Pathology of the Aorta and Aorta as Homograft
Abstract
:1. Introduction
2. Normal Anatomy
3. Pathology
3.1. Congenital Malformations
3.2. Genetically Determined Diseases of Thoracic Aorta
3.3. Degenerative Diseases of the Aorta
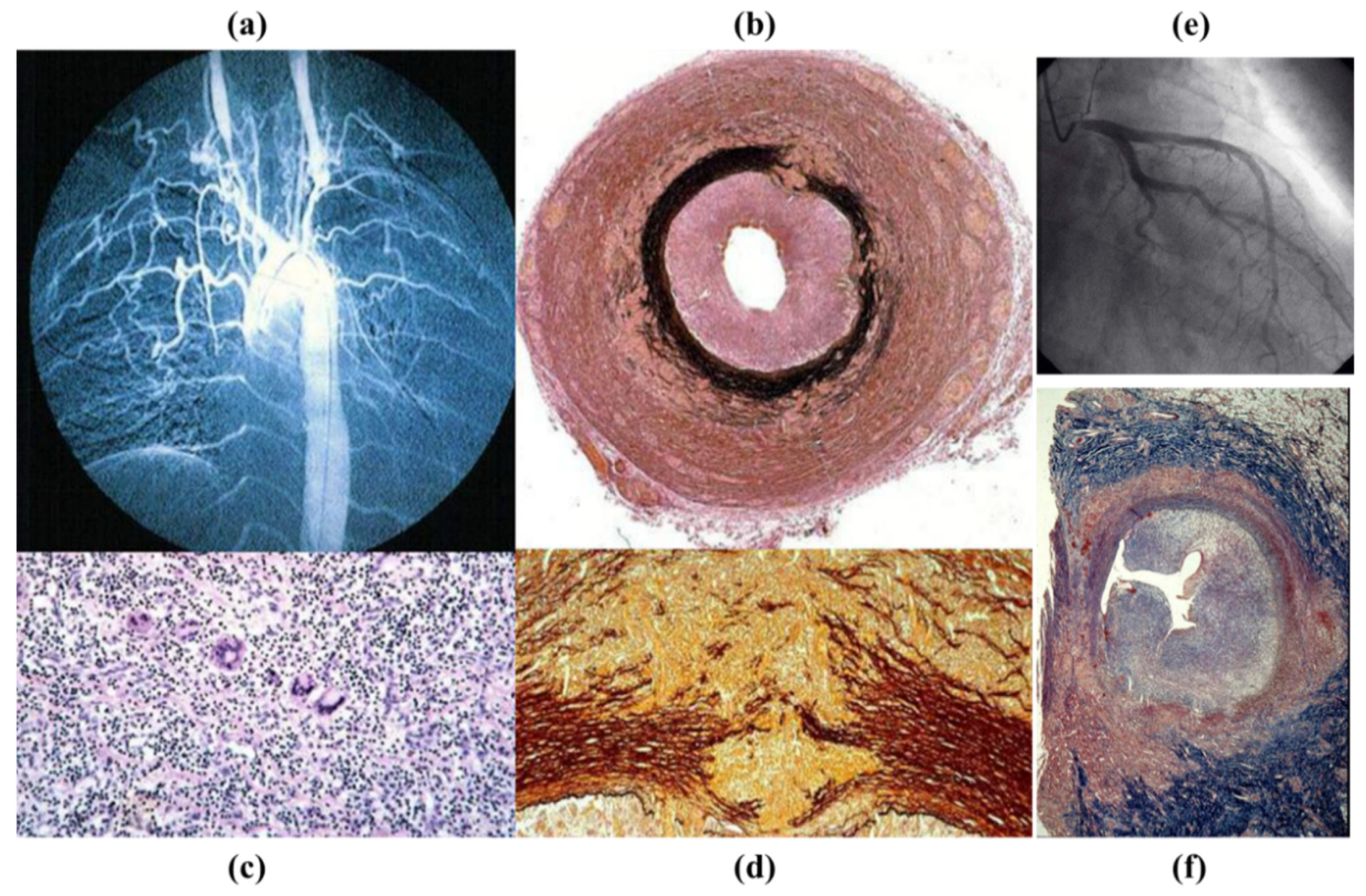
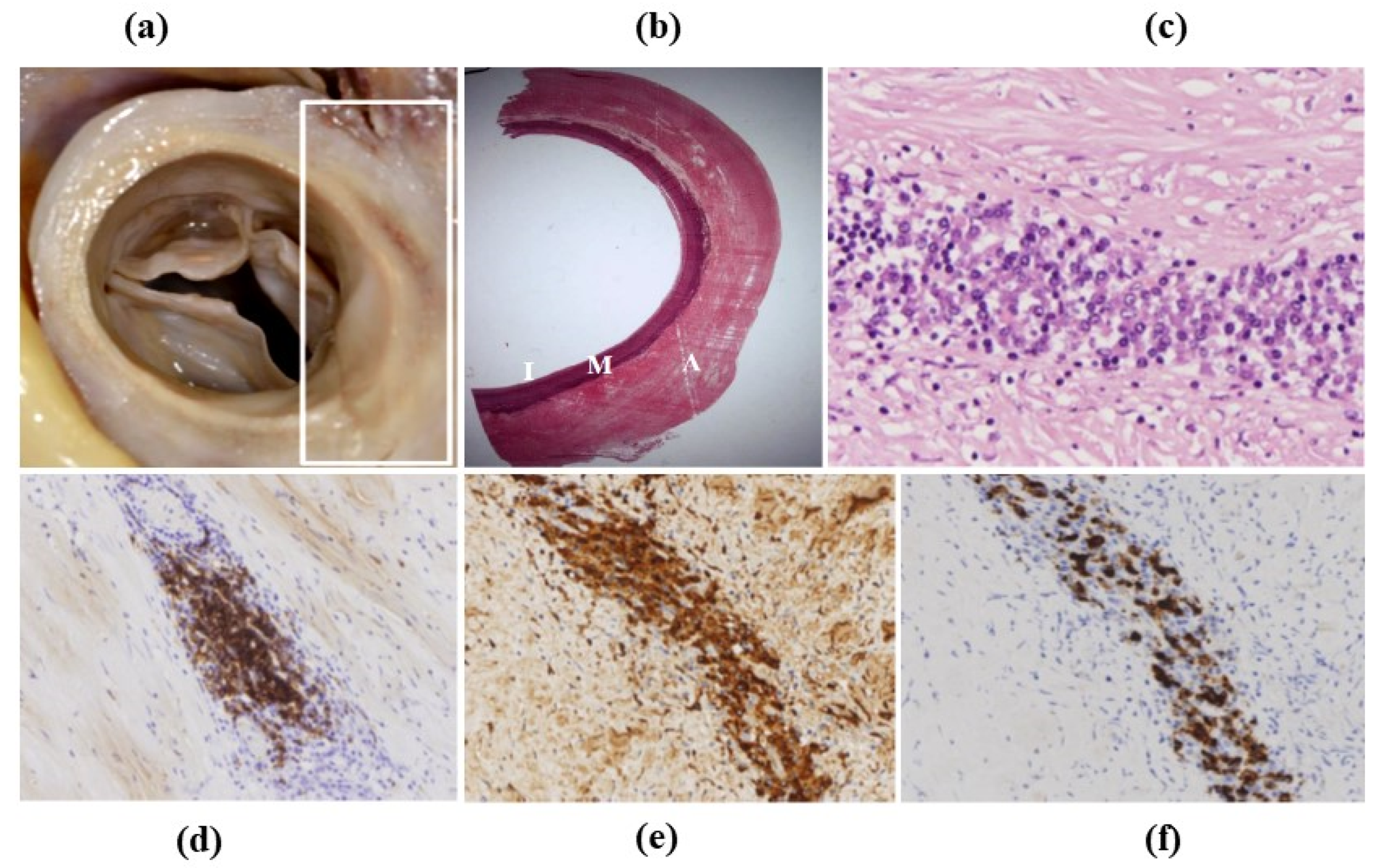
3.4. Inflammatory Diseases of the Aorta
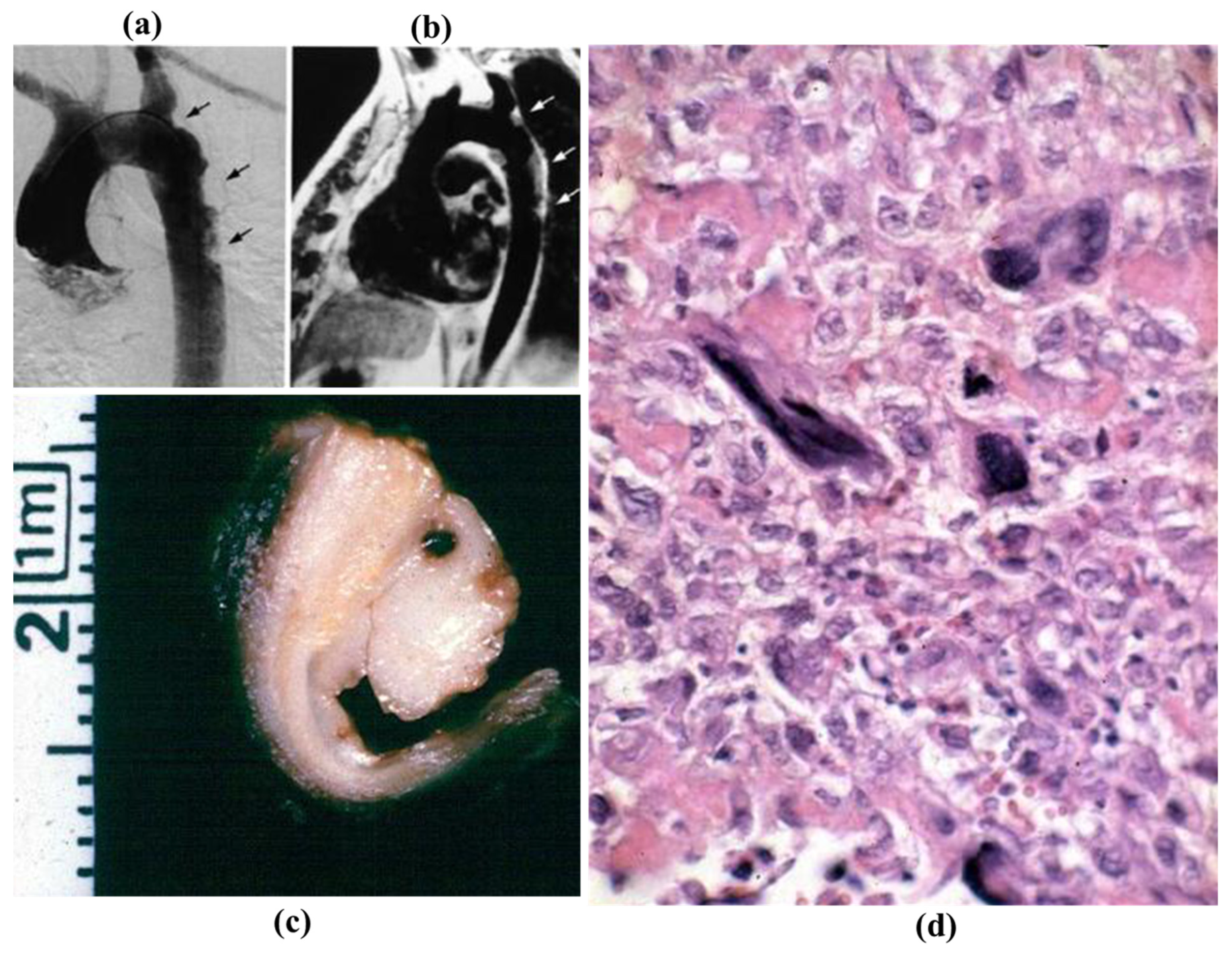
3.5. Neoplasms
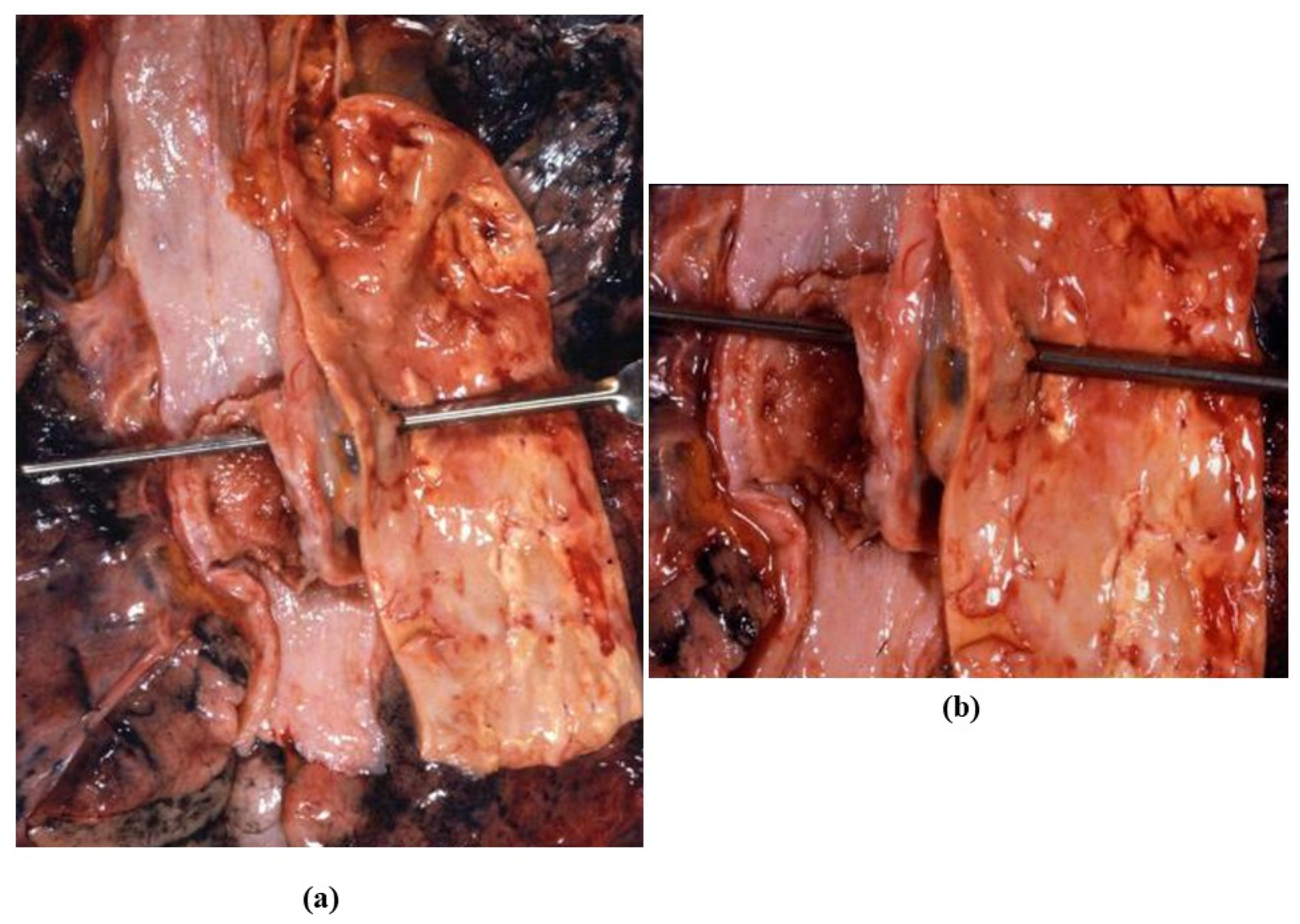
3.6. Thoracic Trauma
4. Aorta as Homograft
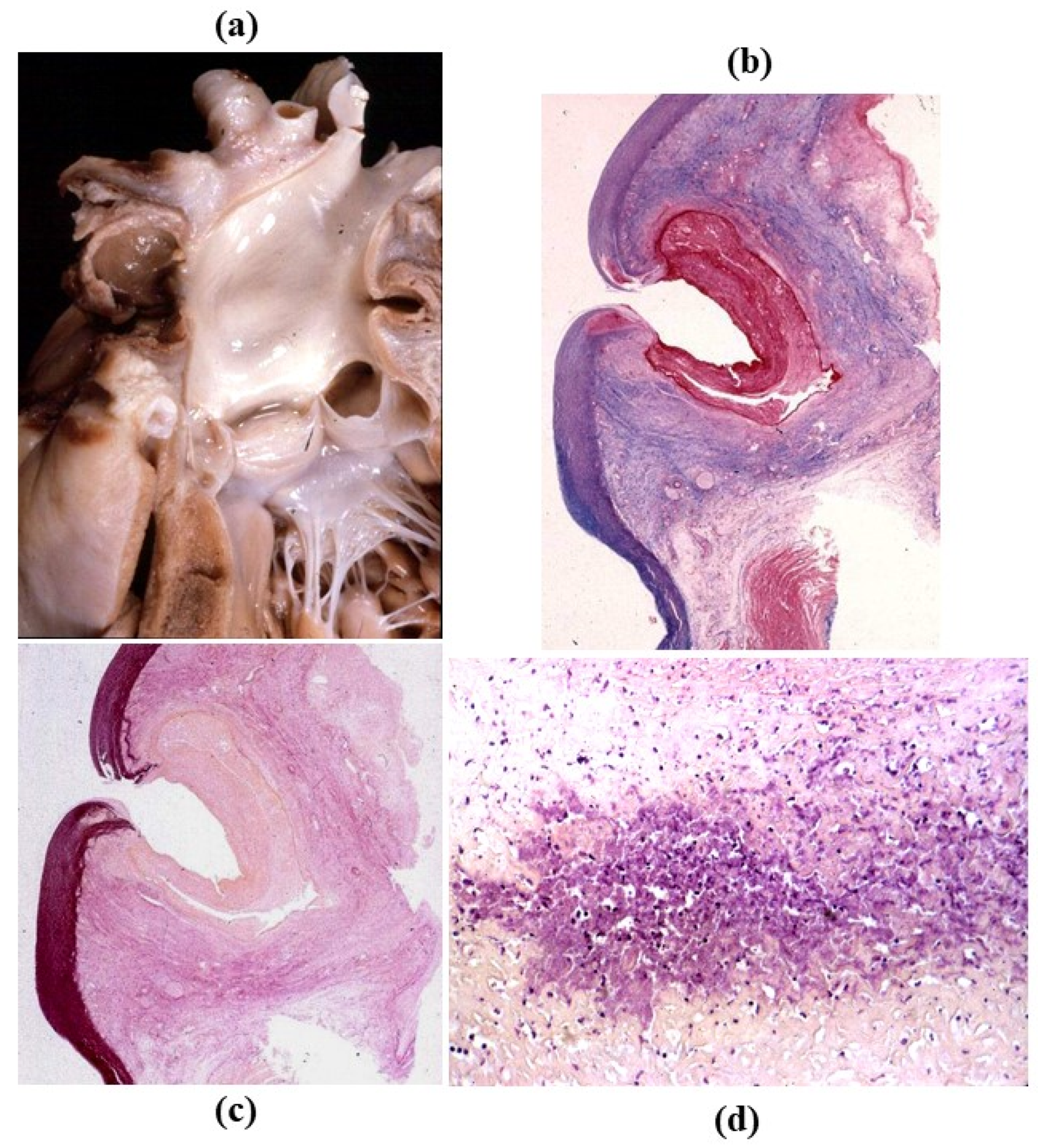
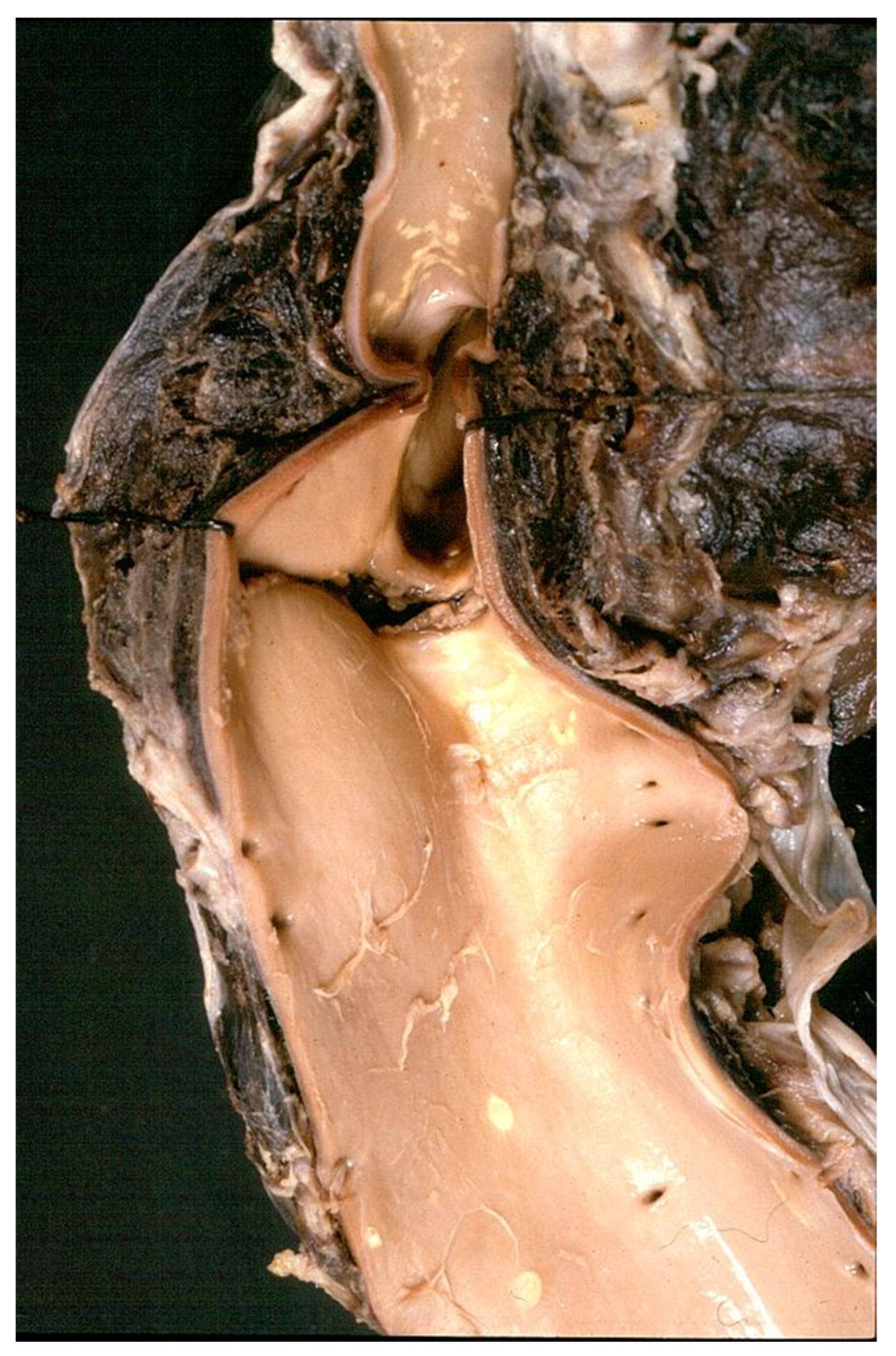
