Isolated Dissection of the Ductus Arteriosus Associated with Sudden Unexpected Intrauterine Death
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Study Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blythe, C.; Vazquez, R.E.Z.; Cabrera, M.S.; Zekic Tomas, S.; OC Anumba, D.; Cohen, M.C. Results of full postmortem examination in a cohort of clinically unexplained stillbirths: Undetected fetal growth restriction and placental insufficiency are prevalent findings. J. Perinatol. 2019, 39, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Frøen, J.F.; Cacciatore, J.; McClure, E.M.; Kuti, O.; Jokhio, A.H.; Islam, M.; Shiffman, J. Stillbirths: Why they matter. Lancet 2011, 377, 1353–1366. [Google Scholar] [CrossRef] [Green Version]
- Reinebrant, H.E.; Leisher, S.H.; Coory, M.; Henry, S.; Wojcieszek, A.M.; Gardener, G.; Lourie, R.; Ellwood, D.; Teoh, Z.; Allanson, E.; et al. Making stillbirths visible: A systematic review of globally reported causes of stillbirth. BJOG An. Int. J. Obstet. Gynaecol. 2018, 125, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Gewillig, M.; Brown, S.C.; De Catte, L.; Debeer, A.; Eyskens, B.; Cossey, V.; Van Schoubroeck, D.; Van Hole, C.; Devlieger, R. Premature foetal closure of the arterial duct: Clinical presentations and outcome. Eur. Heart J. 2009, 30, 1530–1536. [Google Scholar] [CrossRef]
- Tynan, M. The ductus arteriosus and its closure. N. Engl. J. Med. 1993, 329, 1570–1572. [Google Scholar] [CrossRef]
- Leal, S.D.; Cavallé-Garrido, T.; Ryan, G.; Farine, D.; Heilbut, M.; Smallhorn, J.F. Isolated ductal closure in utero diagnosed by fetal echocardiography. Am. J. Perinatol. 1997, 14, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Kawazu, Y.; Kayatani, F.; Inamura, N. Prognostic factors of premature closure of the ductus arteriosus in utero: A systematic literature review. Cardiol. Young 2017, 27, 634–638. [Google Scholar] [CrossRef]
- Operle, M.; Anderson, S. Premature Closure of the Ductus Arteriosus in an Otherwise Healthy Fetus. J. Diagnostic Med. Sonogr. 2019, 35, 235–239. [Google Scholar] [CrossRef]
- Gittenberger-De Groot, A.C.; Peterson, J.C.; Wisse, L.J.; Roest, A.A.W.; Poelmann, R.E.; Bökenkamp, R.; Elzenga, N.J.; Hazekamp, M.; Bartelings, M.M.; Jongbloed, M.R.M.; et al. Pulmonary ductal coarctation and left pulmonary artery interruption; pathology and role of neural crest and second heart field during development. PLoS ONE 2020, 15, e0228478. [Google Scholar] [CrossRef]
