New Interventional Therapies beyond Stenting to Treat ST-Segment Elevation Acute Myocardial Infarction
Abstract
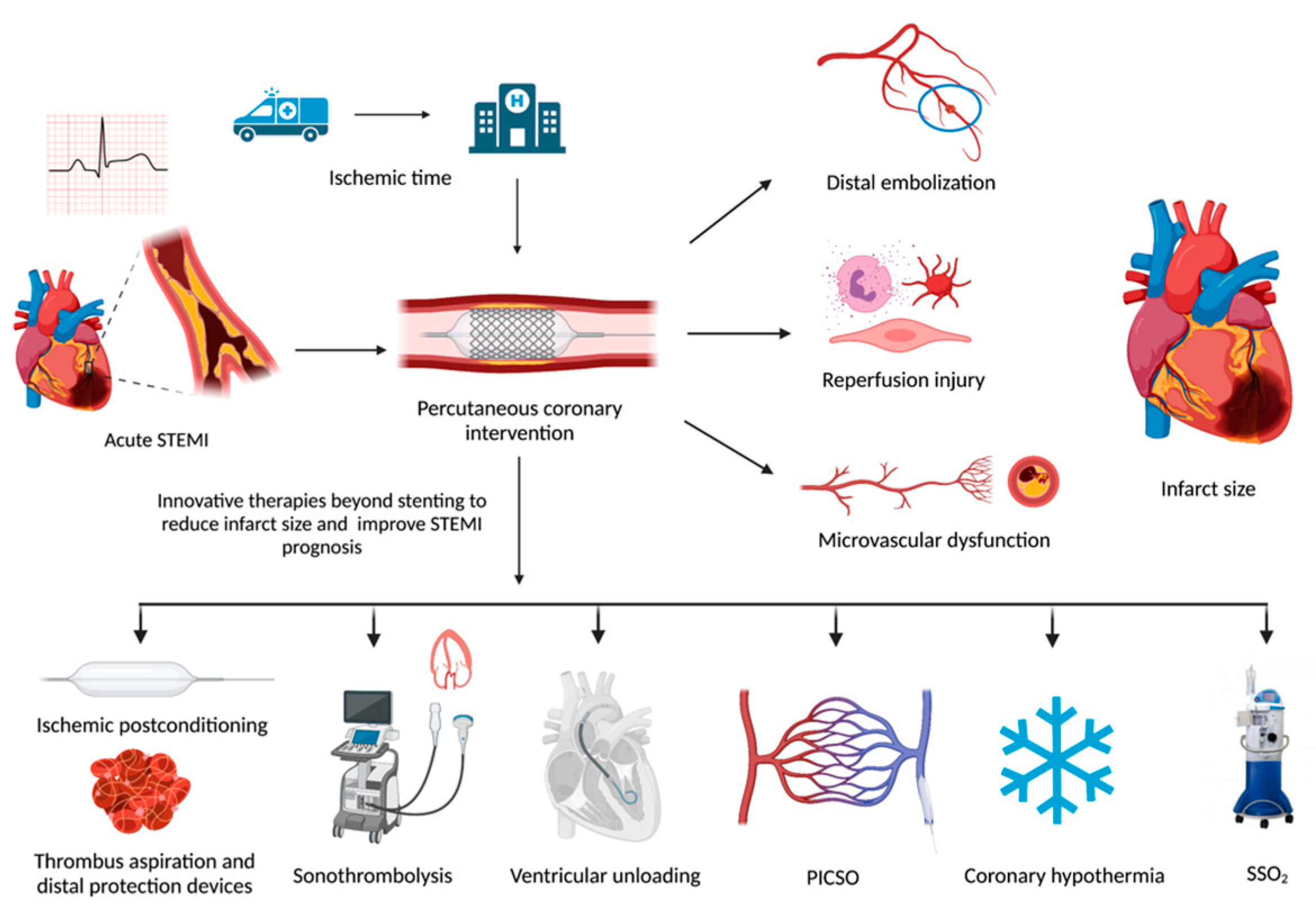
:1. Introduction
2. Prognostic Determinants in Stemi
2.1. Ischemic Time
2.2. Distal Embolization
2.3. Reperfusion Injury
2.4. Microvascular Dysfunction
3. Therapies to Prevent Distal Embolization
3.1. Aspiration Thrombectomy
3.2. Sonothrombolysis
3.3. Distal Protection Devices
4. Ischemic Postconditioning
5. Left Ventricle Unloading
5.1. Intra-Aortic Balloon Counterpulsation
5.2. Assist Devices
6. Supersaturated Oxygen
7. Therapeutic Hypothermia
8. Pressure-Controlled Intermittent Coronary Sinus Occlusion
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nabel, E.G.; Braunwald, E. A tale of coronary artery disease and myocardial infarction. N. Engl. J. Med. 2012, 366, 54–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, F.; Butrymovich, V.; Kelbæk, H.; Wachtell, K.; Helqvist, S.; Kastrup, J.; Holmvang, L.; Clemmensen, P.; Engstrøm, T.; Grande, P.; et al. Short- and long-term cause of death in patients treated with primary PCI for STEMI. J. Am. Coll. Cardiol. 2014, 64, 2101–2108. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.; Teng, T.H.; Finn, J.; Knuiman, M.; Briffa, T.; Stewart, S.; Sanfilippo, F.M.; Ridout, S.; Hobbs, M. Trends from 1996 to 2007 in incidence and mortality outcomes of heart failure after acute myocardial infarction: A population-based study of 20,812 patients with first acute myocardial infarction in Western Australia. J. Am. Heart Assoc. 2013, 2, e000172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menees, D.S.; Peterson, E.D.; Wang, Y.; Curtis, J.P.; Messenger, J.C.; Rumsfeld, J.S.; Gurm, H.S. Door-to-balloon time and mortality among patients undergoing primary PCI. N. Engl. J. Med. 2013, 369, 901–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, G.W.; Selker, H.P.; Thiele, H.; Patel, M.R.; Udelson, J.E.; Ohman, E.M.; Maehara, A.; Eitel, I.; Granger, C.B.; Jenkins, P.L.; et al. Relationship Between Infarct Size and Outcomes Following Primary PCI: Patient-Level Analysis From 10 Randomized Trials. J. Am. Coll. Cardiol. 2016, 67, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- De Waha, S.; Patel, M.R.; Granger, C.B.; Ohman, E.M.; Maehara, A.; Eitel, I.; Ben-Yehuda, O.; Jenkins, P.; Thiele, H.; Stone, G.W. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: An individual patient data pooled analysis from seven randomized trials. Eur. Heart J. 2017, 38, 3502–3510. [Google Scholar] [CrossRef] [PubMed]
- Sulo, G.; Igland, J.; Vollset, S.E.; Nygård, O.; Ebbing, M.; Sulo, E.; Egeland, G.M.; Tell, G.S. Heart Failure Complicating Acute Myocardial Infarction; Burden and Timing of Occurrence: A Nation-wide Analysis Including 86 771 Patients From the Cardiovascular Disease in Norway (CVDNOR) Project. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heusch, P.; Nensa, F.; Heusch, G. Is MRI Really the Gold Standard for the Quantification of Salvage From Myocardial Infarction? Circ. Res. 2015, 117, 222–224. [Google Scholar] [CrossRef] [Green Version]
- Berger, P.B.; Ellis, S.G.; Holmes, D.R., Jr.; Granger, C.B.; Criger, D.A.; Betriu, A.; Topol, E.J.; Califf, R.M. Relationship between delay in performing direct coronary angioplasty and early clinical outcome in patients with acute myocardial infarction: Results from the global use of strategies to open occluded arteries in Acute Coronary Syndromes (GUSTO-IIb) trial. Circulation 1999, 100, 14–20. [Google Scholar] [CrossRef] [Green Version]