- Decellularization in unimplanted allografts appeared complete, both in the lamellar units of the tunica media and valvular interstitium with disappearance of endothelial lining (Figure 31);
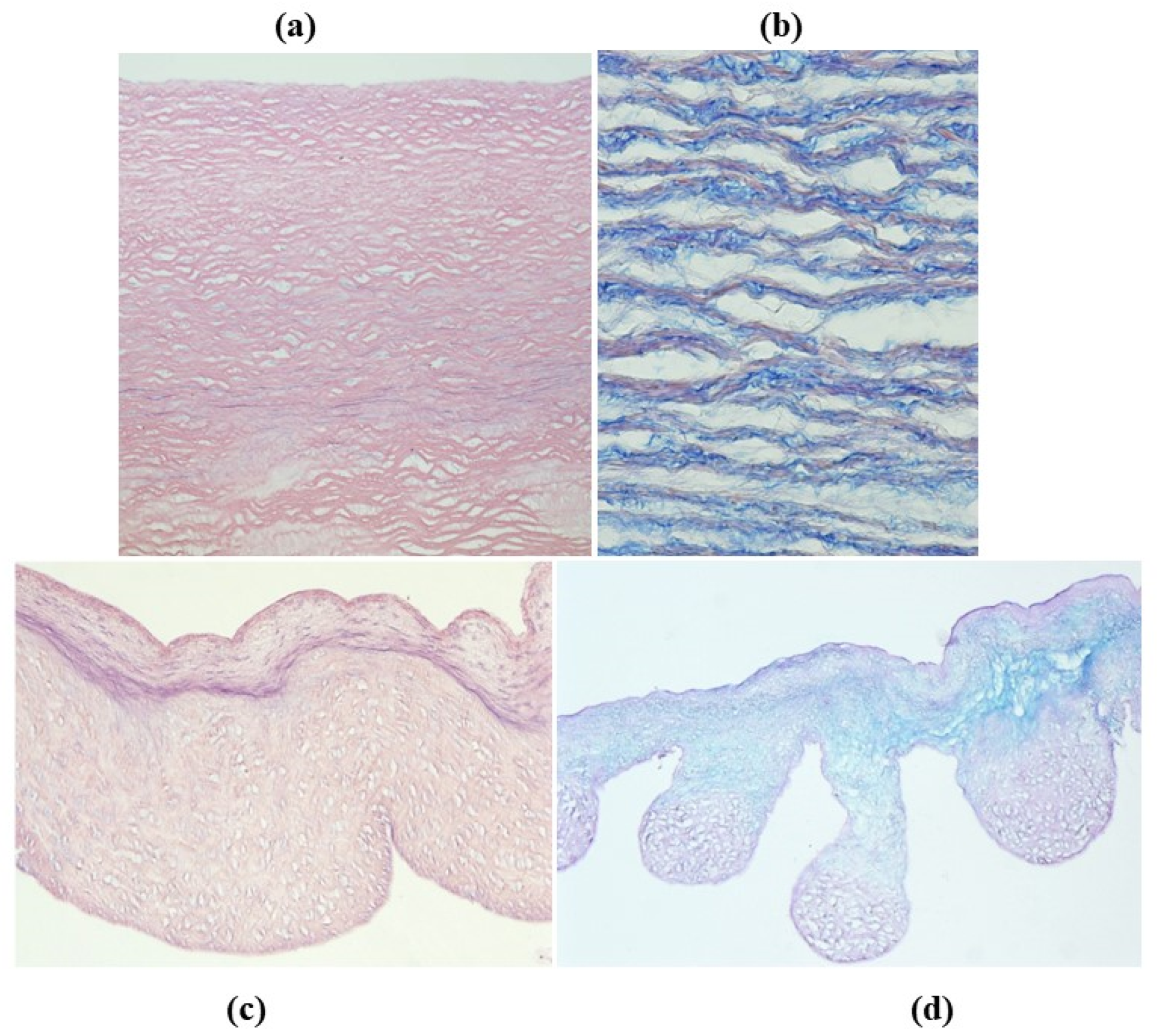
- Cellular repopulation was observed in the outer part of implanted homograft wall by novel smooth muscle cells in the lamellar units (Figure 32a,b) and in the intima with a novel myointimal layer; this layer was noticed also in small animals such as rodents [68]. Novel endothelial cells appeared to line both the aortic wall intima and inflow/outflow at the cusp surface, as well as vasa vasorum, and valve spongiosa appeared repopulated by interstitial cells (Figure 32c,d);
