Post-Acute Sequelae of COVID-19 and Cardiovascular Autonomic Dysfunction: What Do We Know?
Abstract
:1. Introduction
2. Post-Acute Sequelae of COVID-19
How Long Will Symptoms Last?
3. COVID-19 Infection and Dysautonomia
| Authors, Year | Dani et al. [16], 2021 | Johansson et al. [10], 2021 | Blitshteyn et al. [56], 2021 | Shouman et al. [25], 2021 | Wallukat et al. [57], 2021 | Goodman et al. [58], 2021 | Overall |
|---|---|---|---|---|---|---|---|
| Sample size | 5 | 3 | 20 | 20 | 7 | 6 | 61 |
| Age | 43 | 36 | 42 | 44 | 45 | 45 | 42 |
| Female sex | 100% | 33% | 70% | 70% | 57% | 67% | 69% |
| CVAD (n) | OI (4) rTC (1) | POTS (3) | POTS (15), NCS (3), OH (3) | OH (14) POTS (6) | POTS (7) | POTS (4), OH (1), PHTN (3) | POTS 69% of cases |
| Palpitations, tachycardia | 60% | 67% | 80% | 15% | 57% | 67% | 58% |
| Fatigue | 60% | 67% | 60% | 55% | 43% | 100% | 64% |
| Vertigo a | 40% | 67% | 25% | 100% | 0% | 100% | 55% |
| Dyspnea | 20% | 67% | 45% | 45% | 0% | 83% | 43% |
| Presyncope | 20% | 67% | 15% | 5% | 0% | 83% | 32% |
| Chest pain | 20% | 67% | 15% | 25% | 0% | 50% | 30% |
| Headache | 0% | 67% | 15% | 40% | 0% | 67% | 31% |
| Brain fog b | 0% | 67% | 5% | 25% | 14% | 0% | 19% |
| Sleep disturbances | 0% | 67% | 0% | 20% | 0% | 0% | 15% |
When Does CVAD Occur in COVID-19 Patients?
4. Postural Orthostatic Tachycardia Syndrome after COVID-19
5. How Does SARS-CoV-2 Infection Cause Long-Term Dysautonomia?
6. Is PASC Gender-Specific?
Why Would Women Be at Higher Risk of Developing Post-COVID-19 Symptoms?
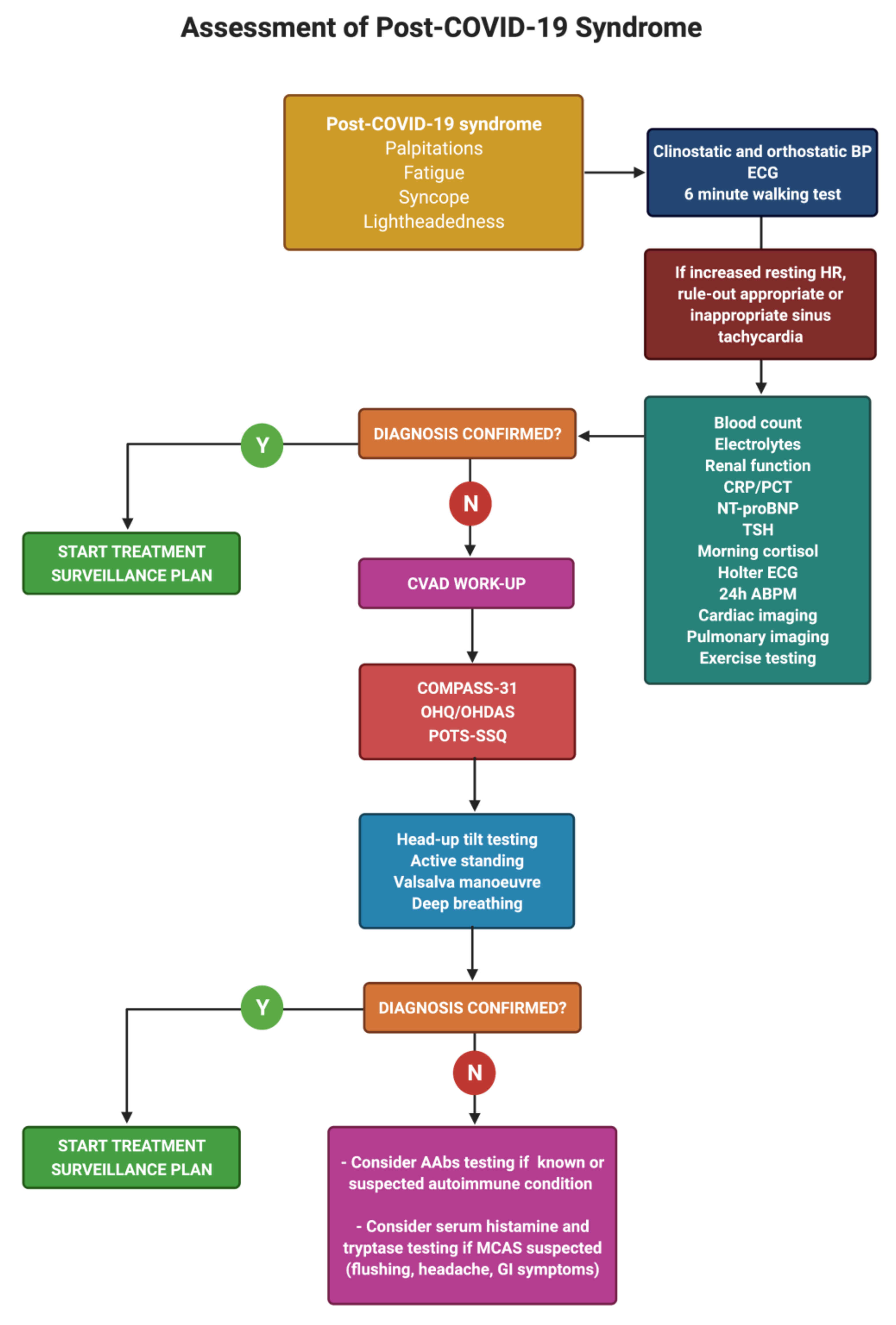
7. Diagnosis of Post-COVID Dysautonomia
8. Management of Post-COVID Dysautonomia
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. WHO COVID-19 Dashboard. 2020. Available online: https://covid19.who.int/ (accessed on 2 September 2021).
- Al-Aly, Z.; Xie, Y.; Bowe, B. High-dimensional characterization of post-acute sequalae of COVID-19. Nature 2021. [Google Scholar] [CrossRef]
- Rando, H.M.; Bennett, T.D.; Byrd, J.B.; Bramante, C.; Callahan, T.J.; Chute, C.G.; Davis, H.E.; Deer, R.; Gagnier, J.; Koraishy, F.M.; et al. Challenges in defining Long COVID: Striking differences across literature, Electronic Health Records, and patient-reported information. medRxiv 2021. [Google Scholar] [CrossRef]
- Raj, S.R.; Arnold, A.C.; Barboi, A.; Claydon, V.E.; Limberg, J.K.; Lucci, V.M.; Numan, M.; Peltier, A.; Snapper, H.; Vernino, S.; et al. Long-COVID postural tachycardia syndrome: An American Autonomic Society statement. Clin. Auton. Res. 2021. [Google Scholar] [CrossRef]
- Kalter, L.; WebMD Health News. Fauci Introduces New Acronym for Long COVID at White House Briefing. Medscape. 2021. Available online: https://www.medscape.com/viewarticle/946419 (accessed on 1 June 2021).
- Greenhalgh, T.; Knight, M.; A’Court, C.; Buxton, M.; Husain, L. Management of post-acute covid-19 in primary care. BMJ 2020, 370, m3026. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Carfi, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Davido, B.; Seang, S.; Tubiana, R.; de Truchis, P. Post-COVID-19 chronic symptoms: A postinfectious entity? Clin. Microbiol. Infect. 2020, 26, 1448–1449. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.; Stahlberg, M.; Runold, M.; Nygren-Bonnier, M.; Nilsson, J.; Olshansky, B.; Bruchfeld, J.; Fedorowski, A. Long-Haul Post-COVID-19 Symptoms Presenting as a Variant of Postural Orthostatic Tachycardia Syndrome: The Swedish Experience. JACC Case Rep. 2021. [Google Scholar] [CrossRef] [PubMed]