- De Luca, G.; Suryapranata, H.; Ottervanger, J.P.; Antman, E.M. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: Every minute of delay counts. Circulation 2004, 109, 1223–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNamara, R.L.; Wang, Y.; Herrin, J.; Curtis, J.P.; Bradley, E.H.; Magid, D.J.; Peterson, E.D.; Blaney, M.; Frederick, P.D.; Krumholz, H.M. Effect of door-to-balloon time on mortality in patients with ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2006, 47, 2180–2186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lønborg, J.; Kelbæk, H.; Helqvist, S.; Holmvang, L.; Jørgensen, E.; Saunamäki, K.; Kløvgaard, L.; Kaltoft, A.; Bøtker, H.E.; Lassen, J.F.; et al. The impact of distal embolization and distal protection on long-term outcome in patients with ST elevation myocardial infarction randomized to primary percutaneous coronary intervention--results from a randomized study. Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G.; Kleinbongard, P.; Böse, D.; Levkau, B.; Haude, M.; Schulz, R.; Erbel, R. Coronary microembolization: From bedside to bench and back to bedside. Circulation 2009, 120, 1822–1836. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, G.M.; Meier, P.; White, S.K.; Yellon, D.M.; Hausenloy, D.J. Myocardial reperfusion injury: Looking beyond primary PCI. Eur. Heart J. 2013, 34, 1714–1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yellon, D.M.; Hausenloy, D.J. Myocardial reperfusion injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, R.; Charron, T.; Puley, G.; Dick, A.; Strauss, B.H. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation 2008, 117, 3152–3156. [Google Scholar] [CrossRef] [Green Version]
- Niccoli, G.; Scalone, G.; Lerman, A.; Crea, F. Coronary microvascular obstruction in acute myocardial infarction. Eur. Heart J. 2016, 37, 1024–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ørn, S.; Manhenke, C.; Greve, O.J.; Larsen, A.I.; Bonarjee, V.V.; Edvardsen, T.; Dickstein, K. Microvascular obstruction is a major determinant of infarct healing and subsequent left ventricular remodelling following primary percutaneous coronary intervention. Eur. Heart J. 2009, 30, 1978–1985. [Google Scholar] [CrossRef] [Green Version]
- Nijveldt, R.; Beek, A.M.; Hirsch, A.; Stoel, M.G.; Hofman, M.B.; Umans, V.A.; Algra, P.R.; Twisk, J.W.; van Rossum, A.C. Functional recovery after acute myocardial infarction: Comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J. Am. Coll. Cardiol. 2008, 52, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Niccoli, G.; Montone, R.A.; Ibanez, B.; Thiele, H.; Crea, F.; Heusch, G.; Bulluck, H.; Hausenloy, D.J.; Berry, C.; Stiermaier, T.; et al. Optimized Treatment of ST-Elevation Myocardial Infarction. Circ. Res. 2019, 125, 245–258. [Google Scholar] [CrossRef]
- Svilaas, T.; Vlaar, P.J.; van der Horst, I.C.; Diercks, G.F.; de Smet, B.J.; van den Heuvel, A.F.; Anthonio, R.L.; Jessurun, G.A.; Tan, E.S.; Suurmeijer, A.J.; et al. Thrombus aspiration during primary percutaneous coronary intervention. N. Engl. J. Med. 2008, 358, 557–567. [Google Scholar] [CrossRef] [Green Version]
- Vlaar, P.J.; Svilaas, T.; van der Horst, I.C.; Diercks, G.F.; Fokkema, M.L.; de Smet, B.J.; van den Heuvel, A.F.; Anthonio, R.L.; Jessurun, G.A.; Tan, E.S.; et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): A 1-year follow-up study. Lancet 2008, 371, 1915–1920. [Google Scholar] [CrossRef]
- Fröbert, O.; Lagerqvist, B.; Olivecrona, G.K.; Omerovic, E.; Gudnason, T.; Maeng, M.; Aasa, M.; Angerås, O.; Calais, F.; Danielewicz, M.; et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N. Engl. J. Med. 2013, 369, 1587–1597. [Google Scholar] [CrossRef] [Green Version]
- Lagerqvist, B.; Fröbert, O.; Olivecrona, G.K.; Gudnason, T.; Maeng, M.; Alström, P.; Andersson, J.; Calais, F.; Carlsson, J.; Collste, O.; et al. Outcomes 1 year after thrombus aspiration for myocardial infarction. N. Engl. J. Med. 2014, 371, 1111–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolly, S.S.; Cairns, J.A.; Yusuf, S.; Meeks, B.; Pogue, J.; Rokoss, M.J.; Kedev, S.; Thabane, L.; Stankovic, G.; Moreno, R.; et al. Randomized trial of primary PCI with or without routine manual thrombectomy. N. Engl. J. Med. 2015, 372, 1389–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabaté, M.; Brugaletta, S. Thrombectomy and Stroke: Guilty or Innocent Bystander? J. Am. Coll. Cardiol. 2018, 72, 1597–1599. [Google Scholar] [CrossRef]