- The ultrastructure of the wall revealed that novel smooth muscle cells have immature aspects, with a central oval nucleus, few contractile filaments and focal densities mainly located close to cytoplasmic membrane and in the paranuclear region; repopulated cells of the cusps are scarcely differentiated cells, in some case showing short intercellular junctions, rough endoplasmic reticulum and focal basal lamina (Figure 33), whereas others exhibited a fibroblast-like morphology;
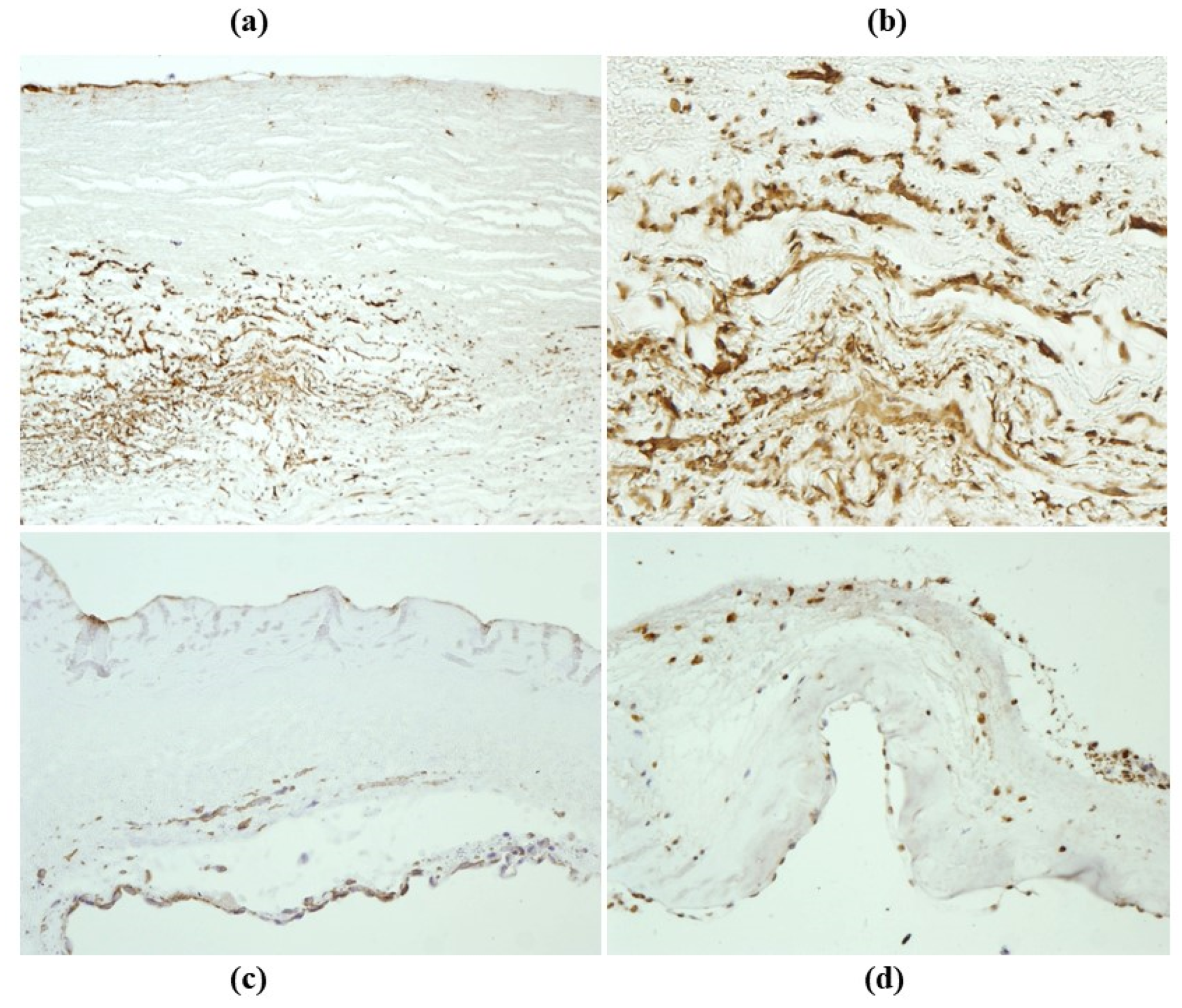