- Nath, A. Long-Haul COVID. Neurology 2020, 95, 559–560. [Google Scholar] [CrossRef]
- Clark, D.V.; Kibuuka, H.; Millard, M.; Wakabi, S.; Lukwago, L.; Taylor, A.; Eller, M.A.; Eller, L.A.; Michael, N.L.; Honko, A.N.; et al. Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: A retrospective cohort study. Lancet Infect. Dis. 2015, 15, 905–912. [Google Scholar] [CrossRef] [Green Version]
- Lo, Y.L. COVID-19, fatigue, and dysautonomia. J. Med. Virol. 2021, 93, 1213. [Google Scholar] [CrossRef]
- O’Sullivan, O. Long-term sequelae following previous coronavirus epidemics. Clin. Med. 2021, 21, e68–e70. [Google Scholar] [CrossRef]
- Cheng, D.; Calderwood, C.; Skyllberg, E.; Ainley, A. Clinical characteristics and outcomes of adult patients admitted with COVID-19 in East London: A retrospective cohort analysis. BMJ Open Respir. Res. 2021, 8, e000813. [Google Scholar] [CrossRef]
- Dani, M.; Dirksen, A.; Taraborrelli, P.; Torocastro, M.; Panagopoulos, D.; Sutton, R.; Lim, P.B. Autonomic dysfunction in ‘long COVID’: Rationale, physiology and management strategies. Clin. Med. 2021, 21, e63–e67. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F. Case report and systematic review suggest that children may experience similar long-term effects to adults after clinical COVID-19. Acta Paediatr. 2021, 110, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Ostergaard, L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: Consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol. Rep. 2021, 9, e14726. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Cholevas, C.; Polyzoidis, K.; Politis, A. Long-COVID syndrome-associated brain fog and chemofog: Luteolin to the rescue. Biofactors 2021, 47, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bollettini, I.; Melloni, E.M.T.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020, 89, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Dennis, A.; Wamil, M.; Alberts, J.; Oben, J.; Cuthbertson, D.J.; Wootton, D.; Crooks, M.; Gabbay, M.; Brady, M.; Hishmeh, L.; et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: A prospective, community-based study. BMJ Open 2021, 11, e048391. [Google Scholar] [CrossRef]
- Osikomaiya, B.; Erinoso, O.; Wright, K.O.; Odusola, A.O.; Thomas, B.; Adeyemi, O.; Bowale, A.; Adejumo, O.; Falana, A.; Abdus-Salam, I.; et al. ‘Long COVID’: Persistent COVID-19 symptoms in survivors managed in Lagos State, Nigeria. BMC Infect. Dis. 2021, 21, 304. [Google Scholar] [CrossRef]
- Peluso, M.J.; Kelly, J.D.; Lu, S.; Goldberg, S.A.; Davidson, M.C.; Mathur, S.; Durstenfeld, M.S.; Spinelli, M.A.; Hoh, R.; Tai, V.; et al. Rapid implementation of a cohort for the study of post-acute sequelae of SARS-CoV-2 infection/COVID-19. medRxiv 2021. [Google Scholar] [CrossRef]
- Perlis, R.H.; Green, J.; Santillana, M.; Lazer, D.; Ognyanova, K.; Simonson, M.; Baum, M.A.; Quintana, A.; Chwe, H.; Druckman, J.; et al. Persistence of symptoms up to 10 months following acute COVID-19 illness. medRxiv 2021. [Google Scholar] [CrossRef]
- Shouman, K.; Vanichkachorn, G.; Cheshire, W.P.; Suarez, M.D.; Shelly, S.; Lamotte, G.J.; Sandroni, P.; Benarroch, E.E.; Berini, S.E.; Cutsforth-Gregory, J.K.; et al. Autonomic dysfunction following COVID-19 infection: An early experience. Clin. Auton. Res. 2021. [Google Scholar] [CrossRef]