- Xie, F.; Lof, J.; Matsunaga, T.; Zutshi, R.; Porter, T.R. Diagnostic ultrasound combined with glycoprotein IIb/IIIa-targeted microbubbles improves microvascular recovery after acute coronary thrombotic occlusions. Circulation 2009, 119, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Lof, J.; Everbach, C.; He, A.; Bennett, R.M.; Matsunaga, T.; Johanning, J.; Porter, T.R. Treatment of acute intravascular thrombi with diagnostic ultrasound and intravenous microbubbles. JACC Cardiovasc. Imaging 2009, 2, 511–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, F.; Slikkerveer, J.; Gao, S.; Lof, J.; Kamp, O.; Unger, E.; Radio, S.; Matsunaga, T.; Porter, T.R. Coronary and microvascular thrombolysis with guided diagnostic ultrasound and microbubbles in acute ST segment elevation myocardial infarction. J. Am. Soc. Echocardiogr. 2011, 24, 1400–1408. [Google Scholar] [CrossRef] [Green Version]
- Mathias, W., Jr.; Tsutsui, J.M.; Tavares, B.G.; Xie, F.; Aguiar, M.O.; Garcia, D.R.; Oliveira, M.T., Jr.; Soeiro, A.; Nicolau, J.C.; Lemos, P.A.N.; et al. Diagnostic Ultrasound Impulses Improve Microvascular Flow in Patients With STEMI Receiving Intravenous Microbubbles. J. Am. Coll. Cardiol. 2016, 67, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- Mathias, W., Jr.; Tsutsui, J.M.; Tavares, B.G.; Fava, A.M.; Aguiar, M.O.D.; Borges, B.C.; Oliveira, M.T., Jr.; Soeiro, A.; Nicolau, J.C.; Ribeiro, H.B.; et al. Sonothrombolysis in ST-Segment Elevation Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2019, 73, 2832–2842. [Google Scholar] [CrossRef] [PubMed]
- El Kadi, S.; Porter, T.R.; van Rossum, A.C.; Kamp, O. Sonothrombolysis in the ambulance for ST-elevation myocardial infarction: Rationale and protocol. Neth. Heart J. 2021, 29, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Baim, D.S.; Wahr, D.; George, B.; Leon, M.B.; Greenberg, J.; Cutlip, D.E.; Kaya, U.; Popma, J.J.; Ho, K.K.; Kuntz, R.E. Randomized trial of a distal embolic protection device during percutaneous intervention of saphenous vein aorto-coronary bypass grafts. Circulation 2002, 105, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Webb, J.; Cox, D.A.; Brodie, B.R.; Qureshi, M.; Kalynych, A.; Turco, M.; Schultheiss, H.P.; Dulas, D.; Rutherford, B.D.; et al. Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction: A randomized controlled trial. JAMA 2005, 293, 1063–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelbaek, H.; Terkelsen, C.J.; Helqvist, S.; Lassen, J.F.; Clemmensen, P.; Kløvgaard, L.; Kaltoft, A.; Engstrøm, T.; Bøtker, H.E.; Saunamäki, K.; et al. Randomized comparison of distal protection versus conventional treatment in primary percutaneous coronary intervention: The drug elution and distal protection in ST-elevation myocardial infarction (DEDICATION) trial. J. Am. Coll. Cardiol. 2008, 51, 899–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.Q.; Corvera, J.S.; Halkos, M.E.; Kerendi, F.; Wang, N.P.; Guyton, R.A.; Vinten-Johansen, J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: Comparison with ischemic preconditioning. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H579–H588. [Google Scholar] [CrossRef] [PubMed]
- Staat, P.; Rioufol, G.; Piot, C.; Cottin, Y.; Cung, T.T.; L’Huillier, I.; Aupetit, J.F.; Bonnefoy, E.; Finet, G.; André-Fouët, X.; et al. Postconditioning the human heart. Circulation 2005, 112, 2143–2148. [Google Scholar] [CrossRef] [PubMed]
- Thuny, F.; Lairez, O.; Roubille, F.; Mewton, N.; Rioufol, G.; Sportouch, C.; Sanchez, I.; Bergerot, C.; Thibault, H.; Cung, T.T.; et al. Post-conditioning reduces infarct size and edema in patients with ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2012, 59, 2175–2181. [Google Scholar] [CrossRef] [Green Version]