- The undifferentiated nature of the repopulated cells is demonstrated by colocalization of some biomarkers. Novel wall cells showed positivity both for α-SMA and vimentin and novel cusp cells for SMA, vWF, VEGF, VEGF R2, α-SMA and CD57 (HNK-1), which is a neural crest marker;
- The origin of repopulated cells may be vasa vasorum for the homograft outer wall and the blood stream itself for cusps. Recently it has been demonstrated in a GFP rodent model that all novel cells belong to the recipient [68]. Bone marrow may be a source of progenitor cells (endothelial and mesenchymal cells) contributing to recruitment of smooth muscle-like cells [69,70]. Circulating bone-marrow-derived endogenous cells can be recruited in vivo by adhering to the intimal surface [71,72] and then recruited, undergoing an endothelial-to-mesenchymal transition (EMT) within the valve, followed by differentiation into interstitial cells that ultimately synthesize and remodel the ECM;
- Cell density, when compared to non-decellularized control allografts, showed 20% repopulation both in the aortic wall and at the cusp level (Figure 34);
- Mean calcium content by spectroscopy in aortic homografts at 14 months from implant was scanty: 4.24 ± 2.17 mg/g dry weight in the wall vs. 0.530 in controls and 5505 ± 2.04 in the cusps vs. 0.936 in the controls.