- Gaber, T.A.K.; Ashish, A.; Unsworth, A. Persistent post-covid symptoms in healthcare workers. Occup. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent fatigue following SARS-CoV-2 infection is common and independent of severity of initial infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef]
- Auwaerter, P.G. The Race to Understand Post–COVID-19 Conditions. Ann. Intern. Med. 2021. [Google Scholar] [CrossRef]
- Petersen, M.S.; Kristiansen, M.F.; Hanusson, K.D.; Danielsen, M.E.; Gaini, S.; Strom, M.; Weihe, P. Long COVID in the Faroe Islands—a longitudinal study among non-hospitalized patients. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Moreno-Perez, O.; Merino, E.; Leon-Ramirez, J.M.; Andres, M.; Ramos, J.M.; Arenas-Jimenez, J.; Asensio, S.; Sanchez, R.; Ruiz-Torregrosa, P.; Galan, I.; et al. Post-acute COVID-19 syndrome. Incidence and risk factors: A Mediterranean cohort study. J. Infect. 2021, 82, 378–383. [Google Scholar] [CrossRef]
- Evans, R.A.; McAuley, H.; Harrison, E.M.; Shikotra, A.; Singapuri, A.; Sereno, M.; Elneima, O.; Docherty, A.B.; Lone, N.I.; Leavy, O.C.; et al. Physical, cognitive and mental health impacts of COVID-19 following hospitalisation—A multi-centre prospective cohort study. medRxiv 2021. [Google Scholar] [CrossRef]
- Halpin, S.J.; McIvor, C.; Whyatt, G.; Adams, A.; Harvey, O.; McLean, L.; Walshaw, C.; Kemp, S.; Corrado, J.; Singh, R.; et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J. Med. Virol. 2021, 93, 1013–1022. [Google Scholar] [CrossRef]
- Townsend, L.; Moloney, D.; Finucane, C.; McCarthy, K.; Bergin, C.; Bannan, C.; Kenny, R.-A. Fatigue following COVID-19 infection is not associated with autonomic dysfunction. PLoS ONE 2021, 16, e0247280. [Google Scholar] [CrossRef] [PubMed]
- van Campen, C.; Verheugt, F.W.A.; Rowe, P.C.; Visser, F.C. Cerebral blood flow is reduced in ME/CFS during head-up tilt testing even in the absence of hypotension or tachycardia: A quantitative, controlled study using Doppler echography. Clin. Neurophysiol. Pract. 2020, 5, 50–58. [Google Scholar] [CrossRef]
- Escorihuela, R.M.; Capdevila, L.; Castro, J.R.; Zaragozà, M.C.; Maurel, S.; Alegre, J.; Castro-Marrero, J. Reduced heart rate variability predicts fatigue severity in individuals with chronic fatigue syndrome/myalgic encephalomyelitis. J. Transl. Med. 2020, 18, 4. [Google Scholar] [CrossRef]
- Nelson, M.J.; Bahl, J.S.; Buckley, J.D.; Thomson, R.L.; Davison, K. Evidence of altered cardiac autonomic regulation in myalgic encephalomyelitis/chronic fatigue syndrome: A systematic review and meta-analysis. Medicine 2019, 98, e17600. [Google Scholar] [CrossRef]
- Kedor, C.; Freitag, H.; Meyer-Arndt, L.; Wittke, K.; Zoller, T.; Steinbeis, F.; Haffke, M.; Rudolf, G.; Heidecker, B.; Volk, H.; et al. Chronic COVID-19 Syndrome and Chronic Fatigue Syndrome (ME/CFS) following the first pandemic wave in Germany—a first analysis of a prospective observational study. medRxiv 2021. [Google Scholar] [CrossRef]
- Sigfrid, L.; Drake, T.M.; Pauley, E.; Jesudason, E.C.; Olliaro, P.; Lim, W.S.; Gillesen, A.; Berry, C.; Lowe, D.J.; McPeake, J.; et al. Long Covid in adults discharged from UK hospitals after COVID-19: A prospective, multicentre cohort study using the ISARIC WHO Clinical Characterisation Protocol. medRxiv 2021. [Google Scholar] [CrossRef]