- Sörensson, P.; Saleh, N.; Bouvier, F.; Böhm, F.; Settergren, M.; Caidahl, K.; Tornvall, P.; Arheden, H.; Rydén, L.; Pernow, J. Effect of postconditioning on infarct size in patients with ST elevation myocardial infarction. Heart 2010, 96, 1710–1715. [Google Scholar] [CrossRef]
- Freixa, X.; Bellera, N.; Ortiz-Pérez, J.T.; Jiménez, M.; Paré, C.; Bosch, X.; De Caralt, T.M.; Betriu, A.; Masotti, M. Ischemic postconditioning revisited: Lack of effects on infarct size following primary percutaneous coronary intervention. Eur. Heart J. 2012, 33, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Limalanathan, S.; Andersen, G.; Kløw, N.E.; Abdelnoor, M.; Hoffmann, P.; Eritsland, J. Effect of ischemic postconditioning on infarct size in patients with ST-elevation myocardial infarction treated by primary PCI results of the POSTEMI (POstconditioning in ST-Elevation Myocardial Infarction) randomized trial. J. Am. Heart Assoc. 2014, 3, e000679. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.R.; Binabdulhak, A.A.; Alastal, Y.; Khan, S.; Faricy-Beredo, B.M.; Luni, F.K.; Lee, W.M.; Khuder, S.; Tinkel, J. Cardioprotective role of ischemic postconditioning in acute myocardial infarction: A systematic review and meta-analysis. Am. Heart J. 2014, 168, 512–521.e514. [Google Scholar] [CrossRef]
- Favaretto, E.; Roffi, M.; Frigo, A.C.; Lee, M.S.; Marra, M.P.; Napodano, M.; Tarantini, G. Meta-analysis of randomized trials of postconditioning in ST-elevation myocardial infarction. Am. J. Cardiol. 2014, 114, 946–952. [Google Scholar] [CrossRef]
- Khalili, H.; Patel, V.G.; Mayo, H.G.; de Lemos, J.A.; Brilakis, E.S.; Banerjee, S.; Bavry, A.A.; Bhatt, D.L.; Kumbhani, D.J. Surrogate and clinical outcomes following ischemic postconditioning during primary percutaneous coronary intervention of ST-segment elevation myocardial infarction: A meta-analysis of 15 randomized trials. Catheter. Cardiovasc. Interv. 2014, 84, 978–986. [Google Scholar] [CrossRef]
- Zhou, C.; Yao, Y.; Zheng, Z.; Gong, J.; Wang, W.; Hu, S.; Li, L. Stenting technique, gender, and age are associated with cardioprotection by ischemic postconditioning in primary coronary intervention: A systematic review of 10 randomized trials. Eur. Heart J. 2012, 33, 3070–3077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heusch, G. Reduction of infarct size by ischemic post-conditioning in humans: Fact or fiction? Eur. Heart J. 2012, 33, 13–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nepper-Christensen, L.; Høfsten, D.E.; Helqvist, S.; Lassen, J.F.; Tilsted, H.H.; Holmvang, L.; Pedersen, F.; Joshi, F.; Sørensen, R.; Bang, L.; et al. Interaction of ischemic postconditioning and thrombectomy in patients with ST-elevation myocardial infarction. Heart 2020, 106, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Swain, L.; Reyelt, L.; Bhave, S.; Qiao, X.; Thomas, C.J.; Zweck, E.; Crowley, P.; Boggins, C.; Esposito, M.; Chin, M.; et al. Transvalvular Ventricular Unloading Before Reperfusion in Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2020, 76, 684–699. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.J.; Aguirre, F.; Bach, R.; Donohue, T.; Siegel, R.; Segal, J. Augmentation of coronary blood flow by intra-aortic balloon pumping in patients after coronary angioplasty. Circulation 1993, 87, 500–511. [Google Scholar] [CrossRef] [Green Version]
