The Role of 2D and 3D Echo in Mitral Stenosis
Abstract
:1. Introduction
2. Rheumatic Mitral Stenosis
2.1. Morphological Findings
- Commissural fusion with valvular thickening, particularly at the free edge of the leaflets. This is usually best seen in the parasternal short-axis view. Later, thickening extends towards the base with further restriction of valve motion.
- Reduced leaflet mobility and restricted opening. The posterior leaflet is usually partially or completely immobile and the anterior leaflet shows a diastolic doming, producing the characteristic “hockey stick” configuration. This is usually best seen in the parasternal long-axis view.
- Affection of the subvalvular apparatus with thickened, fused, and/or shortened chords.
- Calcification of the leaflets and the subvalvular apparatus.
2.2. Evaluation of the Degree of Mitral Stenosis
2.2.1. Mitral Valve Area
Planimetry
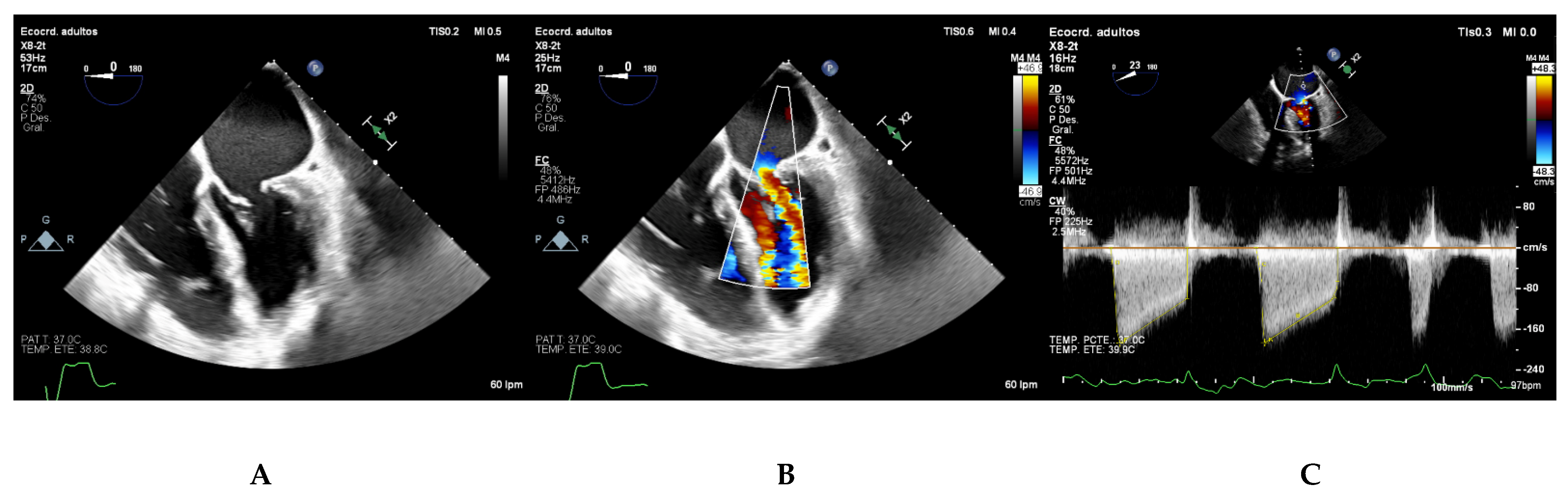
Pressure Half-Time (PHT)
Proximal Isovelocity Surface Area (PISA)
Volumetric Method
2.2.2. Mitral Gradients
2.2.3. Additional Parameters
Left Atrium