- Ortona, E.; Buonsenso, D.; Carfi, A.; Malorni, W.; The Long Covid Kids study group. Long COVID: An estrogen-associated autoimmune disease? Cell Death Discov. 2021, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Funk, A.L.; Florin, T.A.; Dalziel, S.R.; Mintegi, S.; Salvadori, M.I.; Tancredi, D.J.; Neuman, M.I.; Payne, D.C.; Plint, A.C.; Klassen, T.P.; et al. Prospective cohort study of children with suspected SARS-CoV-2 infection presenting to paediatric emergency departments: A Paediatric Emergency Research Networks (PERN) Study Protocol. BMJ Open 2021, 11, e042121. [Google Scholar] [CrossRef] [PubMed]
- Petracek, L.S.; Suskauer, S.J.; Vickers, R.F.; Patel, N.R.; Violand, R.L.; Swope, R.L.; Rowe, P.C. Adolescent and Young Adult ME/CFS After Confirmed or Probable COVID-19. Front. Med. 2021, 8, 525. [Google Scholar] [CrossRef]
- Stephenson, T.; Stephenson, T.; Pereira, S.P.; Shafran, R.; De Stavola, B.; Rojas, N.; McOwat, K.; Simmons, R.; Zavala, M.; O’Mahoney, L.; et al. Long COVID—The physical and mental health of children and non-hospitalised young people 3 months after SARS-CoV-2 infection; a national matched cohort study (The CLoCk) Study. Nat. Portf. 2021. [Google Scholar] [CrossRef]
- Ghosh, R.; Roy, D.; Sengupta, S.; Benito-Leon, J. Autonomic dysfunction heralding acute motor axonal neuropathy in COVID-19. J. Neurovirol. 2020, 26, 964–966. [Google Scholar] [CrossRef] [PubMed]
- Kanjwal, K.; Jamal, S.; Kichloo, A.; Grubb, B.P. New-onset Postural Orthostatic Tachycardia Syndrome Following Coronavirus Disease 2019 Infection. J. Innov. Card Rhythm. Manag. 2020, 11, 4302–4304. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Escobar, M.C.; Kataria, S.; Khan, E.; Subedi, R.; Tandon, M.; Peshwe, K.; Kramer, J.; Niaze, F.; Sriwastava, S. Acute transverse myelitis with Dysautonomia following SARS-CoV-2 infection: A case report and review of literature. J. Neuroimmunol. 2021, 353, 577523. [Google Scholar] [CrossRef]
- Umapathi, T.; Poh, M.Q.W.; Fan, B.E.; Li, K.F.C.; George, J.; Tan, J.Y.L. Acute hyperhidrosis and postural tachycardia in a COVID-19 patient. Clin. Auton. Res. 2020, 30, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Loa, Y.L.; Leonga, H.N.; Hsua, L.Y.; Tana, T.T.; Kurupa, A.; Fook-Chonga, S.; Tana, B.H. Autonomic Dysfunction in Recovered Severe Acute Respiratory Syndrome Patients. Can. J. Neurol. Sci. 2005, 32, 264. [Google Scholar] [CrossRef] [Green Version]
- Ståhlberg, M.; Reistam, U.; Fedorowski, A.; Villacorta, H.; Horiuchi, Y.; Bax, J.; Pitt, B.; Matskeplishvili, S.; Lüscher, T.F.; Weichert, I.; et al. Post-COVID-19 Tachycardia Syndrome: A distinct phenotype of Post-acute COVID-19 Syndrome. Am. J. Med. 2021. [Google Scholar] [CrossRef]
- Fedorowski, A. Postural orthostatic tachycardia syndrome: Clinical presentation, aetiology and management. J. Intern. Med. 2019, 285, 352–366. [Google Scholar] [CrossRef]
- Miglis, M.G.; Prieto, T.; Shaik, R.; Muppidi, S.; Sinn, D.I.; Jaradeh, S. A case report of postural tachycardia syndrome after COVID-19. Clin. Auton. Res. 2020, 30, 449–451. [Google Scholar] [CrossRef]
- Novak, P. Post COVID-19 syndrome associated with orthostatic cerebral hypoperfusion syndrome, small fiber neuropathy and benefit of immunotherapy: A case report. eNeurologicalSci 2020, 21, 100276. [Google Scholar] [CrossRef]