Funding
Acknowledgments
Conflicts of Interest
References
- Isselbacher, E.; Eagle, K.; Desanctis, R. Disease of the aorta. In Heart Disease: A Textbook of Cardiovascular Medicine; Braunwald, E., Ed.; W.B. Saunders Co.: Philadelphia, PA, USA, 1997; pp. 1546–1581. [Google Scholar]
- Buja, L.M.; Butany, J. Cardiovascular Pathology, 4th ed.; Academic Press; Elsevier: London, UK, 2016; ISBN 978-0-12-420219-1. [Google Scholar]
- Wolinsky, H.; Glagov, S. A Lamellar Unit of Aortic Medial Structure and Function in Mammals. Circ. Res. 1967, 20, 99–111. [Google Scholar] [CrossRef] [Green Version]
- El-Hamamsy, I.; Yacoub, M.H. Cellular and molecular mechanisms of thoracic aortic aneurysms. Nat. Rev. Cardiol. 2009, 6, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Majesky, M.W. Developmental Basis of Vascular Smooth Muscle Diversity. Arter. Thromb. Vasc. Biol. 2007, 27, 1248–1258. [Google Scholar] [CrossRef] [Green Version]
- Thiene, G.; Corrado, D.; Basso, C. Sudden Cardiac Death in the Young and Athletes: Text Atlas of Pathology and Clinical Correlates; Springer: Milan, Italy, 2016. [Google Scholar]
- Basso, C.; Boschello, M.; Perrone, C.; Mecenero, A.; Cera, A.; Bicego, D.; Thiene, G.; De Dominicis, E. An echocardiographic survey of primary school children for bicuspid aortic valve. Am. J. Cardiol. 2004, 93, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Nistri, S.; Basso, C.; Marzari, C.; Mormino, P.; Thiene, G. Frequency of bicuspid aortic valve in young male conscripts by echo-cardiogram. Am. J. Cardiol. 2005, 96, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.M.; Khairy, P.; Sanders, S.; Colan, S.D. Bicuspid Aortic Valve Morphology and Interventions in the Young. J. Am. Coll. Cardiol. 2007, 49, 2211–2214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabet, H.Y.; Edwards, W.D.; Tazelaar, H.D.; Daly, R.C. Congenitally Bicuspid Aortic Valves: A Surgical Pathology Study of 542 Cases (1991 through 1996) and a Literature Review of 2715 Additional Cases. In Mayo Clinic Proceedings; Elsevier BV: Amsterdam, The Netherlands, 1999; Volume 74, pp. 14–26. [Google Scholar]
- McKusick, V.A. Association of congenital bicuspid aortic valve and Erdheim’s cystic medial necrosis. Lancet 1972, 1, 1026–1027. [Google Scholar] [CrossRef]
- Roberts, C.S.; Roberts, W.C. Dissection of the aorta associated with congenital malformation of the aortic valve. J. Am. Coll. Cardiol. 1991, 17, 712–716. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Siu, S.C. Aortic Dilatation in Patients with Bicuspid Aortic Valve. New Engl. J. Med. 2014, 370, 1920–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Hamamsy, I.; Yacoub, M.H. A measured approach to managing the aortic root in patients with bicuspid aortic valve dis-ease. Curr. Cardiol. Rep. 2009, 11, 94–100. [Google Scholar] [CrossRef]
- Nistri, S.; Grande-Allen, J.; Noale, M.; Basso, C.; Siviero, P.; Maggi, S.; Crepaldi, G.; Thiene, G. Aortic elasticity and size in bicuspid aortic valve syndrome. Eur. Heart J. 2007, 29, 472–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kappetein, A.P.; Gittenberger-de Groot, A.C.; Zwinderman, A.H.; Rohmer, J.; Poelmann, R.E.; Huysmans, H.A. The neural crest as a possible pathogenetic factor in coarctation of the aorta and bicuspid aortic valve. J. Thorac. Cardiovasc. Surg. 1991, 102, 830–836. [Google Scholar] [CrossRef]
- Sakakibara, S.; Konno, S. Congenital aneurysm of the sinus of Valsalva Anatomy and classification. Am. Heart J. 1962, 63, 405–424. [Google Scholar] [CrossRef]
- Morris, C.A.; Loker, J.; Ensing, G.; Stock, A.D. Supravalvular aortic stenosis cosegregates with a familial 6;7 translocation which disrupts the elastin gene. Am. J. Med. Genet. 1993, 46, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.C.P.; Barratt-Boyes, B.G.; Lowe, J.B. Supravalvular Aortic Stenosis. Circulation 1961, 24, 1311–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietz, H.C.; Cutting, C.R.; Pyeritz, R.E.; Maslen, C.L.; Sakai, L.Y.; Corson, G.M.; Puffenberger, E.; Hamosh, A.; Nanthakumar, E.J.; Curristin, S.M.; et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nat. Cell Biol. 1991, 352, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Loeys, B.L.; Schwarze, U.; Holm, T.; Callewaert, B.L.; Thomas, G.H.; Pannu, H.; De Backer, J.F.; Oswald, G.L.; Symoens, S.; Manouvrier, S.; et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N. Engl. J. Med. 2006, 355, 788–798. [Google Scholar] [CrossRef]
- Neptune, E.R.; Frischmeyer, P.A.; Arking, D.E.; Myers, L.; Bunton, T.E.; Gayraud, B.; Ramirez, F.; Sakai, L.Y.; Dietz, H.C. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003, 33, 407–411. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, A.; Malfait, F. The Ehlers-Danlos syndrome.; a disorder with many faces. Clin. Genet. 2012, 82, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wenstrup, R.J.; A Meyer, R.; Lyle, J.S.; Hoechstetter, L.; Rose, P.S.; Levy, H.P.; A Francomano, C. Prevalence of aortic root dilation in the Ehlers-Danlos syndrome. Genet. Med. 2002, 4, 112–117. [Google Scholar] [CrossRef] [Green Version]
- Turtle, E.J.; A Sule, A.; Webb, D.J.; E Bath, L. Aortic dissection in children and adolescents with Turner syndrome: Risk factors and management recommendations. Arch. Dis. Child. 2015, 100, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.W.; Parks, W.C. Role of Matrix Metalloproteinases in Abdominal Aortic Aneurysms. Ann. N. Y. Acad. Sci. 1996, 800, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Cooley, D.A. Annuloaortic Ectasia. Ann. Thorac. Surg. 1979, 28, 303–304. [Google Scholar] [CrossRef]