- Achour, H.; Boccalandro, F.; Felli, P.; Amirian, J.; Uthman, M.; Buja, M.; Smalling, R.W. Mechanical left ventricular unloading prior to reperfusion reduces infarct size in a canine infarction model. Catheter. Cardiovasc. Interv. 2005, 64, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.F.; Amado, L.C.; Kraitchman, D.L.; Gerber, B.L.; Edvardsen, T.; Osman, N.F.; Rochitte, C.E.; Wu, K.C.; Lima, J.A. The effect of intra-aortic balloon counterpulsation on left ventricular functional recovery early after acute myocardial infarction: A randomized experimental magnetic resonance imaging study. Eur. Heart J. 2005, 26, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Smalling, R.W.; Thiele, H.; Barnhart, H.X.; Zhou, Y.; Chandra, P.; Chew, D.; Cohen, M.; French, J.; Perera, D.; et al. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: The CRISP AMI randomized trial. JAMA 2011, 306, 1329–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassese, S.; de Waha, A.; Ndrepepa, G.; Ranftl, S.; King, L.; Schömig, A.; Kastrati, A. Intra-aortic balloon counterpulsation in patients with acute myocardial infarction without cardiogenic shock. A meta-analysis of randomized trials. Am. Heart J. 2012, 164, 58–65.e51. [Google Scholar] [CrossRef] [PubMed]
- Burkhoff, D.; Sayer, G.; Doshi, D.; Uriel, N. Hemodynamics of Mechanical Circulatory Support. J. Am. Coll. Cardiol. 2015, 66, 2663–2674. [Google Scholar] [CrossRef] [Green Version]
- Sjauw, K.D.; Remmelink, M.; Baan, J., Jr.; Lam, K.; Engström, A.E.; van der Schaaf, R.J.; Vis, M.M.; Koch, K.T.; van Straalen, J.P.; Tijssen, J.G.; et al. Left ventricular unloading in acute ST-segment elevation myocardial infarction patients is safe and feasible and provides acute and sustained left ventricular recovery. J. Am. Coll. Cardiol. 2008, 51, 1044–1046. [Google Scholar] [CrossRef] [Green Version]
- Kapur, N.K.; Qiao, X.; Paruchuri, V.; Morine, K.J.; Syed, W.; Dow, S.; Shah, N.; Pandian, N.; Karas, R.H. Mechanical Pre-Conditioning With Acute Circulatory Support Before Reperfusion Limits Infarct Size in Acute Myocardial Infarction. JACC Heart Fail. 2015, 3, 873–882. [Google Scholar] [CrossRef]
- Kapur, N.K.; Alkhouli, M.A.; DeMartini, T.J.; Faraz, H.; George, Z.H.; Goodwin, M.J.; Hernandez-Montfort, J.A.; Iyer, V.S.; Josephy, N.; Kalra, S.; et al. Unloading the Left Ventricle Before Reperfusion in Patients With Anterior ST-Segment-Elevation Myocardial Infarction. Circulation 2019, 139, 337–346. [Google Scholar] [CrossRef]
- Parikh, M.J.; Schuleri, K.H.; Chakrabarti, A.K.; O’Neill, W.W.; Kapur, N.K.; Wohns, D.H. Door-to-unload: Left ventricular unloading before reperfusion in ST-elevation myocardial infarction. Future Cardiol. 2021, 17, 549–559. [Google Scholar] [CrossRef]
- Buras, J. Basic mechanisms of hyperbaric oxygen in the treatment of ischemia-reperfusion injury. Int. Anesth. Clin. 2000, 38, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Bartorelli, A.L. Hyperoxemic perfusion for treatment of reperfusion microvascular ischemia in patients with myocardial infarction. Am. J. Cardiovasc. Drugs 2003, 3, 253–263. [Google Scholar] [CrossRef]
- O’Neill, W.W.; Martin, J.L.; Dixon, S.R.; Bartorelli, A.L.; Trabattoni, D.; Oemrawsingh, P.V.; Atsma, D.E.; Chang, M.; Marquardt, W.; Oh, J.K.; et al. Acute Myocardial Infarction with Hyperoxemic Therapy (AMIHOT): A prospective, randomized trial of intracoronary hyperoxemic reperfusion after percutaneous coronary intervention. J. Am. Coll. Cardiol. 2007, 50, 397–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, G.W.; Martin, J.L.; de Boer, M.J.; Margheri, M.; Bramucci, E.; Blankenship, J.C.; Metzger, D.C.; Gibbons, R.J.; Lindsay, B.S.; Weiner, B.H.; et al. Effect of supersaturated oxygen delivery on infarct size after percutaneous coronary intervention in acute myocardial infarction. Circ. Cardiovasc. Interv. 2009, 2, 366–375. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; David, S.W.; Khan, Z.A.; Metzger, D.C.; Wasserman, H.S.; Lotfi, A.S.; Hanson, I.D.; Dixon, S.R.; LaLonde, T.A.; Généreux, P.; et al. One-year outcomes of supersaturated oxygen therapy in acute anterior myocardial infarction: The IC-HOT study. Catheter. Cardiovasc. Interv. 2021, 97, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Herring, M.J.; Hale, S.L.; Dai, W.; Oskui, P.M.; Kloner, R.A. Hypothermia in the setting of experimental acute myocardial infarction: A comprehensive review. Hypothermia Temp. Manag. 