- Schofield, J.R. Persistent Antiphospholipid Antibodies, Mast Cell Activation Syndrome, Postural Orthostatic Tachycardia Syndrome and Post-COVID Syndrome: 1 Year On. Eur. J. Case Rep. Intern. Med. 2021, 8, 002378. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing Long COVID in an International Cohort: 7 Months of Symptoms and Their Impact. medRxiv 2021. [Google Scholar] [CrossRef]
- Owens, A.P.; Low, D.A.; Iodice, V.; Critchley, H.D.; Mathias, C.J. The genesis and presentation of anxiety in disorders of autonomic overexcitation. Auton. Neurosci. 2017, 203, 81–87. [Google Scholar] [CrossRef]
- Blitshteyn, S. Is postural orthostatic tachycardia syndrome (POTS) a central nervous system disorder? J. Neurol. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wallukat, G.; Hohberger, B.; Wenzel, K.; Fürst, J.; Schulze-Rothe, S.; Wallukat, A.; Hönicke, A.S.; Müller, J. Functional autoantibodies against G-protein coupled receptors in patients with persistent post-COVID-19 symptoms. J. Transl. Autoimmun. 2021, 4, 100100. [Google Scholar] [CrossRef] [PubMed]
- Goodman, B.P.; Khoury, J.A.; Blair, J.E.; Grill, M.F. COVID-19 Dysautonomia. Front. Neurol. 2021, 12, 624968. [Google Scholar] [CrossRef]
- Kaliyaperumal, D.; Rk, K.; Alagesan, M.; Ramalingam, S. Characterization of cardiac autonomic function in COVID-19 using heart rate variability: A hospital based preliminary observational study. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 247–253. [Google Scholar] [CrossRef]
- Romero-Sanchez, C.M.; Diaz-Maroto, I.; Fernandez-Diaz, E.; Sanchez-Larsen, A.; Layos-Romero, A.; Garcia-Garcia, J.; Gonzalez, E.; Redondo-Penas, I.; Perona-Moratalla, A.B.; Del Valle-Perez, J.A.; et al. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology 2020, 95, e1060–e1070. [Google Scholar] [CrossRef]
- Logmin, K.; Karam, M.; Schichel, T.; Harmel, J.; Wojtecki, L. Non-epileptic seizures in autonomic dysfunction as the initial symptom of COVID-19. J. Neurol. 2020, 267, 2490–2491. [Google Scholar] [CrossRef]
- Eshak, N.; Abdelnabi, M.; Ball, S.; Elgwairi, E.; Creed, K.; Test, V.; Nugent, K. Dysautonomia: An Overlooked Neurological Manifestation in a Critically ill COVID-19 Patient. Am. J. Med. Sci. 2020, 360, 427–429. [Google Scholar] [CrossRef]
- Shaw, B.H.; Stiles, L.E.; Bourne, K.; Green, E.A.; Shibao, C.A.; Okamoto, L.E.; Garland, E.M.; Gamboa, A.; Diedrich, A.; Raj, V.; et al. The face of postural tachycardia syndrome—Insights from a large cross-sectional online community-based survey. J. Intern. Med. 2019, 286, 438–448. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, D.S. The possible association between COVID-19 and postural tachycardia syndrome. Heart Rhythm 2021, 18, 508–509. [Google Scholar] [CrossRef] [PubMed]
- Bryarly, M.; Phillips, L.T.; Fu, Q.; Vernino, S.; Levine, B.D. Postural Orthostatic Tachycardia Syndrome: JACC Focus Seminar. J. Am. Coll. Cardiol. 2019, 73, 1207–1228. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.J. Persistent Brainstem Dysfunction in Long-COVID: A Hypothesis. ACS Chem. Neurosci. 2021, 12, 573–580. [Google Scholar] [CrossRef]
- Lionetti, V.; Bollini, S.; Coppini, R.; Gerbino, A.; Ghigo, A.; Iaccarino, G.; Madonna, R.; Mangiacapra, F.; Miragoli, M.; Moccia, F.; et al. Understanding the heart-brain axis response in COVID-19 patients: A suggestive perspective for therapeutic development. Pharmacol. Res. 2021, 168, 105581. [Google Scholar] [CrossRef]