- Ellis, P.R.; Cooley, D.A.; De Bakey, M.E. Clinical considerations and surgical treatment of annulo-aortic ectasia. J. Thorac. Cardiovasc. Surg. 1961, 42, 363–370. [Google Scholar] [CrossRef]
- Beller, C.J.; Labrosse, M.; Thubrikar, M.J.; Robicsek, F. Role of Aortic Root Motion in the Pathogenesis of Aortic Dissection. Circulation 2004, 109, 763–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evangelista, A.; Mukherjee, D.; Mehta, R.H.; O’Gara, P.T.; Fattori, R.; Cooper, J.V.; Smith, D.E.; Oh, J.K.; Hutchison, S.; Sechtem, U.; et al. International Registry of Aortic Dissection (IRAD) Investigators. Acute in-tramural hematoma of the aorta: A mystery in evolution. Circulation 2005, 111, 1063–1070. [Google Scholar] [CrossRef] [Green Version]
- Larson, E.W.; Edwards, W.D. Risk factors for aortic dissection: A necropsy study of 161 cases. Am. J. Cardiol. 1984, 53, 849–855. [Google Scholar] [CrossRef]
- Goldfinger, J.Z.; Halperin, J.L.; Marin, M.L.; Stewart, A.S.; Eagle, K.A.; Fuster, V. Thoracic Aortic Aneurysm and Dissection. J. Am. Coll. Cardiol. 2014, 64, 1725–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jondeau, G.; Boileau, C. Familial thoracic aortic aneurysms. Curr. Opin. Cardiol. 2014, 29, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Ladich, E.; Butany, J.; Virmani, R. 5-Aneurysms of the Aorta: Ascending, Thoracic and Abdominal and Their Management. In Cardiovascular Pathology, 4th ed.; Buja, L.M., Butany, J., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 169–211. ISBN 9780124202191. [Google Scholar]
- Pyeritz, R.E. Heritable thoracic aortic disorders. Curr. Opin. Cardiol. 2014, 29, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Vaideeswar, P.; Dixit, V.; Butany, J.; David, T.E.; Feindel, C. Surgical pathology of chronic ascending aortic dissections. Pathology 2008, 40, 505–512. [Google Scholar] [CrossRef]
- Gornik, H.L.; Creager, M.A. Aortitis. Circulation 2008, 117, 3039–3051. [Google Scholar] [CrossRef]
- Virmani, R.; Burke, A.P. Pathologic features of aortitis. Cardiovasc. Pathol. 1994, 3, 205–216. [Google Scholar] [CrossRef]
- Virmani, R.; McAllister, H. Pathology of the aorta and major arteries. In Aortitis: Clinical, Pathologic, and Radiographic Aspects; Lande, A., Borkman, Y., McAllister, H., Eds.; Raven Press: New York, NY, USA, 1986; pp. 7–53. [Google Scholar]
- Heggtveit, H.A. Syphilitic aortitis. A clinicopathologic autopsy study of 100 cases, 1950 to 1960. Circulation 1964, 29, 346–355. [Google Scholar] [CrossRef] [Green Version]
- Musci, M.; Weng, Y.; Hübler, M.; Amiri, A.; Pasic, M.; Kosky, S.; Stein, J.; Siniawski, H.; Hetzer, R. Homograft aortic root replacement in native or prosthetic active infective endocarditis: Twenty-year single-center experience. J. Thorac. Cardiovasc. Surg. 2010, 139, 665–673. [Google Scholar] [CrossRef] [Green Version]
- Yanagawa, B.; Mazine, A.; Tam, D.Y.; Jüni, P.; Bhatt, D.L.; Spindel, S.; Puskas, J.D.; Verma, S.; Friedrich, J.O. Homograft Versus Conven-tional Prosthesis for Surgical Management of Aortic Valve Infective Endocarditis: A Systematic Review and Meta-analysis. Innovations 2018, 13, 163–170. [Google Scholar] [CrossRef]
- Evans, J.M.; Bowles, C.A.; Bjornsson, J.; Mullany, C.J.; Hunder, G.G. Thoracic aortic aneurysm and rupture in giant cell arteritis. a descriptive study of 41 cases. Arthritis Rheum. 1994, 37, 1539–1547. [Google Scholar] [CrossRef]
- Lupi-Herrera, E.; Sánchez-Torres, G.; Marcushamer, J.; Mispireta, J.; Horwitz, S.; Vela, J.E. Takayasu’s arteritis. Clinical study of 107 cases. Am. Heart. J. 1977, 93, 94–103. [Google Scholar] [CrossRef]
- Stone, J.H.; Khosroshahi, A.; Deshpande, V.; Stone, J.R. IgG4-related systemic disease accounts for a significant proportion of thoracic lymphoplasmacytic aortitis cases. Arthritis Rheum. 2010, 62, 316–322. [Google Scholar] [CrossRef]
- Ansell, B.M.; Bywaters, E.G.L.; Doniach, I. The aortic lesion of ankylosing spondylitis. Br. Heart J. 1958, 20, 507–515. [Google Scholar] [CrossRef] [Green Version]
- Bulkley, B.H.; Roberts, W.C. Ankylosing spondylitis and aortic regurgitation. Description of the characteristic cardiovascular lesion from study of eight necropsy patients. Circulation 1973, 48, 1014–1027. [Google Scholar] [CrossRef] [Green Version]
- Arnason, J.A.; Patel, A.K.; Rahko, P.S.; Sundstrom, W.R. Transthoracic and transesophageal echocardiographic evaluation of the aortic root and subvalvular structures in ankylosing spondylitis. J. Rheumatol. 1996, 23, 120–123. [Google Scholar] [PubMed]
- Palazzi, C.; D’ Angelo, S.; Lubrano, E.; Olivieri, I. Aortic involvement in ankylosing spondylitis. Clin. Exp. Rheumatol. 2008, 26 (Suppl. 49), S131–S134. [Google Scholar]
- Reimer, K.A.; Rodgers, R.F.; Oyasu, R. Rheumatoid Arthritis With Rheumatoid Heart Disease and Granulomatous Aortitis. JAMA 1976, 235, 2510–2512. [Google Scholar] [CrossRef]
- Paulus, H.E.; Pearson, C.M.; Pitts, W., Jr. Aortic insufficiency in five patients with Reiter’s syndrome. A detailed clinical and pathologic study. Am. J. Med. 1972, 53, 464–472. [Google Scholar] [CrossRef]
- Chajek, T.; Fainaru, M. Behcet’s disease. Report of 41 cases and a review of the literature. Medicine 1975, 54, 179–196. [Google Scholar] [CrossRef]
- Hamza, M. Large artery involvement in Behcet’s disease. J. Rheumatol. 1987, 14, 554–559. [Google Scholar]
