Discriminative Utility of Apelin-to-NT-Pro-Brain Natriuretic Peptide Ratio for Heart Failure with Preserved Ejection Fraction among Type 2 Diabetes Mellitus Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Cohort Identifications
2.2. Determination of Risk Factors and Comorbidities
2.3. General Anthropometric, Clinical and Physical Examinations
2.4. Concomitant Medications
2.5. Echocardiography and Doppler
2.6. Estimating Glomerular Filtration Rate
2.7. Insulin Resistance Determination
2.8. Biomarkers Measurement
2.9. Statistical Analyses
3. Results
3.1. Study Population and Baseline Characteristics
3.2. Correlations between HOMA-IR and Anthropometric Parameters, Age, LV Hypertrophy and Biomarkers
3.3. Correlations between Apelin and Other Variables
3.4. Other Correlations
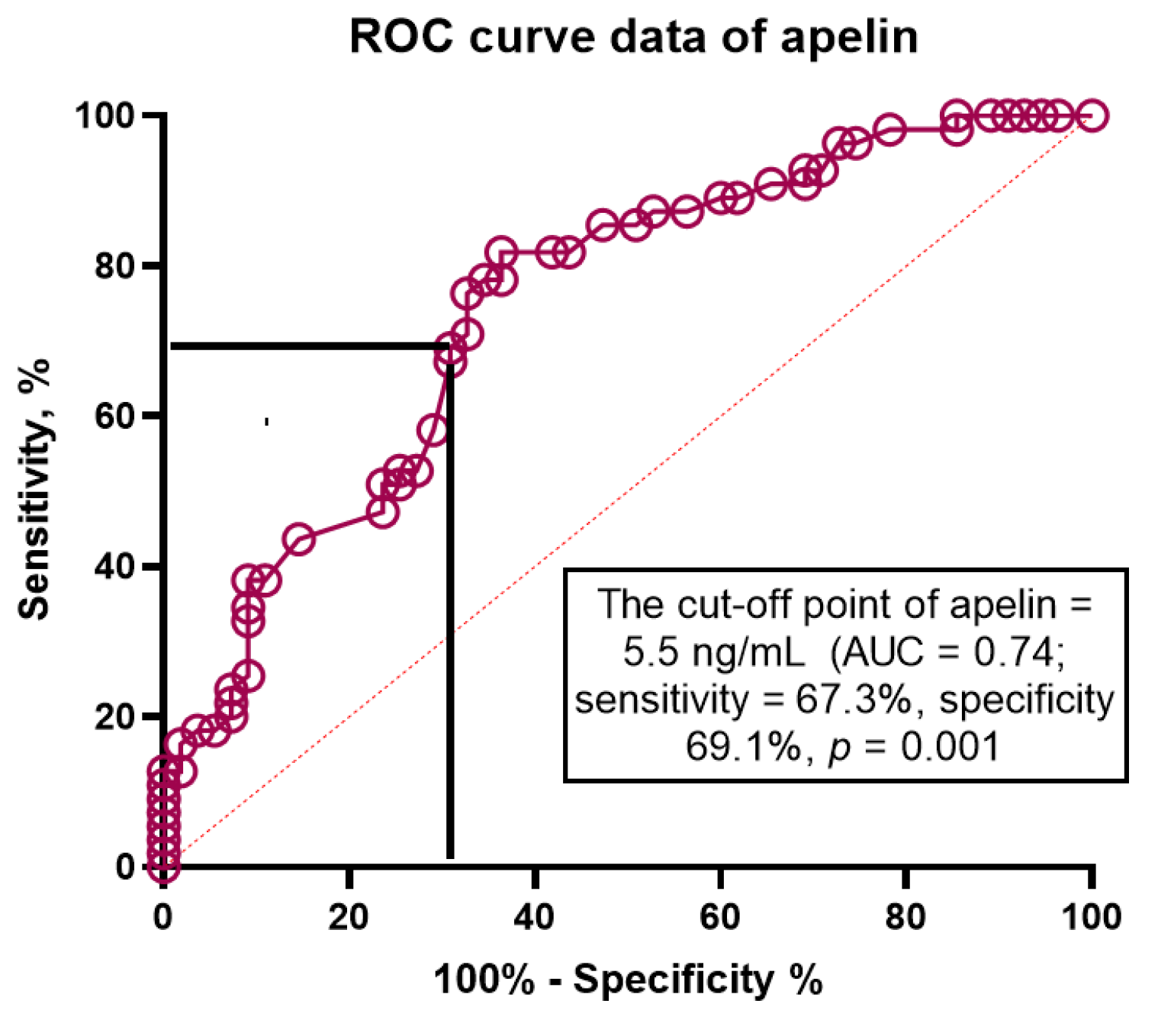
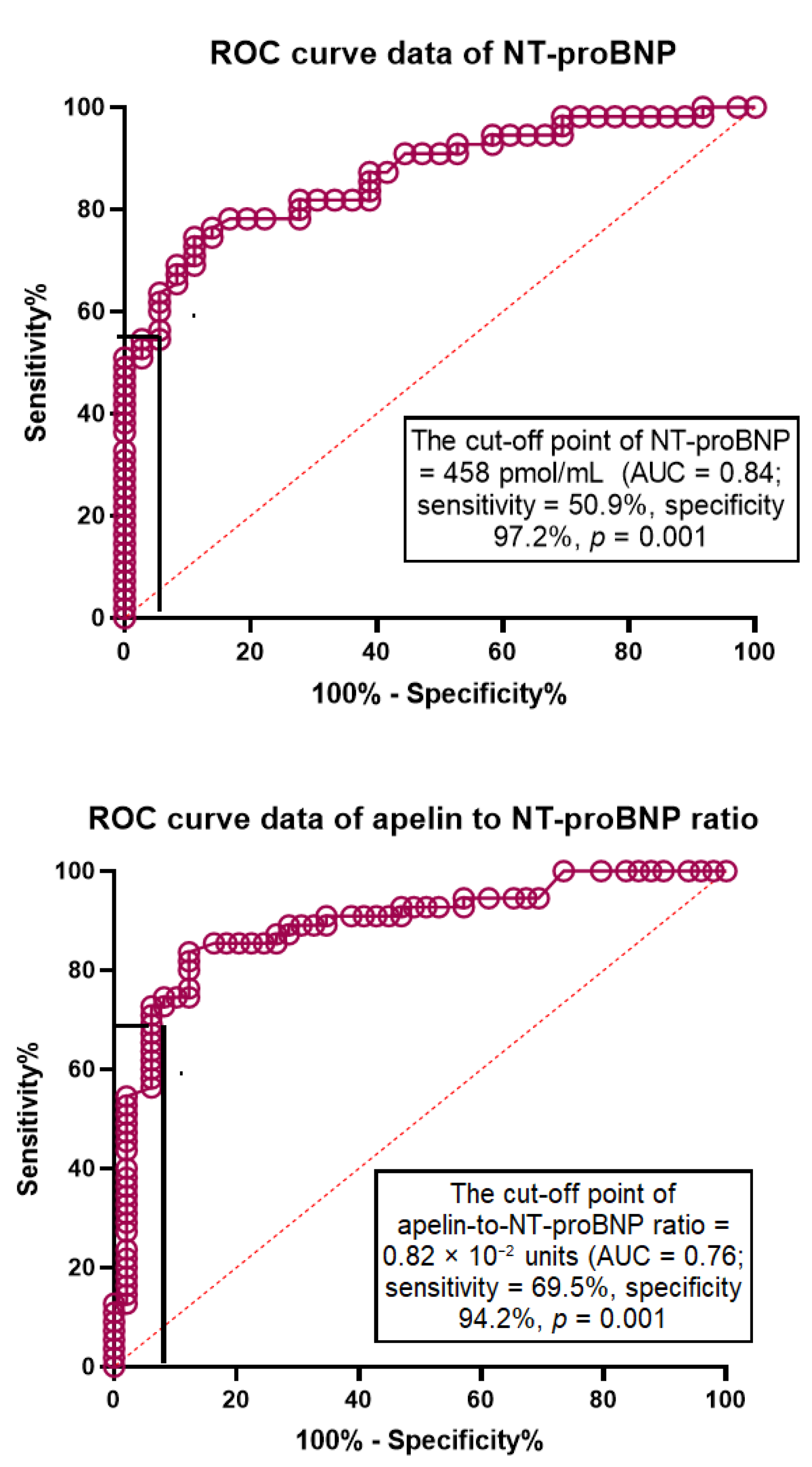
3.5. The ROC Curve
3.6. Univariate and Multivariate Logistic Regressions
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Chen, X.; Savarese, G.; Dahlström, U.; Lund, L.H.; Fu, M. Age-dependent differences in clinical phenotype and prognosis in heart failure with mid-range ejection compared with heart failure with reduced or preserved ejection fraction. Clin. Res. Cardiol. 2019, 108, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Corrà, U.; Magini, A.; Paolillo, S.; Frigerio, M. Comparison among different multiparametric scores for risk stratification in heart failure patients with reduced ejection fraction. Eur. J. Prev. Cardiol. 2020, 27, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Voors, A.A.; Jaarsma, T.; Lang, C.C.; Sama, I.E.; Akkerhuis, K.M.; Boersma, E.; Hillege, H.L.; Postmus, D. A heart failure phenotype stratified model for predicting 1-year mortality in patients admitted with acute heart failure: Results from an individual participant data meta-analysis of four prospective European cohorts. BMC Med. 2021, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, S.J.; Cuijpers, I.; Heymans, S.; Jones, E.A.V. Cellular and Molecular Differences between HFpEF and HFrEF: A Step Ahead in an Improved Pathological Understanding. Cells 2020, 9, 242. [Google Scholar] [CrossRef] [Green Version]