2014, 4, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Erlinge, D. A Review of Mild Hypothermia as an Adjunctive Treatment for ST-Elevation Myocardial Infarction. Hypothermia Temp. Manag. 2011, 1, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Götberg, M.; Olivecrona, G.K.; Engblom, H.; Ugander, M.; van der Pals, J.; Heiberg, E.; Arheden, H.; Erlinge, D. Rapid short-duration hypothermia with cold saline and endovascular cooling before reperfusion reduces microvascular obstruction and myocardial infarct size. BMC Cardiovasc. Disord. 2008, 8, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Götberg, M.; Olivecrona, G.K.; Koul, S.; Carlsson, M.; Engblom, H.; Ugander, M.; van der Pals, J.; Algotsson, L.; Arheden, H.; Erlinge, D. A pilot study of rapid cooling by cold saline and endovascular cooling before reperfusion in patients with ST-elevation myocardial infarction. Circ. Cardiovasc. Interv. 2010, 3, 400–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erlinge, D.; Götberg, M.; Lang, I.; Holzer, M.; Noc, M.; Clemmensen, P.; Jensen, U.; Metzler, B.; James, S.; Bötker, H.E.; et al. Rapid endovascular catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. The CHILL-MI trial: A randomized controlled study of the use of central venous catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. J. Am. Coll. Cardiol. 2014, 63, 1857–1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noc, M.; Laanmets, P.; Neskovic, A.N.; Petrović, M.; Stanetic, B.; Aradi, D.; Kiss, R.G.; Ungi, I.; Merkely, B.; Hudec, M.; et al. A multicentre, prospective, randomised controlled trial to assess the safety and effectiveness of cooling as an adjunctive therapy to percutaneous intervention in patients with acute myocardial infarction: The COOL AMI EU Pivotal Trial. EuroIntervention 2021. [Google Scholar] [CrossRef] [PubMed]
- Otterspoor, L.C.; van Nunen, L.X.; Rosalina, T.T.; Veer, M.V.; Tuijl, S.V.; Stijnen, M.; Rutten, M.C.; van de Vosse, F.N.; Pijls, N.H. Intracoronary hypothermia for acute myocardial infarction in the isolated beating pig heart. Am. J. Transl. Res. 2017, 9, 558–568. [Google Scholar] [PubMed]
- El Farissi, M.; Keulards, D.C.J.; van ‘t Veer, M.; Zelis, J.M.; Berry, C.; De Bruyne, B.; Engstrøm, T.; Fröbert, O.; Piroth, Z.; Oldroyd, K.G.; et al. Selective intracoronary hypothermia in patients with ST-elevation myocardial infarction. Rationale and design of the EURO-ICE trial. EuroIntervention 2021, 16, 1444–1446. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.L.; Hahn, R.S.; Beck, C.S. Revascularization of the myocardium by arterialization of the coronary veins. II. Mechanism of myocardial protection after aorto-sinusoidal graft and partial ligature of the sinus. Rev. Chir. 1952, 71, 81–98. [Google Scholar] [PubMed]
- Mohl, W. The development and rationale of pressure-controlled intermittent coronary sinus occlusion—A new approach to protect ischemic myocardium. Wien. Klin. Wochenschr. 1984, 96, 20–25. [Google Scholar]
- De Maria, G.L.; Kassimis, G.; Raina, T.; Banning, A.P. Reconsidering the back door approach by targeting the coronary sinus in ischemic heart disease. Heart 2016, 102, 1263–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohl, W.; Milasinovic, D.; Faxon, D.P. Amending a dogma. EuroIntervention 2018, 14, e1258–e1261. [Google Scholar] [CrossRef] [PubMed]
- Mohl, W.; Henry, T.D.; Milasinovic, D.; Nguemo, F.; Hescheler, J.; Perin, E.C. From state-of-the-art cell therapy to endogenous cardiac repair. EuroIntervention 2017, 13, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Mohl, W.; Spitzer, E.; Mader, R.M.; Wagh, V.; Nguemo, F.; Milasinovic, D.; Jusić, A.; Khazen, C.; Szodorai, E.; Birkenberg, B.; et al. Acute molecular effects of pressure-controlled intermittent coronary sinus occlusion in patients with advanced heart failure. ESC Heart Fail. 2018, 5, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Syeda, B.; Schukro, C.; Heinze, G.; Modaressi, K.; Glogar, D.; Maurer, G.; Mohl, W. The salvage potential of coronary sinus interventions: Meta-analysis and pathophysiologic consequences. J. Thorac. Cardiovasc. Surg. 2004, 127, 1703–1712. [Google Scholar] [CrossRef]