- Scherptong, R.W.C.; Jongbloed, M.R.M.; Wisse, L.J.; Vicente-steijn, R.; Bartelings, M.M.; Poelmann, R.E.; Schalij, M.J.; Groot, A.C.G. Morphogenesis of Outflow Tract Rotation During Cardiac Development: The Pulmonary Push Concept. Dev. Dyn. 2012, 241, 1413–1422. [Google Scholar] [CrossRef]
- Bergwerff, M.; Deruiter, M.C.; Gittenberger-deGroot, A.C. Comparative anatomy and ontogeny of the ductus arteriosus, a vascular outsider. Anat. Embryol. 1999, 200, 559–571. [Google Scholar] [CrossRef]
- Gittenberger-De Groot, A.C. Persistent ductus arteriosus: Most probably a primary congenital malformation. Br. Heart J. 1977, 39, 610–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen Ho, S.; Anderson, R.H. Anatomical closure of the ductus arteriosus: A study in 35 specimens. J. Anat. 1979, 128, 829–82936. [Google Scholar]
- Gittenberger-de Groot, A.C.; Strengers, J.L.M.; Mentink, M.; Poelmann, R.E.; Patterson, D.F. Histologic studies on normal and persistent ductus arteriosus in the dog. J. Am. Coll. Cardiol. 1985, 6, 394–404. [Google Scholar] [CrossRef] [Green Version]
- Silver, M.M.; Freedom, R.M.; Silver, M.D.; Olley, P.M. The morphology of the human newborn ductus arteriosus: A reappraisal of its structure and closure with special reference to prostaglandin E1 therapy. Hum. Pathol. 1981, 12, 1123–1136. [Google Scholar] [CrossRef]
- Weichert, J.; Hartge, D.R.; Axt-Fliedner, R. The fetal ductus arteriosus and its abnormalities—A review. Congenit. Heart Dis. 2010, 5, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Hofstadler, G.; Tulzer, G.; Altmann, R.; Schmitt, K.; Danford, D.; Huhta, J.C. Spontaneous closure of the human fetal ductus arteriosus—A cause of fetal congestive heart failure. Am. J. Obstet. Gynecol. 1996, 174, 879–883. [Google Scholar] [CrossRef]
- Cruickshank, B.; Marquis, R.M. Spontaneous Aneurysm of the Ductus Arteriosus. A review and report of the tenth adult case. Am. J. Med. 1958, 25, 140–149. [Google Scholar] [CrossRef]
- Tutassaura, H.; Goldman, B.; Moes, C.A.; Mustard, W.T. Spontaneous aneurysm of the ductus arteriosus in childhood. J. Thorac. Cardiovasc. Surg. 1969, 57, 180–184. [Google Scholar] [CrossRef]
- Falcone, M.W.; Perloff, J.K.; Roberts, W.C. Aneurysm of the Nonpatent Ductus Arteriosus in the Newborn. Am. J. Cardiol. 1971, 29, 422–426. [Google Scholar] [CrossRef]