- Berezin, A.E.; Berezin, A.A.; Lichtenauer, M. Emerging Role of Adipocyte Dysfunction in Inducing Heart Failure Among Obese Patients With Prediabetes and Known Diabetes Mellitus. Front. Cardiovasc. Med. 2020, 7, 583175. [Google Scholar] [CrossRef]
- Rørth, R.; Jhund, P.S.; Yilmaz, M.B.; Kristensen, S.L.; Welsh, P.; Desai, A.S.; Køber, L.; Prescott, M.F.; Rouleau, J.L.; Solomon, S.D.; et al. Comparison of BNP and NT-proBNP in Patients With Heart Failure and Reduced Ejection Fraction. Circ. Heart Fail. 2020, 13, e006541. [Google Scholar] [CrossRef] [Green Version]
- Simpson, J.; Jhund, P.S.; Lund, L.H.; Padmanabhan, S.; Claggett, B.L.; Shen, L.; Petrie, M.C.; Abraham, W.T.; Desai, A.S.; Dickstein, K.; et al. Prognostic Models Derived in PARADIGM-HF and Validated in ATMOSPHERE and the Swedish Heart Failure Registry to Predict Mortality and Morbidity in Chronic Heart Failure. JAMA Cardiol. 2020, 5, 432–441. [Google Scholar] [CrossRef]
- Pocock, S.J.; Ferreira, J.P.; Gregson, J.; Anker, S.D.; Butler, J.; Filippatos, G.; Gollop, N.D.; Iwata, T.; Brueckmann, M.; Januzzi, J.L.; et al. Novel biomarker-driven prognostic models to predict morbidity and mortality in chronic heart failure: The EMPEROR-Reduced trial. Eur. Heart J. 2021, 42, 4455–4464. [Google Scholar] [CrossRef]
- Magrì, D.; Gallo, G.; Parati, G.; Cicoira, M.; Senni, M. Risk stratification in heart failure with mild reduced ejection fraction. Eur. J. Prev. Cardiol. 2020, 27, 59–64. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A. Circulating Cardiac Biomarkers in Diabetes Mellitus: A New Dawn for Risk Stratification-A Narrative Review. Diabetes Ther. 2020, 11, 1271–1291. [Google Scholar] [CrossRef]