- Barnden, L.R.; Crouch, B.; Kwiatek, R.; Burnet, R.; Mernone, A.; Chryssidis, S.; Scroop, G.; Del Fante, P. A brain MRI study of chronic fatigue syndrome: Evidence of brainstem dysfunction and altered homeostasis. NMR Biomed. 2011, 24, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Tadic, M.; Larsen, T.H.; Grassi, G.; Mancia, G. Coronavirus disease 2019 and cardiovascular complications: Focused clinical review. J. Hypertens. 2021, 39, 1282–1292. [Google Scholar] [CrossRef]
- Barbieri, L.; Galli, F.; Conconi, B.; Gregorini, T.; Lucreziotti, S.; Mafrici, A.; Pravettoni, G.; Sommaruga, M.; Carugo, S. Takotsubo syndrome in COVID-19 era: Is psychological distress the key? J. Psychosom. Res. 2021, 140, 110297. [Google Scholar] [CrossRef]
- Kir, D.; Beer, N.; De Marchena, E.J. Takotsubo cardiomyopathy caused by emotional stressors in the coronavirus disease 2019 (COVID-19) pandemic era. J. Card. Surg. 2021, 36, 764–769. [Google Scholar] [CrossRef]
- Suzuki, H.; Yasuda, S.; Shimokawa, H. Brain-heart connection in Takotsubo syndrome before onset. Eur. Heart J. 2021, 42, 1909–1911. [Google Scholar] [CrossRef]
- Vernino, S.; Stiles, L.E. Autoimmunity in postural orthostatic tachycardia syndrome: Current understanding. Auton. Neurosci. 2018, 215, 78–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharraziha, I.; Axelsson, J.; Ricci, F.; Martino, G.D.; Persson, M.; Sutton, R.; Fedorowski, A.; Hamrefors, V. Serum Activity Against G Protein-Coupled Receptors and Severity of Orthostatic Symptoms in Postural Orthostatic Tachycardia Syndrome. J. Am. Heart Assoc. 2020, 9, e015989. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.; Forsythe, E.; Okamoto, L.E.; Yu, X. Implications of Antimuscarinic Autoantibodies in Postural Tachycardia Syndrome. J. Cardiovasc. Transl. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Molina, V.; Shoenfeld, Y. Infection, vaccines and other environmental triggers of autoimmunity. Autoimmunity 2005, 38, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Dotan, A.; Muller, S.; Kanduc, D.; David, P.; Halpert, G.; Shoenfeld, Y. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun. Rev. 2021, 20, 102792. [Google Scholar] [CrossRef] [PubMed]
- Kohno, R.; Cannom, D.S.; Olshansky, B.; Xi, S.C.; Krishnappa, D.; Adkisson, W.O.; Norby, F.L.; Fedorowski, A.; Benditt, D.G. Mast Cell Activation Disorder and Postural Orthostatic Tachycardia Syndrome: A Clinical Association. J. Am. Heart Assoc. 2021, e021002. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Khunti, K.; Nafilyan, V.; Maddox, T.; Humberstone, B.; Diamond, I.; Banerjee, A. Post-covid syndrome in individuals admitted to hospital with covid-19: Retrospective cohort study. BMJ 2021, 372, n693. [Google Scholar] [CrossRef]
- Torjesen, I. COVID-19: Middle aged women face greater risk of debilitating long term symptoms. BMJ 2021, 372, n829. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, S.; Shokry, D.; Gomaa, S.M. Post-COVID-19 fatigue and anhedonia: A cross-sectional study and their correlation to post-recovery period. Neuropsychopharmacol. Rep. 2021, 41, 50–55. [Google Scholar] [CrossRef]