Pulmonary Artery Systolic Pressure (PSP)
3. Degenerative Mitral Stenosis
4. Congenital Mitral Stenosis
5. Two-Dimensional Transthoracic Echocardiography Assessment
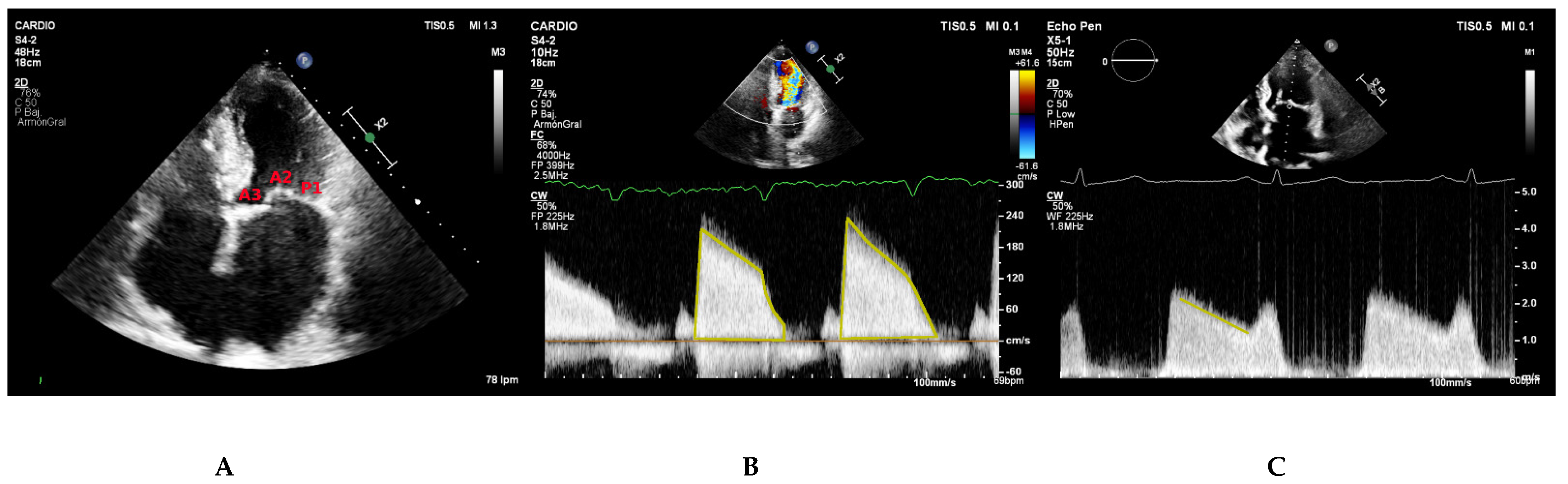
5.1. Parasternal Long-Axis View
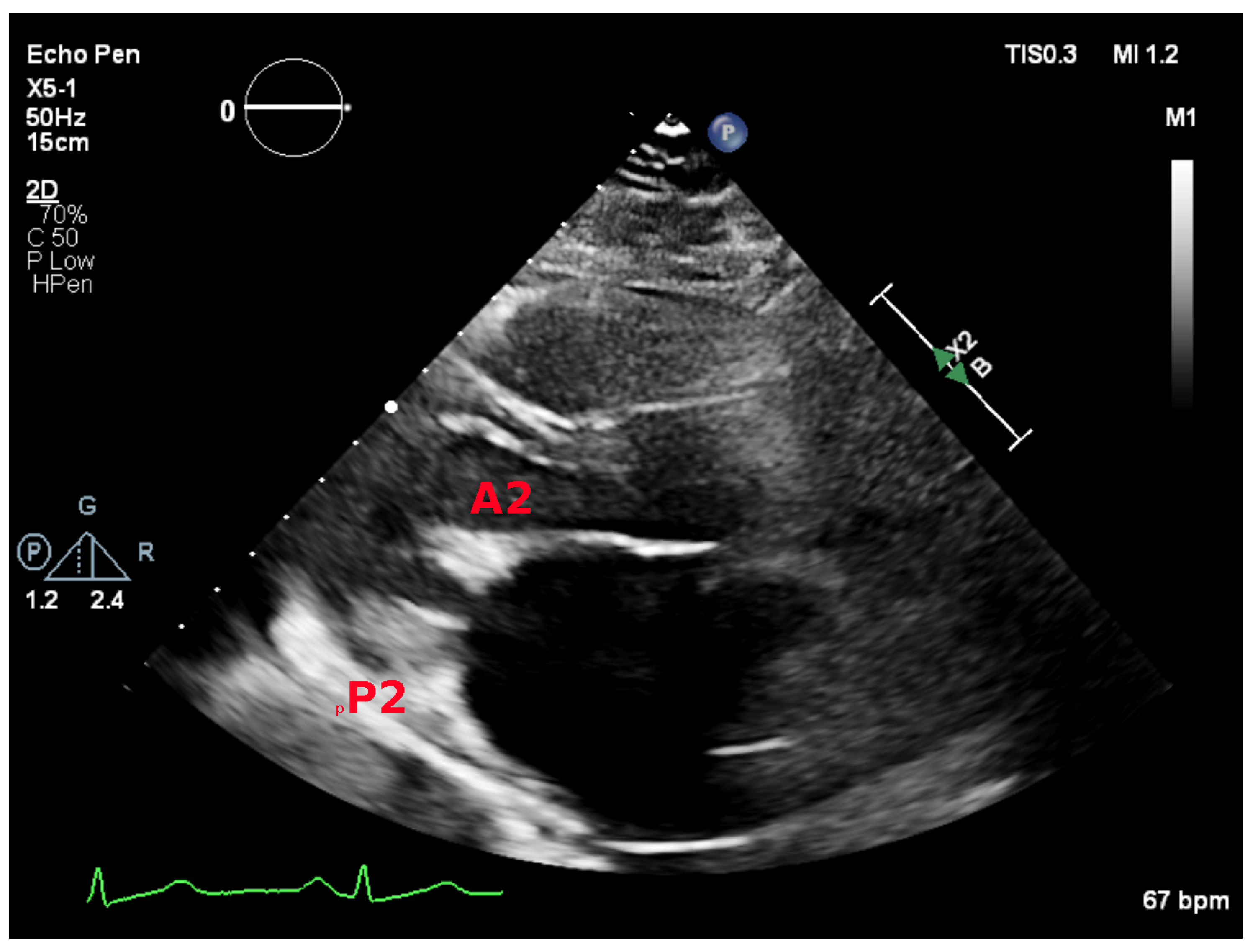
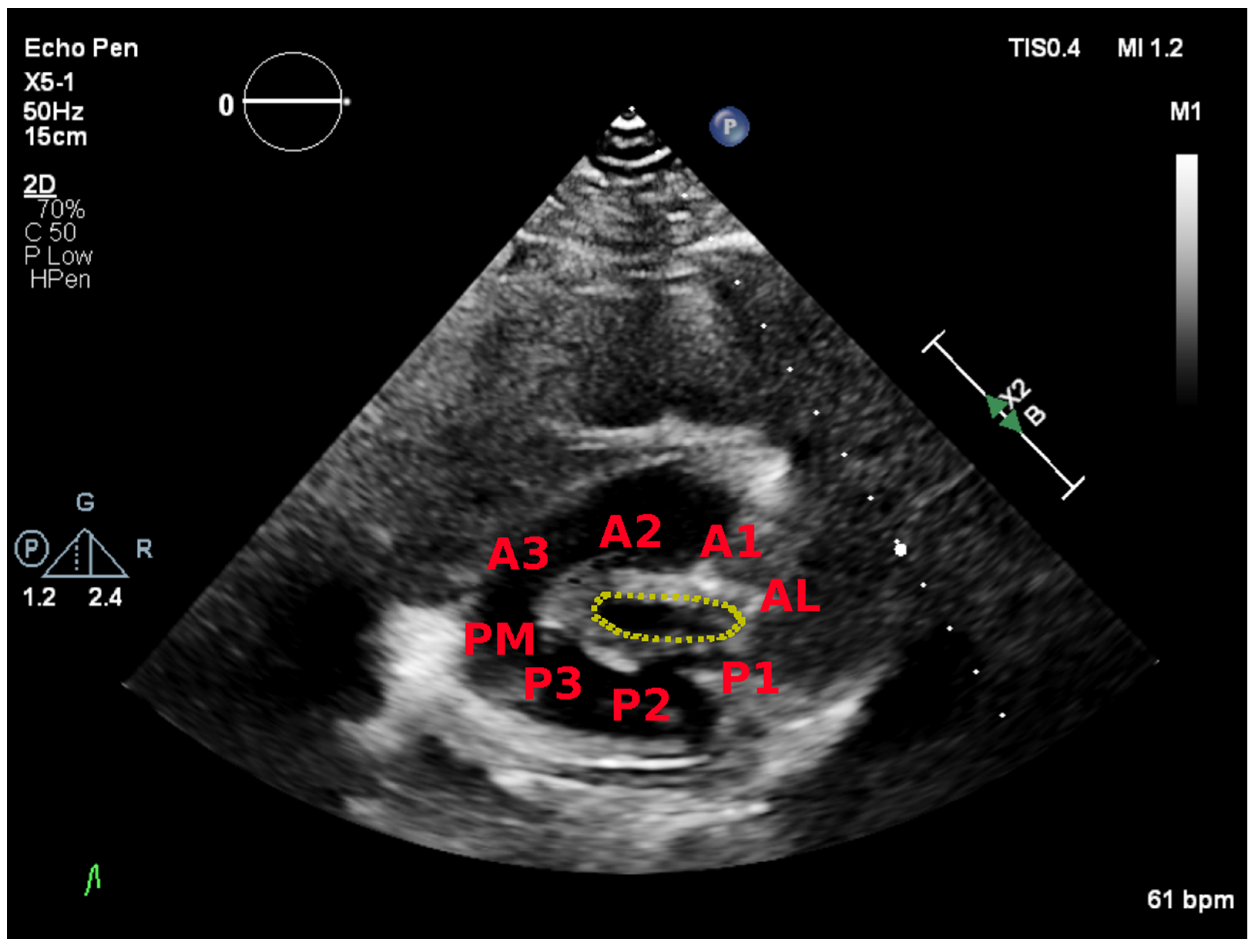
5.2. Parasternal Short-Axis View
5.3. Apical Four Chamber View