- Van de Hoef, T.P.; Nolte, F.; Delewi, R.; Henriques, J.P.; Spaan, J.A.; Tijssen, J.G.; Siebes, M.; Wykrzykowska, J.J.; Stone, G.W.; Piek, J.J. Intracoronary hemodynamic effects of pressure-controlled intermittent coronary sinus occlusion (PICSO): Results from the First-In-Man Prepare PICSO Study. J. Interv. Cardiol. 2012, 25, 549–556. [Google Scholar] [CrossRef] [Green Version]
- Van de Hoef, T.P.; Nijveldt, R.; van der Ent, M.; Neunteufl, T.; Meuwissen, M.; Khattab, A.; Berger, R.; Kuijt, W.J.; Wykrzykowska, J.; Tijssen, J.G.; et al. Pressure-controlled intermittent coronary sinus occlusion (PICSO) in acute ST-segment elevation myocardial infarction: Results of the Prepare RAMSES safety and feasibility study. EuroIntervention 2015, 11, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Maria, G.L.; Alkhalil, M.; Borlotti, A.; Wolfrum, M.; Gaughran, L.; Dall’Armellina, E.; Langrish, J.P.; Lucking, A.J.; Choudhury, R.P.; Kharbanda, R.K.; et al. Index of microcirculatory resistance-guided therapy with pressure-controlled intermittent coronary sinus occlusion improves coronary microvascular function and reduces infarct size in patients with ST-elevation myocardial infarction: The Oxford Acute Myocardial Infarction—Pressure-controlled Intermittent Coronary Sinus Occlusion study (OxAMI-PICSO study). EuroIntervention 2018, 14, e352–e359. [Google Scholar] [CrossRef] [PubMed]
- Egred, M.; Bagnall, A.; Spyridopoulos, I.; Purcell, I.F.; Das, R.; Palmer, N.; Grech, E.D.; Jain, A.; Stone, G.W.; Nijveldt, R.; et al. Effect of Pressure-controlled intermittent Coronary Sinus Occlusion (PiCSO) on infarct size in anterior STEMI: PiCSO in ACS study. Int. J. Cardiol. Heart Vasc. 2020, 28, 100526. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Study and Design | n | Intervention | Main Results | Notes | Ref. |
|---|---|---|---|---|---|---|
| Thrombus aspiration | TASTE (2014) RCT | 7244 | Thrombus aspiration | No difference in 1-year mortality | [25] | |
| TOTAL (2015) RCT | 10732 | Thrombus aspiration | No difference in CV death, recurrent MI, cardiogenic shock, NYHA class IV HF in 180 days | ↑ stroke in 30 days (0.7% vs. 0.3%) * | [26] | |
| Sono thrombolysis | Mathias et al. (2019) RCT | 100 | Diagnostic ultrasound with contrast agent plus HMI pulses | 58% ↑ angiographic recanalization * 27% ↓ MI size by CMR at 72 h * 11% ↑ LV ejection fraction at 6 months * | [32] | |
| Distal protectiondevices | EMERALD (2005) RCT | 501 | Aspiration distal microcirculatory protection system | No difference in ST resolution, MI size at 5 days or MACE at 6 months | [35] | |
| Ischemic post conditioning | Khalili et al. (2014) Metanalysis | 1545 | Coronary inflation/deflation cycles with angioplasty balloon | No difference in ST resolution, MI size, mortality, recurrent MI, stent thrombosis or MACE | 15 RCTs reviewing clinical outcomes | [45] |
| DANAMI 3-iPOST (2017) RCT | 1234 | 4 repeated 30-s balloon occlusions followed by 30-s reperfusion | No difference in all-cause mortality and HF hospitalization at 38 months | 44% ↓ all-cause mortality and HF hospitalization in patients without thrombectomy * | [48] | |
| Ventricular unloading | CRISP-AMI (2011) RCT | 337 | IABC unloading before PCI | No difference in MI size by CMR at 3–5 days | [53] | |
| DTU-STEMI pilot trial (2019) RCT | 50 | Impella CP® unloading during 30 min before primary PCI | No difference in MACE or MI size by CMR at 30 days | Safety and feasibility trial | [58] | |