- Ithuralde, M.; Halloran, K.H.; Fishbone, G.; Brill, S.; Downing, S.E. Dissecting Aneurysm of the Ductus Arteriosus in the Newborn Infant. Am. J. Dis. Child. 1971, 122, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Lund, J.T.; Jensen, M.B.; Hielms, E. Aneurysm of the ductus arteriosus. A review of the literature and the surgical implications. Eur. J. Cario-Thorac. Surg. 1991, 5, 566–570. [Google Scholar] [CrossRef]
- Lund, J.T.; Hansen, D.; Brocks, V.; Jensen, M.B.; Jacobsen, J.R. Aneurysm of the ductus arteriosus in the neonate: Three case reports with a review of the literature. Pediatr. Cardiol. 1992, 13, 222–226. [Google Scholar] [CrossRef]
- Dyamenahalli, U.; Smallhorn, J.F.; Geva, T.; Fouron, J.C.; Cairns, P.; Jutras, L.; Hughes, V.; Rabinovitch, M.; Mason, C.A.E.; Hornberger, L.K. Isolated ductus arteriosus aneurysm in the fetus and infant: A multi-institutional experience. J. Am. Coll. Cardiol. 2000, 36, 262–269. [Google Scholar] [CrossRef] [Green Version]
- Jan, S.L.; Hwang, B.; Fu, Y.C.; Chai, J.W.; Chi, C.S. Isolated neonatal ductus arteriosus aneurysm. J. Am. Coll. Cardiol. 2002, 39, 342–347. [Google Scholar] [CrossRef] [Green Version]
- Fukuta, M.; Horita, T.; Seko-Nakamura, Y.; Kato, H.; Kanno, S.; Monma-Otaki, J.; Aoki, Y. Sudden Death Caused by Rupture of Spontaneous Ductus Arteriosus Aneurysm in an Adult. J. Forensic Sci. 2020, 65, 1004–1008. [Google Scholar] [CrossRef]
- Komai, H.; Naito, Y.; Fujiwara, K. Ductal aneurysm of adult patients. Jpn. J. Thorac. Cardiovasc. Surg. 2000, 48, 139–141. [Google Scholar] [CrossRef]
- Pastuszko, P.; Eisenberg, J.A.; Diehl, J.T. Ductus arteriosus aneurysm in an adult patient presenting with hoarseness. J. Card. Surg. 2005, 20, 386–388. [Google Scholar] [CrossRef]
- Ardhanari, M.; Swaminathan, S. Congenital ductus arteriosus aneurysm in association with MYH11 mutation: A case report. Cardiol. Young 2020, 30, 123–125. [Google Scholar] [CrossRef]
- Gittenberger-de Groot, A.C.; van Ertbruggen, I.; Moulaert, A.J.M.G.; Harinck, E. The ductus arteriosus in the preterm infant: Histologic and clinical observations. J. Pediatr. 1980, 96, 88–93. [Google Scholar] [CrossRef]
- Bökenkamp, R.; Deruiter, M.C.; Van Munsteren, C.; Gittenberger-De Groot, A.C. Insights into the pathogenesis and genetic background of patency of the ductus arteriosus. Neonatology 2010, 98, 6–17. [Google Scholar] [CrossRef]