- Antushevich, H.; Wójcik, M. Review: Apelin in disease. Clin. Chim. Acta 2018, 483, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Castan-Laurell, I.; Dray, C.; Attané, C.; Duparc, T.; Knauf, C.; Valet, P. Apelin, diabetes, and obesity. Endocrine 2011, 40, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, A.; Cao, J.; Chen, L. Apelin/APJ system: An emerging therapeutic target for respiratory diseases. Cell Mol. Life Sci. 2020, 77, 2919–2930. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chen, L.; Li, L. Apelin/APJ system: A novel promising therapy target for pathological angiogenesis. Clin. Chim. Acta 2017, 466, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lv, S.Y.; Lyu, S.K.; Wu, D.; Chen, Q. The protective effect of apelin on ischemia/reperfusion injury. Peptides 2015, 63, 43–46. [Google Scholar] [CrossRef]
- Shao, Z.Q.; Dou, S.S.; Zhu, J.G.; Wang, H.Q.; Wang, C.M.; Cheng, B.H.; Bai, B. Apelin-13 inhibits apoptosis and excessive autophagy in cerebral ischemia/reperfusion injury. Neural Regen. Res. 2021, 16, 1044–1051. [Google Scholar] [CrossRef]
- Zhong, S.; Guo, H.; Wang, H.; Xing, D.; Lu, T.; Yang, J.; Wang, C. Apelin-13 alleviated cardiac fibrosis via inhibiting the PI3K/Akt pathway to attenuate oxidative stress in rats with myocardial infarction-induced heart failure. Biosci Rep. 2020, 40, BSR20200040. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekaran, B.; Dar, O.; McDonagh, T. The role of apelin in cardiovascular function and heart failure. Eur. J. HeartFail. 2008, 10, 725–732. [Google Scholar] [CrossRef] [Green Version]
- Castan-Laurell, I.; El Boustany, R.; Pereira, O.; Potier, L.; Marre, M.; Fumeron, F.; Valet, P.; Gourdy, P.; Velho, G.; Roussel, R. Plasma Apelin and Risk of Type 2 Diabetes in a Cohort From the Community. Diabetes Care 2020, 43, e15–e16. [Google Scholar] [CrossRef] [Green Version]
- Parikh, V.N.; Liu, J.; Shang, C.; Woods, C.; Chang, A.C.; Zhao, M.; Charo, D.N.; Grunwald, Z.; Huang, Y.; Seo, K.; et al. Apelin and APJ orchestrate complex tissue-specific control of cardiomyocyte hypertrophy and contractility in the hypertrophy-heart failure transition. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H348–H356. [Google Scholar] [CrossRef] [PubMed]
