Collagen Fibrillogenesis in the Mitral Valve: It’s a Matter of Compliance
Abstract
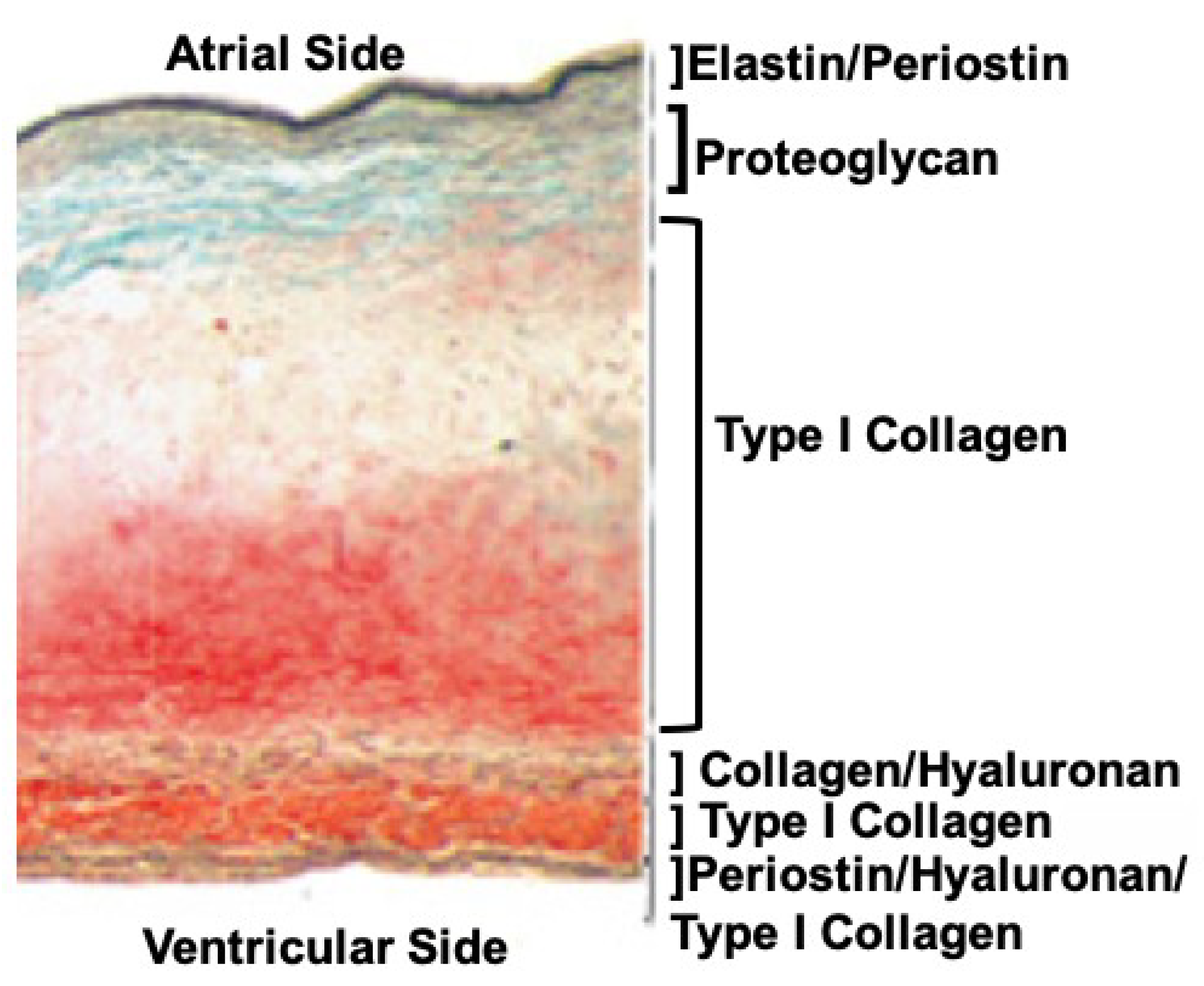
:1. Collagen in the Mitral Valve
2. Collagens, Fibrils, and Fibers of the Mitral Valve
3. Collagen Fibers and Mitral Dysfunction
4. Conclusions
Funding
Conflicts of Interest
References
- Kheradvar, A.; Groves, E.M.; Dasi, L.P.; Alavi, S.H.; Tranquillo, R.T.; Grande-Allen, K.J.; Simmons, C.A.; Griffith, B.E.; Falahatpisheh, A.; Goergen, C.J.; et al. Emerging Trends in Heart Valve Engineering: Part I. Solutions for Future. Ann. Biomed. Eng. 2015, 43, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Grande-Allen, K.J.; Liao, J. The heterogeneous biomechanics and mechanobiology of the mitral valve: Implications for tissue engineering. Curr. Cardiol. Rep. 2011, 13, 113–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Vlaming, A.; Sauls, K.; Hajdu, Z.; Visconti, R.P.; Mehesz, A.N.; Levine, R.A.; Slaugenhaupt, S.A.; Hagège, A.; Chester, A.H.; Markwald, R.R.; et al. Atrioventricular valve development: New perspectives on an old theme. Differentiation 2012, 84, 103–116. [Google Scholar] [CrossRef] [Green Version]
- Liao, J.; Vesely, I. A structural basis for the size-related mechanical properties of mitral valve chordae tendineae. J. Biomech. 2003, 36, 1125–1133. [Google Scholar] [CrossRef]
- Fidler, A.L.; Boudko, S.P.; Rokas, A.; Hudson, B.G. The triple helix of collagens—An ancient protein structure that enabled animal multicellularity and tissue evolution. J. Cell Sci. 2018, 131, jcs203950. [Google Scholar] [CrossRef] [Green Version]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [Green Version]
- Hulmes, D.J. Building collagen molecules, fibrils, and suprafibrillar structures. J. Struct. Biol. 2002, 137, 2–10. [Google Scholar] [CrossRef]
- Kodigepalli, K.M.; Thatcher, K.; West, T.; Howsmon, D.P.; Schoen, F.J.; Sacks, M.S.; Breuer, C.K.; Lincoln, J. Biology and Biomechanics of the Heart Valve Extracellular Matrix. J. Cardiovasc. Dev. Dis. 2020, 7, 57. [Google Scholar] [CrossRef]
- Cescon, M.; Gattazzo, F.; Chen, P.; Bonaldo, P. Collagen VI at a glance. J. Cell Sci. 2015, 128, 3525–3531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norris, R.A.; Moreno-Rodriguez, R.A.; Sugi, Y.; Hoffman, S.; Amos, J.; Hart, M.M.; Potts, J.D.; Goodwin, R.L.; Markwald, R.R. Periostin regulates atrioventricular valve maturation. Dev. Biol. 2008, 316, 200–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, C.J.; Zheng, J.; Ma, L.; Wu, Y.; Lee, C.H. Mechanics and Microstructure of the Atrioventricular Heart Valve Chordae Tendineae: A Review. Bioengineering 2020, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Kunzelman, K.S.; Cochran, R.P. Mechanical properties of basal and marginal mitral valve chordae tendineae. ASAIO Trans. 1990, 36, M405–M408. [Google Scholar]