- Gittenberger-De Groot, A.C. Influence of Prostaglandin E1 on the Histology of the Normal and the Persistent Ductus Arteriosus. In Proceedings of the Selected Topics in Cardiac Surgery, Symposium Hel, Padova, Italy, 25–26 May 1979; Gallucci, V., Bini, R.M., Thiene, G., Eds.; Patron Editore Bologna: Bologna, Italy, 1979; pp. 159–168. [Google Scholar]
- Matsui, H.; McCarthy, K.; Ho, S. Morphology of the patent arterial duct: Features relevant to treatment. Images Paediatr. Cardiol. 2008, 10, 27–38. [Google Scholar]
- Dagle, J.M.; Lepp, N.T.; Cooper, M.E.; Schaa, K.L.; Kelsey, K.J.P.; Orr, K.L.; Caprau, D.; Zimmerman, C.R.; Steffen, K.M.; Johnson, K.J.; et al. Determination of Genetic Predisposition to Patent Ductus Arteriosus in Preterm Infants. Pediatrics 2009, 123, 1116–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khong, T.Y.; Mooney, E.E.; Ariel, I.; Balmus, N.C.M.; Boyd, T.K.; Brundler, M.A.; Derricott, H.; Evans, M.J.; Faye-Petersen, O.M.; Gillan, J.E.; et al. Sampling and definitions of placental lesions Amsterdam placental workshop group consensus statement. Arch. Pathol. Lab. Med. 2016, 140, 698–713. [Google Scholar] [CrossRef] [Green Version]
- Gardosi, J.; Kady, S.M.; McGeown, P.; Francis, A.; Tonks, A. Classification of stillbirth by relevant condition at death (ReCoDe): Population based cohort study. Br. Med. J. 2005, 331, 1113–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlatmann, T.J.M.; Becker, A.E. Pathogenesis of dissecting aneurysm of aorta. Comparative histopathologic study of significance of medial changes. Am. J. Cardiol. 1977, 39, 21–26. [Google Scholar] [CrossRef]
- Halushka, M.K.; Angelini, A.; Bartoloni, G.; Basso, C.; Batoroeva, L.; Bruneval, P.; Buja, L.M.; Butany, J.; D’Amati, G.; Fallon, J.T.; et al. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association for European Cardiovascular Pathology: II. Noninflammatory degenerative diseases-Nomenclature and diagnostic criteria. Cardiovasc. Pathol. 2016, 25, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Visentin, S.; Londero, A.P.; Calanducci, M.; Grisan, E.; Bongiorno, M.C.; Marin, L.; Cosmi, E. Fetal Abdominal Aorta: Doppler and Structural Evaluation of Endothelial Function in Intrauterine Growth Restriction and Controls. Ultraschall Med. 2019, 40, 55–63. [Google Scholar] [CrossRef]