- Goidescu, C.M.; Vida-Simiti, L.A. The Apelin-APJ System in the Evolution of Heart Failure. Clujul Med. 2015, 88, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, T.; Sato, C.; Kadowaki, A.; Watanabe, H.; Ho, L.; Ishida, J.; Yamaguchi, T.; Kimura, A.; Fukamizu, A.; Penninger, J.M.; et al. ELABELA-APJ axis protects from pressure overload heart failure and angiotensin II-induced cardiac damage. Cardiovasc. Res. 2017, 113, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Tycinska, A.M.; Lisowska, A.; Musial, W.J.; Sobkowicz, B. Apelin in acute myocardial infarction and heart failure induced by ischemia. Clin. Chim. Acta 2012, 413, 406–410. [Google Scholar] [CrossRef]
- Szczurek, W.; Gąsior, M.; Skrzypek, M.; Szyguła-Jurkiewicz, B. Apelin Improves Prognostic Value of HFSS (Heart Failure Survival Score) and MAGGIC (Meta-Analysis Global Group in Chronic Heart Failure) Scales in Ambulatory Patients with End-Stage Heart Failure. J. Clin. Med. 2020, 9, 2300. [Google Scholar] [CrossRef]
- Sans-Roselló, J.; Casals, G.; Rossello, X.; González de la Presa, B.; Vila, M.; Duran-Cambra, A.; Morales-Ruiz, M.; Ferrero-Gregori, A.; Jiménez, W.; Sionis, A. Prognostic value of plasma apelin concentrations at admission in patients with ST-segment elevation acute myocardial infarction. Clin. Biochem. 2017, 50, 279–284. [Google Scholar] [CrossRef]
- Dalzell, J.R.; Jackson, C.E.; Chong, K.S.; McDonagh, T.A.; Gardner, R.S. Do plasma concentrations of apelin predict prognosis in patients with advanced heart failure? Biomark. Med. 2014, 8, 807–813. [Google Scholar] [CrossRef]
- Standards of medical care in diabetes—2017: Summary of revisions. Diabetes Care 2017, 40, S4–S5. [CrossRef] [Green Version]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidemias: The Task Force for the Management of Dyslipidemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 2016, 253, 281–344. [Google Scholar]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail 2016, 18, 891–975. [Google Scholar] [CrossRef] [PubMed]
