, S.Y.; Redmann, K.; Sanchez-Quintana, D.; Lunkenheimer, P.P. The anatomical arrangement of the myocardial cells making up the ventricular mass. Eur. J. Cardiothorac. Surg. 2005, 28, 517–525. [Google Scholar] [CrossRef]
| Cell Types | Advantages | Disadvantages |
|---|---|---|
| Skeletal myoblasts |
|
|
| Mesenchymal stem cells |
|
|
| Embryonic stem cells |
|
|
| Induced pluripotent stem cells |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, D.; Wang, X.; Ye, L. Cardiac Tissue Engineering for the Treatment of Myocardial Infarction. J. Cardiovasc. Dev. Dis. 2021, 8, 153. https://doi.org/10.3390/jcdd8110153
Yu D, Wang X, Ye L. Cardiac Tissue Engineering for the Treatment of Myocardial Infarction. Journal of Cardiovascular Development and Disease. 2021; 8(11):153. https://doi.org/10.3390/jcdd8110153
Chicago/Turabian StyleYu, Dongmin, Xiaowei Wang, and Lei Ye. 2021. "Cardiac Tissue Engineering for the Treatment of Myocardial Infarction" Journal of Cardiovascular Development and Disease 8, no. 11: 153. https://doi.org/10.3390/jcdd8110153
APA StyleYu, D., Wang, X., & Ye, L. (2021). Cardiac Tissue Engineering for the Treatment of Myocardial Infarction. Journal of Cardiovascular Development and Disease, 8(11), 153. https://doi.org/10.3390/jcdd8110153