- Zhu, L.; Vranckx, R.; Van Kien, P.K.; Lalande, A.; Boisset, N.; Mathieu, F.; Wegman, M.; Glancy, L.; Gasc, J.M.; Brunotte, F.; et al. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat. Genet. 2006, 38, 343–349. [Google Scholar] [CrossRef]
- Tongsong, T.; Chanprapaph, P.; Sittiwangkul, R.; Sirichotiyakul, S. Rupture of Fetal Ductus Arteriosus Aneurysm. Am. Coll. Obstet. Gynecol. 2005, 105, 1275–1278. [Google Scholar] [CrossRef]
- Stewart, A.; Dyamenahalli, U.; Greenberg, S.B.; Drummond-Webb, J. Ductus arteriosus aneurysm with community-acquired methicillin-resistant Staphylococcus aureus infection and spontaneous rupture: A potentially fatal quandary. Pediatrics 2006, 117, e1259–e1262. [Google Scholar] [CrossRef] [PubMed]
- Muthialu, N.; Robertson, A.; Mortensen, K.; Khambadkone, S. Case report on surgical repair of unusual dissection of arterial duct involving main pulmonary artery in a child with vein of Galen malformation. Eur. Hear. J. Case Rep. 2018, 2, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.J.; Jan, S.L. Fetal echocardiographic diagnosis of isolated ductus arteriosus aneurysm: A longitudinal study from 32 weeks of gestation to term. Ultrasound Obstet. Gynecol. 2005, 26, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Luchese, S.; Mânica, J.L.; Zielinsky, P. Intrauterine ductus arteriosus constriction: Analysis of a historic cohort of 20 cases. Arq. Bras. Cardiol. 2003, 81, 405–410. [Google Scholar] [CrossRef]
- Mielke, G.; Steil, E.; Breuer, J.; Goelz, R. Circulatory changes following intrauterine closure of the ductus arteriosus in the human fetus and newborn. Prenat. Diagn. 1998, 18, 139–145. [Google Scholar] [CrossRef]
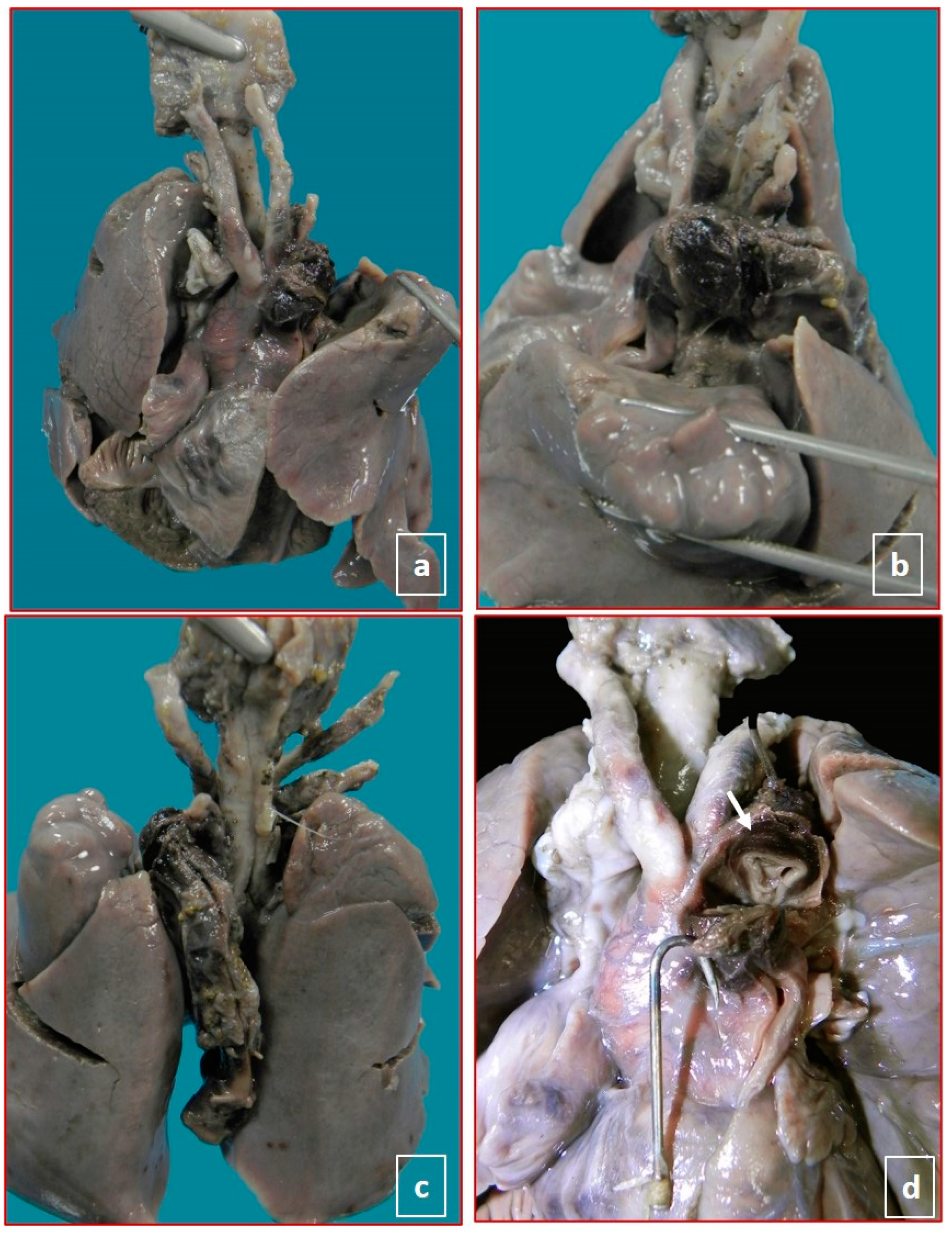
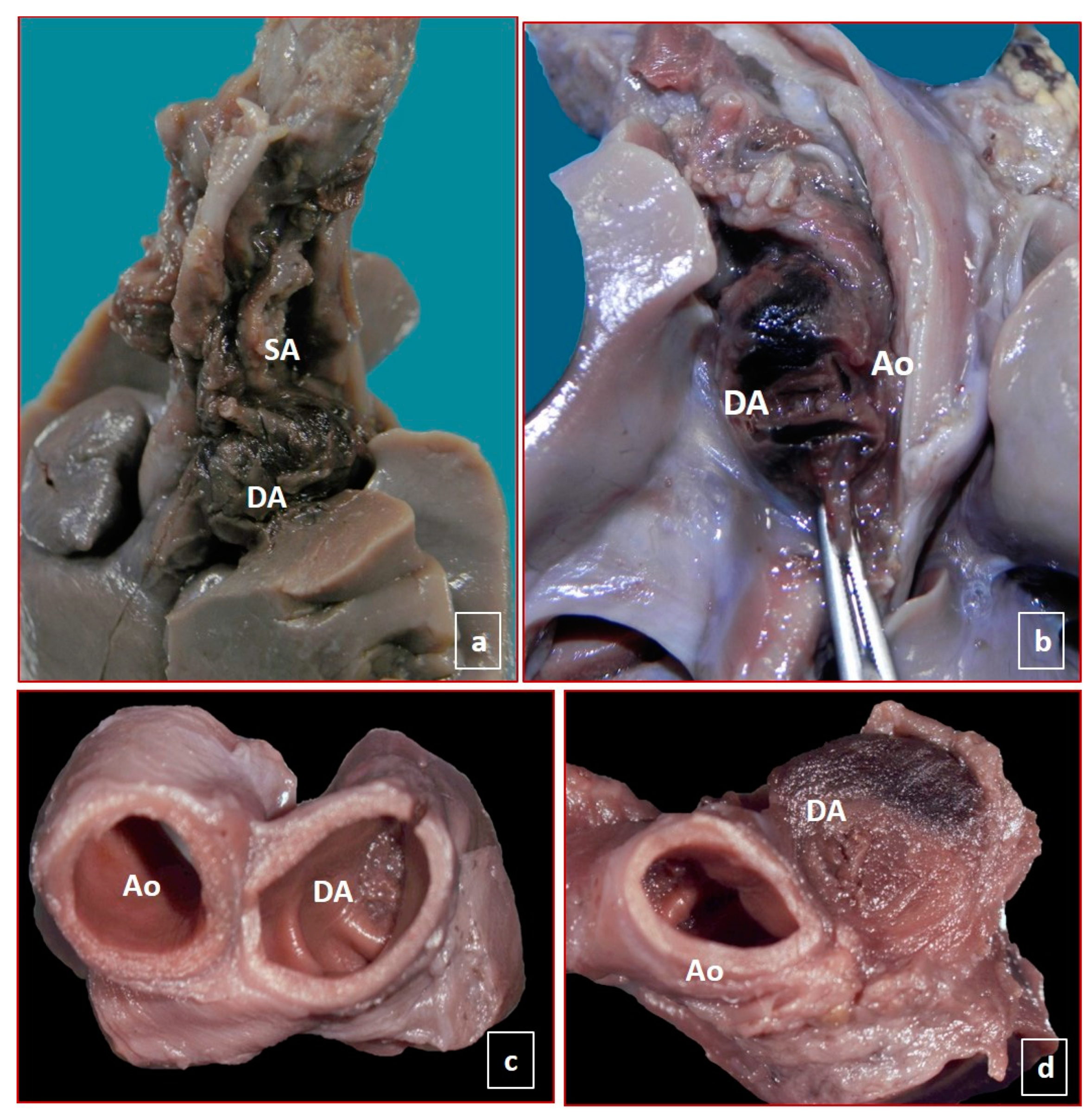



| Case | Sex | GA | Ethnicity | Risk Factors of Mother | Pregnancy | Medications during Pregnancy | Reported Symptoms during Pregnancy |
|---|---|---|---|---|---|---|---|
| 1 | M | 33 | Caucasian | Smoking during pregnancy | First | None | None |
| 2 | M | 32 | Caucasian | No risk factors | Second | None | None |
| 3 | M | 32 | Caucasian | Hypertension, smoking during pregnancy | First | None | Hypertension |
| 4 | M | 26 | Caucasian | No risk factors | First | None | None |
| 5 | M | 33 | Caucasian | Pre-eclampsia | First | None | Hypertension |
| Case | Body Weight | Heart Weight | Wall Thickness RV/S/LV mm | GM | DA Anatomical Macroscopic Description | Foramen Ovale |
|---|---|---|---|---|---|---|
| 1 | 1750 g | 8.7 g | 2.5/2.8/2.8 | III | Dissecting DA | Restrictive |