- Marwick, T.H.; Gillebert, T.C.; Aurigemma, G.; Chirinos, J.; Derumeaux, G.; Galderisi, M.; Gottdiener, J.; Haluska, B.; Ofili, E.; Segers, P.; et al. Recommendations on the Use of Echocardiography in Adult Hypertension: A Report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). J. Am. Soc. Echocardiogr. 2015, 28, 727–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 27, ehab368. [Google Scholar] [CrossRef]
- Seferovic, P.M.; Ponikowski, P.; Anker, S.D.; Bauersachs, J.; Chioncel, O.; Cleland, J.G.F.; de Boer, R.A.; Drexel, H.; Ben Gal, T.; Hill, L.; et al. Clinical practice update on heart failure 2019: Pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail 2019, 21, 1169–1186. [Google Scholar] [CrossRef]
- Lavine, S.J.; Murtaza, G.; Rahman, Z.U.; Kelvas, D.; Paul, T.K. Diastolic function grading by American Society of Echocardiography guidelines and prediction of heart failure readmission and all-cause mortality in a community-based cohort. Echocardiography 2021, 38, 1988–1998. [Google Scholar] [CrossRef]
- Shah, A.M.; Cikes, M.; Prasad, N.; Li, G.; Getchevski, S.; Claggett, B.; Rizkala, A.; Lukashevich, I.; O’Meara, E.; Ryan, J.J.; et al. Echocardiographic Features of Patients With Heart Failure and Preserved Left Ventricular Ejection Fraction. J. Am. Coll. Cardiol. 2019, 74, 2858–2873. [Google Scholar] [CrossRef]
- Kapłon-Cieślicka, A.; Laroche, C.; Crespo-Leiro, M.G.; Coats, A.J.S.; Anker, S.D.; Filippatos, G.; Maggioni, A.P.; Hage, C.; Lara-Padrón, A.; Fucili, A.; et al. Is heart failure misdiagnosed in hospitalized patients with preserved ejection fraction? From the European Society of Cardiology—Heart Failure Association EURObservational Research Programme Heart Failure Long-Term Registry. ESC Heart Fail. 2020, 7, 2098–2112. [Google Scholar] [CrossRef]