5.4. Classification of Mitral Stenosis Severity
5.5. Assessment of Feasibility of Percutaneous Mitral Commissurotomy
6. Two-Dimensional Transesophageal Echocardiography Assessment
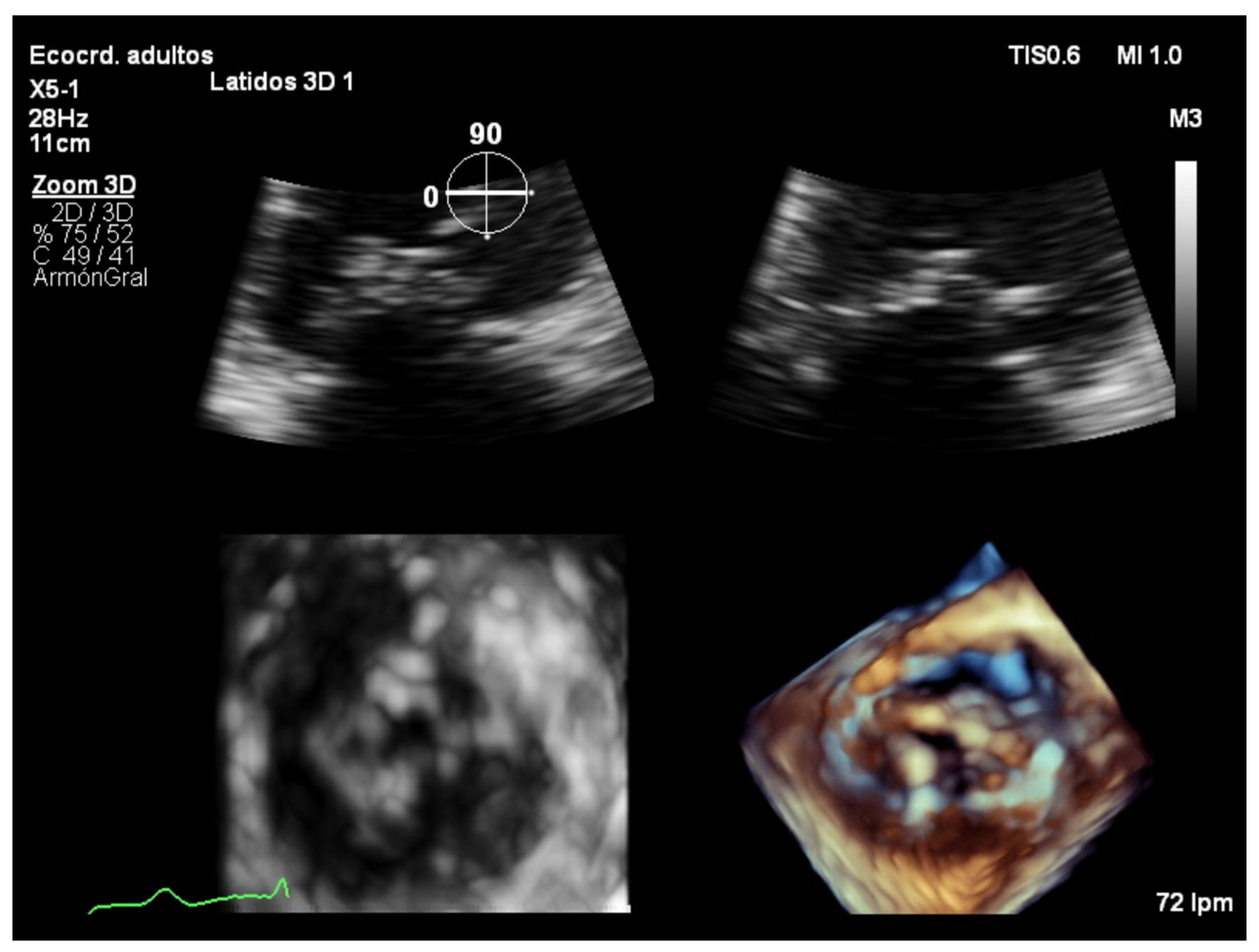
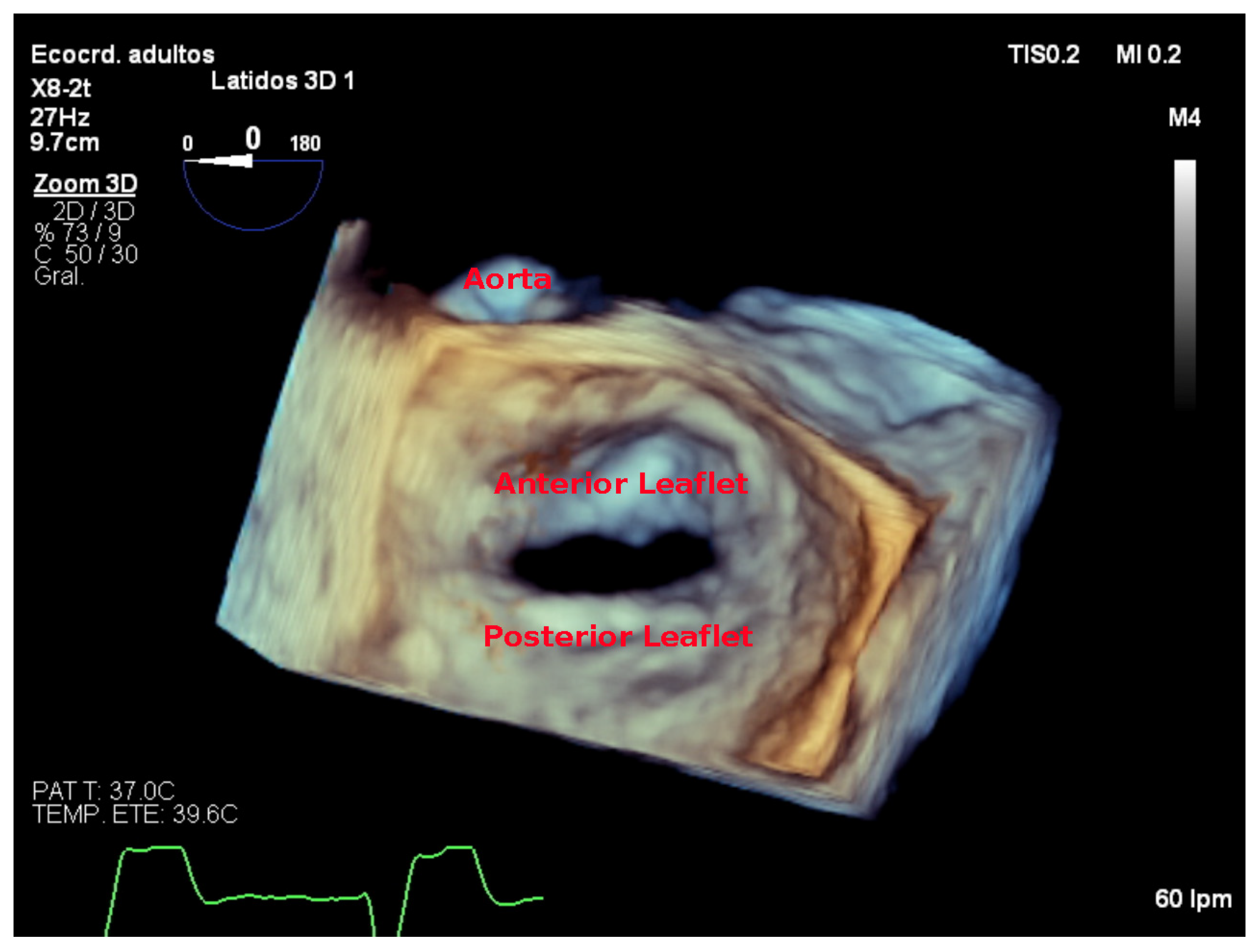
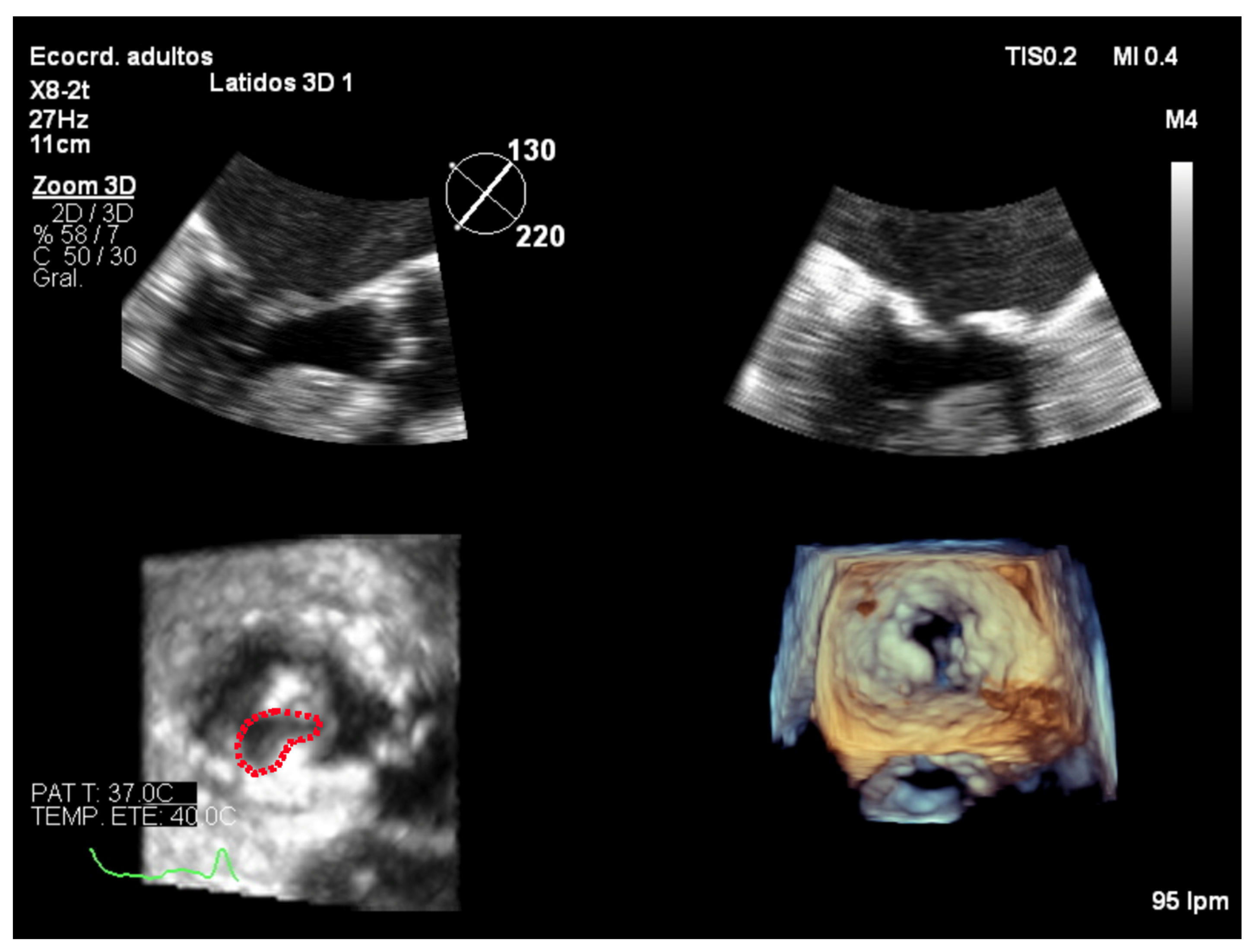
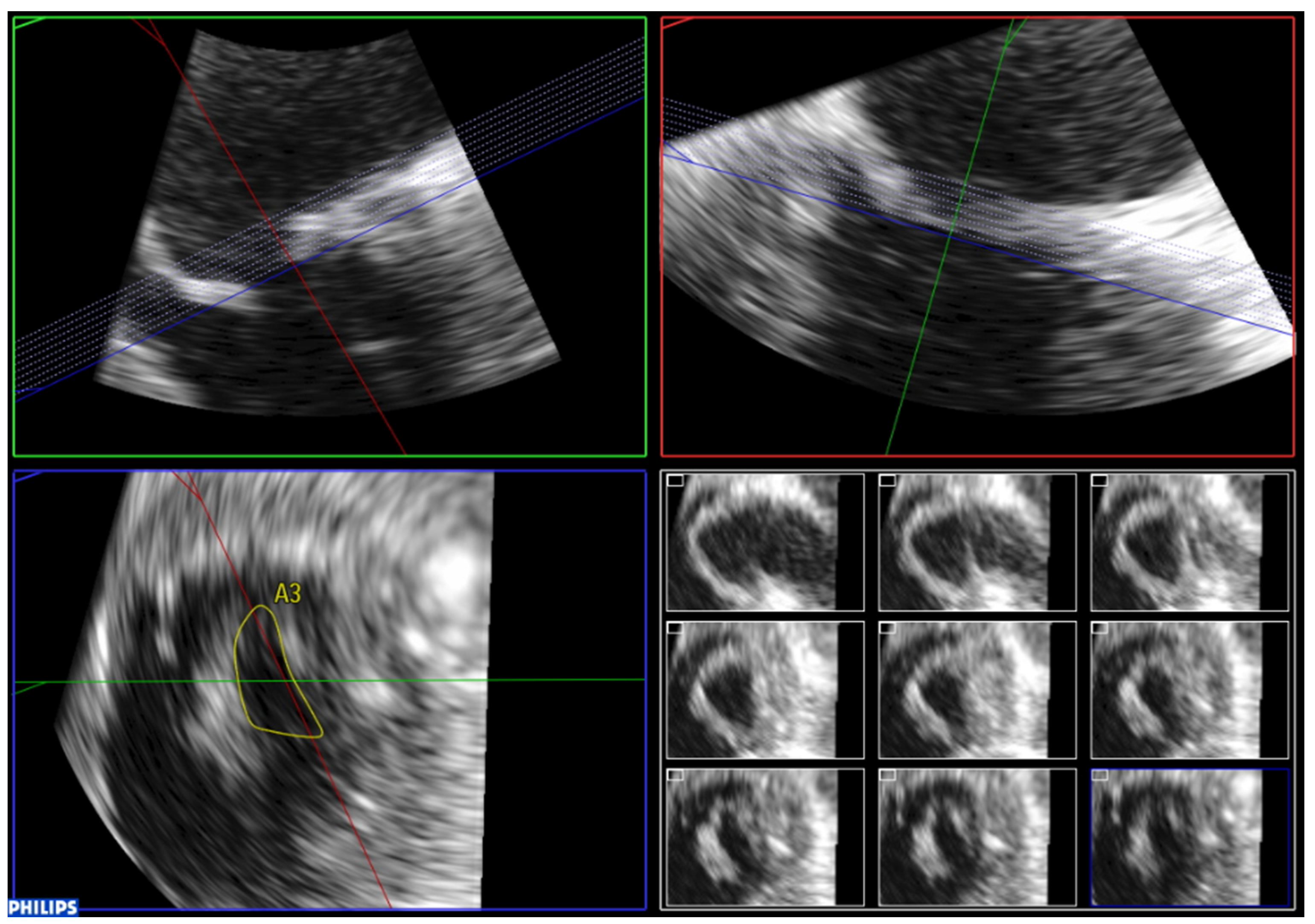
7. Three-Dimensional Echocardiography Assessment
- It measures all three spatial dimensions. Therefore, it is not reliant on plane positioning and does not require geometric assumptions of cardiac structures.
- Images can be rotated and viewed from different perspectives. This allows for a better understanding of the relationship between structures and makes 3D images more intuitive.