- Wang, H.; Abhilash, A.S.; Chen, C.S.; Wells, R.G.; Shenoy, V.B. Long-range force transmission in fibrous matrices enabled by tension-driven alignment of fibers. Biophys. J. 2014, 107, 2592–2603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kheradvar, A.; Groves, E.M.; Falahatpisheh, A.; Mofrad, M.R.K.; Alavi, S.H.; Tranquillo, R.; Dasi, L.P.; Simmons, C.A.; Goergen, C.J.; Baaijens, F.; et al. Emerging Trends in Heart Valve Engineering: Part IV. Computational Modeling and Experimental Studies. Ann. Biomed. Eng. 2015, 43, 2314–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacks, M.S.; Smith, D.B.; Hiester, E.D. The aortic valve microstructure: Effects of transvalvular pressure. J. Biomed. Mater. Res. 1998, 41, 131–141. [Google Scholar] [CrossRef]
- Driessen, N.J.; Peters, G.W.; Huyghe, J.M.; Bouten, C.V.; Baaijens, F.P. Remodelling of continuously distributed collagen fibres in soft connective tissues. J. Biomech. 2003, 36, 1151–1158. [Google Scholar] [CrossRef]
- Driessen, N.J.; Cox, M.A.; Bouten, C.V.; Baaijens, F.P. Remodelling of the angular collagen fiber distribution in cardiovascular tissues. Biomech. Modeling Mechanobiol. 2008, 7, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Peacock, J.D.; Lu, Y.; Koch, M.; Kadler, K.E.; Lincoln, J. Temporal and spatial expression of collagens during murine atrioventricular heart valve development and maintenance. Dev. Dyn. 2008, 237, 3051–3058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lincoln, J.; Lange, A.W.; Yutzey, K.E. Hearts and bones: Shared regulatory mechanisms in heart valve, cartilage, tendon, and bone development. Dev. Biol. 2006, 294, 292–302. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Biechler, S.; Junor, L.; Yost, M.J.; Dean, D.; Li, J.; Potts, J.D.; Goodwin, R.L. Fluid flow forces and rhoA regulate fibrous development of the atrioventricular valves. Dev. Biol. 2013, 374, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Biechler, S.V.; Junor, L.; Evans, A.N.; Eberth, J.F.; Price, R.L.; Potts, J.D.; Yost, M.J.; Goodwin, R.L. The impact of flow-induced forces on the morphogenesis of the outflow tract. Front. Physiol. 2014, 5, 225. [Google Scholar] [CrossRef]
- Falahatpisheh, A.; Pahlevan, N.M.; Kheradvar, A. Effect of the Mitral Valve’s Anterior Leaflet on Axisymmetry of Transmitral Vortex Ring. Ann. Biomed. Eng. 2015, 43, 2349–2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hjortnaes, J.; Keegan, J.; Bruneval, P.; Schwartz, E.; Schoen, F.J.; Carpentier, A.; Levine, R.A.; Hagège, A.; Aikawa, E. Comparative Histopathological Analysis of Mitral Valves in Barlow Disease and Fibroelastic Deficiency. Semin. Thorac. Cardiovasc. Surg. 2016, 28, 757–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, A.J.; Xu, N.; Yutzey, K.E. Macrophage lineages in heart valve development and disease. Cardiovasc. Res. 2021, 117, 663–673. [Google Scholar] [CrossRef]
- Kim, A.J.; Xu, N.; Umeyama, K.; Hulin, A.; Ponny, S.R.; Vagnozzi, R.J.; Green, E.A.; Hanson, P.; McManus, B.M.; Nagashima, H.; et al. Deficiency of Circulating Monocytes Ameliorates the Progression of Myxomatous Valve Degeneration in Marfan Syndrome. Circulation 2020, 141, 132–146. [Google Scholar] [CrossRef]