- Tuzun, H.; Besirli, K.; Sayin, A.; Vural, F.S.; Hamuryudan, V.; Hizli, N.; Yurdakul, S.; Yazici, H. Management of aneurysms in Behcet’s syndrome: An analysis of 24 patients. Surgery 1997, 121, 150–156. [Google Scholar] [CrossRef]
- Blockmans, D.; Baeyens, H.; Van Loon, R.; Lauwers, G.; Bobbaers, H. Periaortitis and aortic dissection due to Wegener’s granu-lomatosis. Clin. Rheumatol. 2000, 19, 161–164. [Google Scholar] [CrossRef]
- Unlü, C.; Willems, M.; Ten Berge, I.J.; Legemate, D.A. Aortitis with aneurysm formation as a rare complication of Wegener’s granulomatosis. J. Vasc. Surg. 2011, 54, 1485–1487. [Google Scholar] [CrossRef]
- Chiche, L.; Mongrédien, B.; Brocheriou, I.; Kieffer, E. Primary Tumors of the Thoracoabdominal Aorta: Surgical Treatment of 5 Patients and Review of the Literature. Ann. Vasc. Surg. 2003, 17, 354–364. [Google Scholar] [CrossRef]
- Neschis, D.G.; Scalea, T.M.; Flinn, W.R.; Griffith, B.P. Blunt Aortic Injury. N. Eng. J. Med. 2008, 359, 1708–1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eugene, H.B.; Frank, L.H.; James, K.K.; Nicholas, T. Kouchoukos Kirklin/Barratt-Boyes. Cardiac Surgery; Expert Consult. Saunders: Milan, Italy, 2012; ISBN 9781416063919. [Google Scholar]
- Mendelson, K.; Schoen, F.J. Heart valve tissue engineering: Concepts, approaches, progress, and challenges. Ann. Biomed. Eng. 2006, 34, 1799–1819. [Google Scholar] [CrossRef] [Green Version]
- Flaumenhaft, R.; Moscatelli, D.; Saksela, O.; Rifkin, D.B. Role of extracellular matrix in the action of basic fibroblast growth factor: Ma-trix as a source of growth factor for long-term stimulation of plasminogen activator production and DNA synthesis. J. Cell. Physiol. 1989, 140, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Baraki, H.; Tudorache, I.; Braun, M.; Höffler, K.; Görler, A.; Lichtenberg, A.; Bara, C.; Calistru, A.; Brandes, G.; Hewicker-Trautwein, M.; et al. Orthotopic replacement of the aortic valve with decellularized allograft in a sheep model. Biomaterials 2009, 30, 6240–6246. [Google Scholar] [CrossRef]
- Grauss, R.W.; Hazekamp, M.G.; Oppenhuizen, F.; Van Munsterena, C.J.; Groot, A.C.G.-D.; DeRuiter, M.C. Histological evaluation of decellularised porcine aortic valves: Matrix changes due to different decellularisation methods. Eur. J. Cardio-Thorac. Surg. 2005, 27, 566–571. [Google Scholar] [CrossRef]
- Cebotari, S.; Lichtenberg, A.; Tudorache, I.; Hilfiker, A.; Mertsching, H.; Leyh, R.; Breymann, T.; Kallenbach, K.; Maniuc, L.; Batrinac, A.; et al. Clinical application of tissue engineered human heart valves using autolo-gous progenitor cells. Circulation 2006, 114, I132–I137. [Google Scholar] [CrossRef] [Green Version]
- Cebotari, S.; Tudorache, I.; Jaekel, T.; Hilfiker, A.; Dorfman, S.; Ternes, W.; Haverich, A.; Lichtenberg, A. Detergent Decellularization of Heart Valves for Tissue Engineering: Toxicological Effects of Residual Detergents on Human Endothelial Cells. Artif. Organs 2010, 34, 206–210. [Google Scholar] [CrossRef]
- Tudorache, I.; Theodoridis, K.; Samir, S.; Bara, C.; Meyer, T.; Höffler, K.; Hartung, D.; Hilfiker, A.; Haverich, A.; Cebotari, S. Decellular-ized aortic allograft vs pulmonary autograft for aortic valve replacement in growing sheep model: Haemodynamic and morphological results after implantation for 20 months. Circulation 2014, 130, A20027. [Google Scholar]
- Della Barbera, M.; Valente, M.; Basso, C.; Thiene, G. Morphologic studies of cell endogenous repopulation in decellularized aortic and pulmonary homografts implanted in sheep. Cardiovasc Pathol. 2015, 24, 102–109. [Google Scholar] [CrossRef]
- Dedja, A.; Padalino, M.A.; Della Barbera, M.; Rasola, C.; Pesce, P.; Milan, A.; Pozzobon, M.; Sacerdoti, D.; Thiene, G.; Stellin, G. Heterotopic Implantation of Decellularized Pulmonary Artery Homografts In A Rodent Model: Technique Description and Preliminary Report. J. Investig. Surg. 2017, 31, 282–291. [Google Scholar] [CrossRef]
- Sata, M.; Fukuda, D.; Tanaka, K.; Kaneda, Y.; Yashiro, H.; Shirakawa, I. The role of circulating precursors in vascular repair and lesion formation. J. Cell. Mol. Med. 2005, 9, 557–568. [Google Scholar] [CrossRef]
- Frid, M.G.; Kale, V.A.; Stenmark, K.R. Mature vascular endothelium can give rise to smooth muscle cells via endotheli-al-mesenchymal transdifferentiation: In vitro analysis. Circ. Res. 2002, 90, 1189–1196. [Google Scholar] [CrossRef] [Green Version]
- Deb, A.; Wang, S.H.; Skelding, K.; Miller, D.; Simper, D.; Caplice, N. Bone marrow-derived myofibroblasts are present in adult human heart valves. J. Heart Valve Dis. 2005, 14, 674–678. [Google Scholar]
- Armstrong, E.J.; Bischoff, J. Heart valve development: Endothelial cell signaling and differentiation. Circ. Res. 2004, 95, 459–470. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiene, G.; Basso, C.; Della Barbera, M. Pathology of the Aorta and Aorta as Homograft. J. Cardiovasc. Dev. Dis. 2021, 8, 76. https://doi.org/10.3390/jcdd8070076
Thiene G, Basso C, Della Barbera M. Pathology of the Aorta and Aorta as Homograft. Journal of Cardiovascular Development and Disease. 2021; 8(7):76. https://doi.org/10.3390/jcdd8070076
Chicago/Turabian StyleThiene, Gaetano, Cristina Basso, and Mila Della Barbera. 2021. "Pathology of the Aorta and Aorta as Homograft" Journal of Cardiovascular Development and Disease 8, no. 7: 76. https://doi.org/10.3390/jcdd8070076
APA StyleThiene, G., Basso, C., & Della Barbera, M. (2021). Pathology of the Aorta and Aorta as Homograft. Journal of Cardiovascular Development and Disease, 8(7), 76. https://doi.org/10.3390/jcdd8070076