- Berezin, A.E. Biomarkers for cardiovascular risk in patients with diabetes. Heart 2016, 102, 1939–1941. [Google Scholar] [CrossRef]
- Preda, A.; Liberale, L.; Montecucco, F. Imaging techniques for the assessment of adverse cardiac remodeling in metabolic syndrome. Heart Fail Rev. 2021, 1–15. [Google Scholar] [CrossRef]
- Januzzi, J.L., Jr.; Myhre, P.L. The Challenges of NT-proBNP Testing in HFpEF: Shooting Arrows in the Wind. JACC Heart Fail. 2020, 8, 382–385. [Google Scholar] [CrossRef]
- Cortés, R.; Portolés, M.; Roselló-Lletí, E.; Martínez-Dolz, L.; Almenar, L.; Grigorian, L.; Bertomeu, V.; Rivera, M. Impact of glomerular filtration rate on urinary BNP and NT-proBNP levels in heart failure. Peptides 2012, 33, 354–358. [Google Scholar] [CrossRef]
- Nalivaeva, N.N.; Zhuravin, I.A.; Turner, A.J. Neprilysin expression and functions in development, ageing and disease. Mech. Ageing Dev. 2020, 192, 111363. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Leptin-Aldosterone-Neprilysin Axis: Identification of Its Distinctive Role in the Pathogenesis of the Three Phenotypes of Heart Failure in People With Obesity. Circulation 2018, 137, 1614–1631. [Google Scholar] [CrossRef] [PubMed]
- Dawood, A.F.; Sabry, M.M.; Estaphan, S.A.; Mohamed, E.A.; Younes, S.F.; Rashed, L.A.; Elzainy, A.W. Cross-talk between apelin and vasopressin in response to different osmotic stimuli in type 2 diabetic rats. J. Biol. Regul. Homeost. Agents. 2018, 32, 1117–1127. [Google Scholar]
- Hu, G.; Wang, Z.; Zhang, R.; Sun, W.; Chen, X. The Role of Apelin/Apelin Receptor in Energy Metabolism and Water Homeostasis: A Comprehensive Narrative Review. Front Physiol. 2021, 12, 632886. [Google Scholar] [CrossRef] [PubMed]
- Toczylowski, K.; Hirnle, T.; Harasiuk, D.; Zabielski, P.; Lewczuk, A.; Dmitruk, I.; Ksiazek, M.; Sulik, A.; Gorski, J.; Chabowski, A.; et al. Plasma concentration and expression of adipokines in epicardial and subcutaneous adipose tissue are associated with impaired left ventricular filling pattern. J. Transl. Med. 2019, 17, 310. [Google Scholar] [CrossRef]