| 2 | 1650 g | 8.5 g | 2.4/2.5/2.5 | III | Dissecting DA | Patent |
| 3 | 1300 g | 7 g | 2/2/2.2 | III | Dissecting DA | Patent |
| 4 | 810 g | 5 g | 1.2/1.3/1.8 | III | Dissecting DA | Patent |
| 5 | 1670 g | 9 g | 2.3/2.5/2.5 | I | Dissecting DA | Patent |
| Case | Intimal Flap | Intima | Media-Adventitia | Inflammation | Apoptosis (TUNEL) |
|---|---|---|---|---|---|
| 1 | Absent | No subendothelial cushions, thrombotic lumen occlusion | Outer dissecting hematoma, no cystic medial necrosis | Absent | + |
| 2 | Present | No subendothelial cushions | Outer dissecting hematoma, cystic medial necrosis | Absent | +++ |
| 3 | Present | Subendothelial cushions, thrombus stratification | Outer dissecting hematoma, cystic medial necrosis | Absent | + |
| 4 | Present | No subendothelial cushions | Outer dissecting hematoma, no cystic medial necrosis | Absent | +++ |
| 5 | Present | Subendothelial cushions | Dissecting hematoma, cystic medial necrosis | Absent | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedrigo, M.; Visentin, S.; Veronese, P.; Barison, I.; Giarraputo, A.; Cosmi, E.; Thiene, G.; Gervasi, M.T.; Basso, C.; Angelini, A. Isolated Dissection of the Ductus Arteriosus Associated with Sudden Unexpected Intrauterine Death. J. Cardiovasc. Dev. Dis. 2021, 8, 91. https://doi.org/10.3390/jcdd8080091
Fedrigo M, Visentin S, Veronese P, Barison I, Giarraputo A, Cosmi E, Thiene G, Gervasi MT, Basso C, Angelini A. Isolated Dissection of the Ductus Arteriosus Associated with Sudden Unexpected Intrauterine Death. Journal of Cardiovascular Development and Disease. 2021; 8(8):91. https://doi.org/10.3390/jcdd8080091
Chicago/Turabian StyleFedrigo, Marny, Silvia Visentin, Paola Veronese, Ilaria Barison, Alessia Giarraputo, Erich Cosmi, Gaetano Thiene, Maria Teresa Gervasi, Cristina Basso, and Annalisa Angelini. 2021. "Isolated Dissection of the Ductus Arteriosus Associated with Sudden Unexpected Intrauterine Death" Journal of Cardiovascular Development and Disease 8, no. 8: 91. https://doi.org/10.3390/jcdd8080091
APA StyleFedrigo, M., Visentin, S., Veronese, P., Barison, I., Giarraputo, A., Cosmi, E., Thiene, G., Gervasi, M. T., Basso, C., & Angelini, A. (2021). Isolated Dissection of the Ductus Arteriosus Associated with Sudden Unexpected Intrauterine Death. Journal of Cardiovascular Development and Disease, 8(8), 91. https://doi.org/10.3390/jcdd8080091