- Blitshteyn, S.; Whitelaw, S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: A case series of 20 patients. Immunol. Res. 2021, 69, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.J.; Cheshire, W.P.; Claydon, V.E.; Norcliffe-Kaufmann, L.; Peltier, A.; Singer, W.; Snapper, H.; Vernino, S.; Raj, S.R. Autonomic function testing in the COVID-19 pandemic: An American Autonomic Society position statement. Clin. Auton. Res. 2020, 30, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Moya, A.; de Lange, F.J.; Deharo, J.-C.; Elliott, P.M.; Fanciulli, A.; Fedorowski, A.; Furlan, R.; Kenny, R.A.; Martín, A.; et al. Practical Instructions for the 2018 ESC Guidelines for the diagnosis and management of syncope. Eur. Heart J. 2018, 39, e43–e80. [Google Scholar] [CrossRef]
- Sletten, D.M.; Suarez, G.A.; Low, P.A.; Mandrekar, J.; Singer, W. COMPASS 31: A refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin. Proc. 2012, 87, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Buoite Stella, A.; Furlanis, G.; Frezza, N.A.; Valentinotti, R.; Ajcevic, M.; Manganotti, P. Autonomic dysfunction in post-COVID patients with and witfhout neurological symptoms: A prospective multidomain observational study. J. Neurol. 2021. [Google Scholar] [CrossRef]
- Kim, Y.; Seok, J.; Park, J.; Kim, K.-H.; Min, J.-H.; Cho, J.W.; Park, S.; Kim, H.-J.; Kim, B.; Youn, J. The composite autonomic symptom scale 31 is a useful screening tool for patients with Parkinsonism. PLoS ONE 2017, 12, e0180744. [Google Scholar] [CrossRef] [Green Version]
- Raj, S.R.; Guzman, J.C.; Harvey, P.; Richer, L.; Schondorf, R.; Seifer, C.; Thibodeau-Jarry, N.; Sheldon, R.S. Canadian Cardiovascular Society Position Statement on Postural Orthostatic Tachycardia Syndrome (POTS) and Related Disorders of Chronic Orthostatic Intolerance. Can. J. Cardiol. 2020, 36, 357–372. [Google Scholar] [CrossRef] [Green Version]
- Thijs, R.D.; Brignole, M.; Falup-Pecurariu, C.; Fanciulli, A.; Freeman, R.; Guaraldi, P.; Jordan, J.; Habek, M.; Hilz, M.; Traon, A.P.; et al. Recommendations for tilt table testing and other provocative cardiovascular autonomic tests in conditions that may cause transient loss of consciousness: Consensus statement of the European Federation of Autonomic Societies (EFAS) endorsed by the American Autonomic Society (AAS) and the European Academy of Neurology (EAN). Clin. Auton. Res. 2021. [Google Scholar] [CrossRef]
- Hinduja, A.; Moutairou, A.; Calvet, J.H. Sudomotor dysfunction in patients recovered from COVID-19. Neurophysiol. Clin. 2021, 51, 193–196. [Google Scholar] [CrossRef]
- Ricci, F.; De Caterina, R.; Fedorowski, A. Orthostatic Hypotension: Epidemiology, Prognosis, and Treatment. J. Am. Coll. Cardiol. 2015, 66, 848–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente-Moragón, A.; Martínez-Milla, J.; Oliver, E.; Santos, A.; Flandes, J.; Fernández, I.; Rodríguez-González, L.; Serrano del Castillo, C.; Ioan, A.-M.; López-Álvarez, M.; et al. Metoprolol in Critically Ill Patients With COVID-19. J. Am. Coll. Cardiol. 2021, 78, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Moragon, A.; Gomez, M.; Villena-Gutierrez, R.; Lalama, D.V.; Garcia-Prieto, J.; Martinez, F.; Sanchez-Cabo, F.; Fuster, V.; Oliver, E.; Ibanez, B. Metoprolol exerts a non-class effect against ischaemia-reperfusion injury by abrogating exacerbated inflammation. Eur. Heart J. 2020, 41, 4425–4440. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, Y.; Liao, Y.; Du, J. Efficacy of β-Blockers on Postural Tachycardia Syndrome in Children and Adolescents: A Systematic Review and Meta-Analysis. Front. Pediatr. 2019, 7, 460. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Authors, Year | Miglis et al. [50], 2020 | Kanjwal et al. [44], 2020 | Novak et al. [51], 2020 | Umapathi et al. [46], 2020 | Schofield et al. [52], 2021 |