- Li, L.; Yang, G.; Li, Q.; Tang, Y.; Yang, M.; Yang, H.; Li, K. Changes and relations of circulating visfatin, apelin, and resistin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Exp. Clin. Endocrinol. Diabetes. 2006, 114, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Azizi, Y.; Faghihi, M.; Imani, A.; Roghani, M.; Nazari, A. Post-infarct treatment with [Pyr1]-apelin-13 reduces myocardial damage through reduction of oxidative injury and nitric oxide enhancement in the rat model of myocardial infarction. Peptides 2013, 46, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, D.; Li, L.; Chen, L. Apelin/APJ System: A Novel Therapeutic Target for Myocardial Ischemia/Reperfusion Injury. DNA Cell Biol. 2016, 35, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Massari, F.; Scicchitano, P.; Ciccone, M.M.; Caldarola, P.; Aspromonte, N.; Iacoviello, M.; Barro, S.; Pantano, I.; Valle, R. Bioimpedance vector analysis predicts hospital length of stay in acute heart failure. Nutrition 2019, 61, 56–60. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Braunwald, E. Mechanisms of Cardiorenal Effects of Sodium-Glucose Cotransporter 2 Inhibitors: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 422–434. [Google Scholar] [CrossRef] [PubMed]

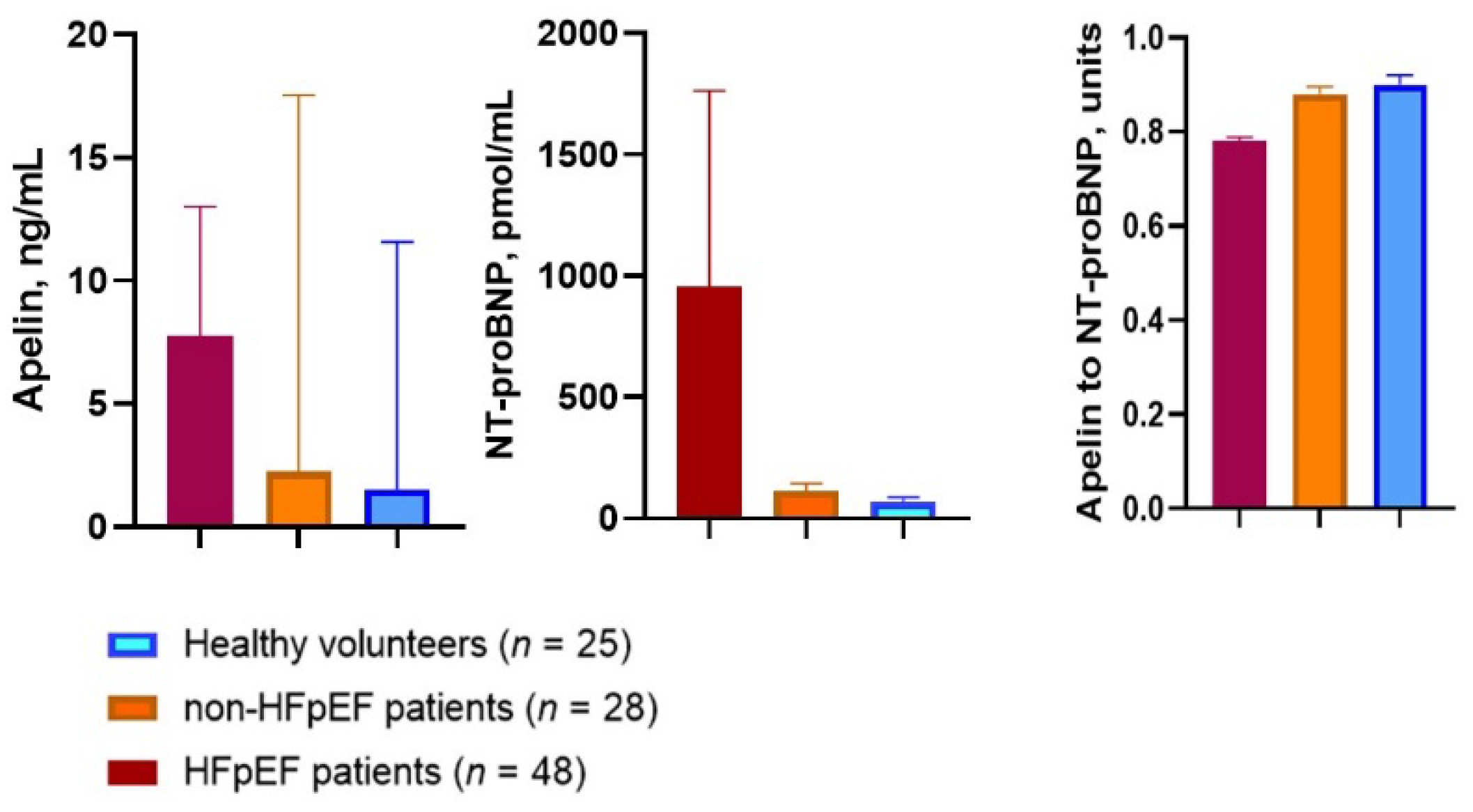
| Variables | Healthy Volunteers (n = 25) | Entire Patient Cohort (n = 76) | T2DM Patients (n = 76) | p-Value | |
|---|---|---|---|---|---|
| HFpEF (n = 48) | Non-HFpEF (n = 28) | ||||
| Age, year | 48 (42–55) | 51 (41–62) | 52(43–62) | 51(41–60) | NS |
| Male, n (%) | 17 (68.0) | 49 (64.5) | 31 (64.6) | 18 (64.3) | NS |
| Dyslipidemia, n (%) | - | 62 (81.6) # | 38 (79.1) | 24 (85.7) | NS |
| Hypertension, n (%) | - | 66 (86.8) # | 43 (89.5) | 23 (82.1) | NS |
| Smoking, n (%) | 5 (20.0) | 37 (48.7) # | 21 (43.8) | 16 (57.1) | 0.05 |
| Abdominal obesity, n (%) | - | 34 (44.7) # | 22 (45.8) | 12 (42.9) | NS |
| Microalbuminuria, n (%) | - | 23 (30.2) # | 14 (29.1) | 9 (32.1) | NS |