- Gensemer, C.; Burks, R.; Kautz, S.; Judge, D.P.; Lavallee, M.; Norris, R.A. Hypermobile Ehlers-Danlos syndromes: Complex phenotypes, challenging diagnoses, and poorly understood causes. Dev. Dyn. 2021, 250, 318–344. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, V.; Morlino, S.; Di Stolfo, G.; Mastroianno, S.; Mazza, T.; Castori, M. Cardiac valvular Ehlers-Danlos syndrome is a well-defined condition due to recessive null variants in COL1A2. Am. J. Med. Genet. 2019, 179, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Morlino, S.; Micale, L.; Ritelli, M.; Rohrbach, M.; Zoppi, N.; Vandersteen, A.; Mackay, S.; Agolini, E.; Cocciadiferro, D.; Sasaki, E.; et al. COL1-related overlap disorder: A novel connective tissue disorder incorporating the osteogenesis imperfecta/Ehlers-Danlos syndrome overlap. Clin. Genet. 2020, 97, 396–406. [Google Scholar] [CrossRef]
- Olson, L.J.; Subramanian, R.; Ackermann, D.M.; Orszulak, T.A.; Edwards, W.D. Surgical pathology of the mitral valve: A study of 712 cases spanning 21 years. Mayo Clin. Proc. 1987, 62, 22–34. [Google Scholar] [CrossRef]
- Anyanwu, A.C.; Adams, D.H. Etiologic classification of degenerative mitral valve disease: Barlow’s disease and fibroelastic deficiency. Semin. Thorac. Cardiovasc. Surg. 2007, 19, 90–96. [Google Scholar] [CrossRef] [Green Version]
- van Wijngaarden, A.L.; Kruithof, B.P.T.; Vinella, T.; Barge-Schaapveld, D.Q.C.M.; Ajmone Marsan, N. Characterization of Degenerative Mitral Valve Disease: Differences between Fibroelastic Deficiency and Barlow’s Disease. J. Cardiovasc. Dev. Dis. 2021, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Passos, L.S.A.; Nunes, M.C.P.; Aikawa, E. Rheumatic Heart Valve Disease Pathophysiology and Underlying Mechanisms. Front. Cardiovasc. Med. 2021, 7, 612716. [Google Scholar] [CrossRef] [PubMed]
- Toomer, K.A.; Yu, M.; Fulmer, D.; Guo, L.; Moore, K.S.; Moore, R.; Drayton, K.D.; Glover, J.; Peterson, N.; Ramos-Ortiz, S.; et al. Primary cilia defects causing mitral valve prolapse. Sci. Transl. Med. 2019, 11, eaax0290. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goodwin, R.L.; Kheradvar, A.; Norris, R.A.; Price, R.L.; Potts, J.D. Collagen Fibrillogenesis in the Mitral Valve: It’s a Matter of Compliance. J. Cardiovasc. Dev. Dis. 2021, 8, 98. https://doi.org/10.3390/jcdd8080098
Goodwin RL, Kheradvar A, Norris RA, Price RL, Potts JD. Collagen Fibrillogenesis in the Mitral Valve: It’s a Matter of Compliance. Journal of Cardiovascular Development and Disease. 2021; 8(8):98. https://doi.org/10.3390/jcdd8080098
Chicago/Turabian StyleGoodwin, Richard L., Arash Kheradvar, Russell A. Norris, Robert L. Price, and Jay D. Potts. 2021. "Collagen Fibrillogenesis in the Mitral Valve: It’s a Matter of Compliance" Journal of Cardiovascular Development and Disease 8, no. 8: 98. https://doi.org/10.3390/jcdd8080098
APA StyleGoodwin, R. L., Kheradvar, A., Norris, R. A., Price, R. L., & Potts, J. D. (2021). Collagen Fibrillogenesis in the Mitral Valve: It’s a Matter of Compliance. Journal of Cardiovascular Development and Disease, 8(8), 98. https://doi.org/10.3390/jcdd8080098