| SSO2 | AMIHOT-II (2009) RCT | 301 | Intracoronary SSO2 in LAD during 90 min | 26% ↓ MI size by Tc-99m-sestamibi SPECT and non-inferior MACE at 30 days (3.8% vs. 5.4%) * | ↑ Hemorrhagic complications and stentthrombosis | [63] |
| IC-HOT (2021) RCT | 100 | “Optimized” intracoronary SSO2 therapy in LAD during 60 min | ↓ all-cause 1 year mortality or new HF onset/hospitalization (0.0% vs. 12.3%) * | No difference in stent thrombosis between groups | [64] | |
| Coronary hypothermia | COOL-AMI EU (2021) RCT | 111 | Hypothermia with intravascular cooling system | No difference in MI size by CMR ↑ MACE in the hypothermia group | Discontinuation due to 44-min ↑ ischemic time in hypothermia group | [70] |
| PICSO | Ox-AMIPICSO (2018) Observational | 105 | PICSO after flow restoration andbefore stenting during 33 min | ↑ microvascular function and 21% ↓ MI size by CMR at 6 months * | Patients were stratified based on IMR | [82] |
| PICSO in ACS (2020)Observational | 92 | PICSO after flow restoration andbefore stenting during 30 min | 33% ↓ MI size by CMR at 5 days * | [83] |
| Treatment | Study | Estimated Enrollment (n) | Condition | Intervention | Primary Endpoint | Estimated Completion Date |
|---|---|---|---|---|---|---|
| Sono thrombolysis | SONOSTEMILYSIS trial | 60 | High-risk STEMI (>2 mm in ECG) undergoing fibrinolysis | Diagnostic ultrasound with contrast agent plus HMI pulses vs. diagnostic ultrasound plus standard therapy alone | Complete ST- segment resolution 90 min post- fibrynolisis | May 2023 |
| Ischemic post conditioning | iPOST2 trial | 1800 | STEMI with TIMI flow 0–1 | Ischemic postconditioning with balloon (4 cycles 60 s reperfusion/60 s re-occlusion) without thrombectomy vs. standard PCI | All-cause mortality or HFhospitalization | January 2024 |
| Left ventricle unloading | STEMI DTU pivotal trial | 668 | Anterior STEMI | Impella CP® placement through a femoral arterial sheath and activation during 30 min prior to primary PCI vs. standard PCI | Infarct size 3–5 days post-procedure by CMR | October 2027 |
| SSO2 | ISO SHOCK trial | 60 | STEMI withcardiogenic shock | PCI + Impella CP® + 60-min adjunctive reperfusion of SSO2 into culprit artery vs. PCI + Impella CP® | All-cause mortality at 30 days | June 2025 |
| Coronary hypothermia | EURO ICE trial | 200 | Anterior STEMI with TIMI flow 0–1 | Selective intracoronary hypothermia during 20 min (10 min of occlusion phase and 10 min of reperfusion phase) followed by PCI vs. standard PCI | Infarct size 3 months after STEMI by CMR | January 2022 |
| PICSO | PICSO AMI I trial | 144 | Anterior STEMI with TIMI flow 0–1 | Coronary sinus cannulation through femoral vein and PICSO placement, followed by stenting; then PICSO therapy during 45 min vs. standard PCI | Infarct size 5 days after STEMI by CMR | July 2025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal-Calés, P.; Cepas-Guillén, P.L.; Brugaletta, S.; Sabaté, M. New Interventional Therapies beyond Stenting to Treat ST-Segment Elevation Acute Myocardial Infarction. J. Cardiovasc. Dev. Dis. 2021, 8, 100. https://doi.org/10.3390/jcdd8090100
Vidal-Calés P, Cepas-Guillén PL, Brugaletta S, Sabaté M. New Interventional Therapies beyond Stenting to Treat ST-Segment Elevation Acute Myocardial Infarction. Journal of Cardiovascular Development and Disease. 2021; 8(9):100. https://doi.org/10.3390/jcdd8090100
Chicago/Turabian StyleVidal-Calés, Pablo, Pedro L. Cepas-Guillén, Salvatore Brugaletta, and Manel Sabaté. 2021. "New Interventional Therapies beyond Stenting to Treat ST-Segment Elevation Acute Myocardial Infarction" Journal of Cardiovascular Development and Disease 8, no. 9: 100. https://doi.org/10.3390/jcdd8090100
APA StyleVidal-Calés, P., Cepas-Guillén, P. L., Brugaletta, S., & Sabaté, M. (2021). New Interventional Therapies beyond Stenting to Treat ST-Segment Elevation Acute Myocardial Infarction. Journal of Cardiovascular Development and Disease, 8(9), 100. https://doi.org/10.3390/jcdd8090100