|---|---|---|---|---|---|
| Follow-up (months) | 5.5 | 1 | 3 | 2.5 | 12 |
| Mean age (years) | 26 | 36 | 64 | 39 | 50 |
| Sex | F | F | F | M | F |
| Diagnosis | POTS, OH | POTS | OCHOS | POTS | POTS |
| Palpitations, tachycardia | Yes | Yes | - | Yes | - |
| Fatigue | Yes | Yes | Yes | - | - |
| Vertigo a | Yes | Yes | - | - | - |
| Dyspnea | Yes | - | - | - | Yes |
| Presyncope | Yes | - | - | - | Yes |
| Chest pain | Yes | Yes | - | - | Yes |
| Headache | - | Yes | Yes | - | - |
| Brain fog b | Yes | - | Yes | - | - |
| Sleep disturbances | Yes | - | - | - | - |
| Study Features | Autonomic Testing Modality | Treatment Strategy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors, year | Study type | HUT | Active standing | Deep breathing | Valsalva | Sudomotor test | Holter monitoring | NPT a | Volume expanders b | HR Inhibitors c | Sympatholytic Drugs d | IT e |
| Miglis et al. [61], 2020 | Report | Yes | - | - | Yes | - | - | - | - | Yes | Yes | - |
| Kanjwal et al. [42], 2020 | Report | Yes | - | - | - | - | - | Yes | - | Yes | - | - |
| Novak et al. [73], 2020 | Report | Yes | - | Yes | Yes | Yes | - | - | - | - | - | Yes |
| Umapathi et al. [44], 2020 | Report | Yes | Yes | - | - | - | - | Yes | Yes | Yes | - | - |
| Schofield et al. [63], 2021 | Report | - | Yes | - | - | - | - | N.A. | ||||
| Dani et al. [16], 2021 | Series | Yes | Yes | - | - | - | Yes | N.A. | ||||
| Townsend et al. [32], 2021 | Series | - | Yes | Yes | Yes | Yes | Yes | N.A. | ||||
| Johansson et al. [10], 2021 | Series | Yes | Yes | - | Yes | - | - | Yes | - | Yes | - | - |
| Blitshteyn et al. [77], 2021 | Series | Yes | Yes | - | - | - | - | Yes | Yes | - | - | - |
| Shouman et al. [25], 2021 | Series | Yes | - | - | Yes | Yes | - | Yes | - | Yes | - | - |
| Goodman et al. [80], 2021 | Series | Yes | - | Yes | Yes | Yes | - | N.A. | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bisaccia, G.; Ricci, F.; Recce, V.; Serio, A.; Iannetti, G.; Chahal, A.A.; Ståhlberg, M.; Khanji, M.Y.; Fedorowski, A.; Gallina, S. Post-Acute Sequelae of COVID-19 and Cardiovascular Autonomic Dysfunction: What Do We Know? J. Cardiovasc. Dev. Dis. 2021, 8, 156. https://doi.org/10.3390/jcdd8110156
Bisaccia G, Ricci F, Recce V, Serio A, Iannetti G, Chahal AA, Ståhlberg M, Khanji MY, Fedorowski A, Gallina S. Post-Acute Sequelae of COVID-19 and Cardiovascular Autonomic Dysfunction: What Do We Know? Journal of Cardiovascular Development and Disease. 2021; 8(11):156. https://doi.org/10.3390/jcdd8110156
Chicago/Turabian StyleBisaccia, Giandomenico, Fabrizio Ricci, Vittoria Recce, Antonio Serio, Giovanni Iannetti, Anwar A. Chahal, Marcus Ståhlberg, Mohammed Yunus Khanji, Artur Fedorowski, and Sabina Gallina. 2021. "Post-Acute Sequelae of COVID-19 and Cardiovascular Autonomic Dysfunction: What Do We Know?" Journal of Cardiovascular Development and Disease 8, no. 11: 156. https://doi.org/10.3390/jcdd8110156
APA StyleBisaccia, G., Ricci, F., Recce, V., Serio, A., Iannetti, G., Chahal, A. A., Ståhlberg, M., Khanji, M. Y., Fedorowski, A., & Gallina, S. (2021). Post-Acute Sequelae of COVID-19 and Cardiovascular Autonomic Dysfunction: What Do We Know? Journal of Cardiovascular Development and Disease, 8(11), 156. https://doi.org/10.3390/jcdd8110156