7.1. Simultaneous Biplane Imaging
7.2. Full Volume Imaging
7.3. Real-Time 3D Imaging
7.4. Focused Wide-Sector (3D Zoom) Imaging
7.5. Assessment of Feasibility of Percutaneous Mitral Commissurotomy Using 3D Echocardiography
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Seckeler, M.D.; Hoke, T.R. The Worldwide Epidemiology of Acute Rheumatic Fever and Rheumatic Heart Disease. Clin. Epidemiol. 2011, 3, 67–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abramowitz, Y.; Jilaihawi, H.; Chakravarty, T.; Mack, M.J.; Makkar, R.R. Mitral Annulus Calcification. J. Am. Coll. Cardiol. 2015, 66, 1934–1941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andell, P.; Li, X.; Martinsson, A.; Andersson, C.; Stagmo, M.; Zöller, B.; Sundquist, K.; Smith, J.G. Epidemiology of Valvular Heart Disease in a Swedish Nationwide Hospital-Based Register Study. Heart 2017, 103, 1696–1703. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2021, 60, 727–800. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef]
- Garbi, M.; Monaghan, M.J. Quantitative Mitral Valve Anatomy and Pathology. Echo. Res. Pract. 2015, 2, R63–R72. [Google Scholar] [CrossRef] [Green Version]
- Lancellotti, P.; Moura, L.; Pierard, L.A.; Agricola, E.; Popescu, B.A.; Tribouilloy, C.; Hagendorff, A.; Monin, J.-L.; Badano, L.; Zamorano, J.L.; et al. European Association of Echocardiography Recommendations for the Assessment of Valvular Regurgitation. Part 2: Mitral and Tricuspid Regurgitation (Native Valve Disease). Eur. J. Echocardiogr. 2010, 11, 307–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faletra, F.; Pezzano, A.; Fusco, R.; Mantero, A.; Corno, R.; Crivellaro, W.; De Chiara, F.; Vitali, E.; Gordini, V.; Magnani, P.; et al. Measurement of Mitral Valve Area in Mitral Stenosis: Four Echocardiographic Methods Compared with Direct Measurement of Anatomic Orifices. J. Am. Coll. Cardiol. 1996, 28, 1190–1197. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, M.A.; Child, J.S.; Krivokapich, J. Comparison of Two-Dimensional and Doppler Echocardiography and Intracardiac Hemodynamics for Quantification of Mitral Stenosis. Am. J. Cardiol. 1987, 60, 327–332. [Google Scholar] [CrossRef]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Evangelista, A.; Griffin, B.P.; Iung, B.; Otto, C.M.; Pellikka, P.A.; Quiñones, M.; et al. Echocardiographic Assessment of Valve Stenosis: EAE/ASE Recommendations for Clinical Practice. Eur. J. Echocardiogr. 2009, 10, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Massera, D.; Kizer, J.R.; Dweck, M.R. Mechanisms of Mitral Annular Calcification. Trends Cardiovasc. Med. 2020, 30, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Abascal, V.M.; Moreno, P.R.; Rodriguez, L.; Monterroso, V.M.; Palacios, I.F.; Weyman, A.E.; Davidoff, R. Comparison of the Usefulness of Doppler Pressure Half-Time in Mitral Stenosis in Patients <65 and > or = 65 Years of Age. Am. J. Cardiol. 1996, 78, 1390–1393. [Google Scholar] [PubMed]
- Bertrand, P.B.; Churchill, T.W.; Yucel, E.; Namasivayam, M.; Bernard, S.; Nagata, Y.; He, W.; Andrews, C.T.; Picard, M.H.; Weyman, A.E.; et al. Prognostic Importance of the Transmitral Pressure Gradient in Mitral Annular Calcification with Associated Mitral Valve Dysfunction. Eur. Heart J. 2020, 41, 4321–4328. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Padang, R.; Scott, C.G.; Guerrero, M.; Pislaru, S.V.; Pellikka, P.A. The Natural History of Severe Calcific Mitral Stenosis. J. Am. Coll. Cardiol. 2020, 75, 3048–3057. [Google Scholar] [CrossRef]
- Silbiger, J.J. Mitral Annular Calcification and Calcific Mitral Stenosis: Role of Echocardiography in Hemodynamic Assessment and Management. J. Am. Soc. Echocardiogr. 2021, 34, 923–931. [Google Scholar] [CrossRef]
- Wilkins, G.T.; Weyman, A.E.; Abascal, V.M.; Block, P.C.; Palacios, I.F. Percutaneous Balloon Dilatation of the Mitral Valve: An Analysis of Echocardiographic Variables Related to Outcome and the Mechanism of Dilatation. Br. Heart J. 1988, 60, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Sugeng, L.; Coon, P.; Weinert, L.; Jolly, N.; Lammertin, G.; Bednarz, J.E.; Thiele, K.; Lang, R.M. Use of Real-Time 3-Dimensional Transthoracic Echocardiography in the Evaluation of Mitral Valve Disease. J. Am. Soc. Echocardiogr. 2006, 19, 413–421. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Tsang, W.; Adams, D.H.; Agricola, E.; Buck, T.; Faletra, F.F.; Franke, A.; Hung, J.; de Isla, L.P.; et al. EAE/ASE Recommendations for Image Acquisition and Display Using Three-Dimensional Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 1–46. [Google Scholar] [CrossRef]
- Zamorano, J.; Cordeiro, P.; Sugeng, L.; Perez de Isla, L.; Weinert, L.; Macaya, C.; Rodríguez, E.; Lang, R.M. Real-Time Three-Dimensional Echocardiography for Rheumatic Mitral Valve Stenosis Evaluation: An Accurate and Novel Approach. J. Am. Coll. Cardiol. 2004, 43, 2091–2096. [Google Scholar] [CrossRef]
- Sugeng, L.; Weinert, L.; Lammertin, G.; Thomas, P.; Spencer, K.T.; Decara, J.M.; Mor-Avi, V.; Huo, D.; Feldman, T.; Lang, R.M. Accuracy of Mitral Valve Area Measurements Using Transthoracic Rapid Freehand 3-Dimensional Scanning: Comparison with Noninvasive and Invasive Methods. J. Am. Soc. Echocardiogr. 2003, 16, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.M.; Attia, W.M.; Nosir, Y.F.M.; Soliman, O.I.I.; Mosad, M.A.; Othman, M.; Geleijnse, M.L.; El-Amin, A.M.; Ten Cate, F.J. Validation of a New Score for the Assessment of Mitral Stenosis Using Real-Time Three-Dimensional Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 13–22. [Google Scholar] [CrossRef] [PubMed]

| Mild | Moderate | Severe | |
|---|---|---|---|
| Specific Findings Mitral Valve Area (cm2) | >1.5 | 1.0–1.5 | <1.0 |
| Supportive Findings Mean Transmitral Gradient (mmHg) PSP (mmHg) | <5 <30 | 5–10 30–50 | >10 >50 |
| Grade | Mobility | Thickening | Subvalvular Thickening | Calcification |
|---|---|---|---|---|
| 1 | Highly mobile valve with only leaflet tips restricted | Leaflets near normal in thickness (4–5 mm) | Minimal thickening just below the mitral leaflets | A single area of increased echo brightness |
| 2 | Leaflet mid and base portions have normal mobility | Mid leaflets normal, considerable thickening of margins (5–8 mm) | Thickening of chordal structures extending to one third of the chordal length | Scattered areas of brightness confined to leaflet margins |
| 3 | Valve continues to move forward in diastole, mainly from the base | Thickening extending through the entire leaflet (5–8 mm) | Thickening extended to distal third of the chords | Brightness extending into the mid portions of the leaflets |
| 4 | No or minimal forward movement of the leaflets in diastole | Considerable thickening of all leaflet tissue (>8–10 mm) | Extensive thickening and shortening of all chordal structures extending down to the papillary muscles | Extensive brightness through out much of the leaflet tissue |
| Anterior Leaflet | Posterior Leaflet | |||||
| Thickness (0–6) (0 = normal, 1 = thickened) | A1 | A2 | A3 | P1 | P2 | P3 |
| Mobility (0–6) (0 = normal, 1 = limited) | 0–1 | 0–1 | 0–1 | 0–1 | 0–1 | 0–1 |
| Calcification (0–10) (0 = no, 1–2 = calcified) | 0–2 | 0–1 | 0–2 | 0–2 | 0–1 | 0–2 |
| Subvalvular Apparatus | ||||||
| Proximal Third | Middle Third | Distal Third | ||||
| Thickness (0–3) (0 = normal, 1 = thickened) | 0–1 | 0–1 | 0–1 | |||
| Separation (0–6) (0 = normal, 1 = partial, 2 = no) | 0–2 | 0–2 | 0–2 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteagudo Ruiz, J.M.; Zamorano Gómez, J.L. The Role of 2D and 3D Echo in Mitral Stenosis. J. Cardiovasc. Dev. Dis. 2021, 8, 171. https://doi.org/10.3390/jcdd8120171
Monteagudo Ruiz JM, Zamorano Gómez JL. The Role of 2D and 3D Echo in Mitral Stenosis. Journal of Cardiovascular Development and Disease. 2021; 8(12):171. https://doi.org/10.3390/jcdd8120171
Chicago/Turabian StyleMonteagudo Ruiz, Juan Manuel, and José Luis Zamorano Gómez. 2021. "The Role of 2D and 3D Echo in Mitral Stenosis" Journal of Cardiovascular Development and Disease 8, no. 12: 171. https://doi.org/10.3390/jcdd8120171
APA StyleMonteagudo Ruiz, J. M., & Zamorano Gómez, J. L. (2021). The Role of 2D and 3D Echo in Mitral Stenosis. Journal of Cardiovascular Development and Disease, 8(12), 171. https://doi.org/10.3390/jcdd8120171