| LV hypertrophy, n (%) | - | 60 (78.9) # | 41 (85.4) | 19 (67.9) | 0.001 |
| BMI, kg/m2 | 21.9 ± 0.5 | 25.8 ± 2.1 # | 25.5 ± 2.4 | 26.3 ± 2.6 | NS |
| Waist circumference, sm | 75.0 ± 2.6 | 85.6 ± 2.90 # | 85.0 ± 3.20 | 86.5 ± 3.10 | NS |
| WHR, units | 0.78 ± 0.02 | 0.86 ± 0.03 # | 0.85 ± 0.04 | 0.87 ± 0.03 | NS |
| SBP, mm Hg | 127 ± 4 | 132 ± 5 | 130 ± 4 | 135 ± 5 | NS |
| DBP, mm Hg | 75 ± 3 | 80 ± 4 | 78 ± 4 | 84 ± 3 | NS |
| LVEDV, mL | 88 ± 4 | 154 ± 9 # | 159 ±5 | 147 ± 6 | NS |
| LVESV, mL | 30 ± 3 | 62 ± 7 # | 66 ± 4 | 59 ± 3 | 0.04 |
| LVEF, % | 66 ± 2 | 59 ± 6 # | 58 ± 3 | 60 ± 2 | NS |
| LVMMI, g/m2 | 80.7 ± 0.06 | 142 ± 6.12 # | 149 ± 4.0 | 137 ± 3.0 | 0.02 |
| LAVI, mL/m2 | 22 ± 4 | 33 ± 8 | 36 ± 4 | 30 ± 5 | 0.03 |
| E/e’, unit | 5.40 ± 0.10 | 8.90 ± 0.20 # | 12.8 ± 0.10 | 7.2 ± 0.20 | 0.001 |
| Variables | Healthy Volunteers (n = 25) | Entire Patient Cohort (n = 76) | T2DM Patients (n = 76) | p-Value | |
|---|---|---|---|---|---|
| HFpEF (n = 48) | Non-HFpEF (n = 28) | ||||
| eGFR, mL/min/1.73 m2 | 108 ± 5.10 | 83 ± 6.0 | 81 ± 4.2 | 86 ± 3.5 | NS |
| HOMA-IR | 1.53 ± 0.30 | 7.65 ± 3.7 # | 7.90 ± 3.0 | 7.15 ± 2.4 | NS |
| Fasting glucose, mmol/L | 4.22 ± 0.70 | 5.84 ± 1.2 # | 5.70 ± 1.5 | 5.92 ± 1.3 | NS |
| Creatinine, mcmol/L | 52.5 ± 9.15 | 98.4 ± 11.60 | 103.7 ± 9.8 | 95.1 ± 10.4 | NS |
| HbA1c, % | 4.20 ± 0.95 | 6.65 ± 0.04 # | 6.54 ± 0.03 | 6.70 ± 0.05 | NS |
| TC, mmol/L | 4.6 ± 0.09 | 6.39 ± 0.04 # | 6.37 ± 0.68 | 6.42 ± 0.55 | NS |
| HDL-C, mmol/L | 1.2 ± 0.03 | 0.95 ± 0.21 # | 0.97 ± 0.22 | 0.93 ± 0.24 | NS |
| LDL-C, mmol/L | 2.8 ± 0.05 | 4.43 ± 0.20 # | 4.42 ± 0.12 | 4.51 ± 0.15 | 0.042 |
| TG, mmol/L | 1.3 ± 0.04 | 2.26 ± 0.04 # | 2.23 ± 0.19 | 2.30 ± 1.12 | NS |
| hs-CRP, mg/L | 3.21 ± 0.25 | 6.92 ± 1.03 # | 7.56 ± 0.94 | 6.25 ± 0.42 | NS |
| hs-cTnT, ng/mL | 0.02 ± 0.20 | 0.09 ± 0.42 # | 0.12 ± 0.36 | 0.07 ± 0.24 | 0.046 |
| Variables | Depending Variable: HFpEF | |||||
|---|---|---|---|---|---|---|
| Univariate Log Regression | Multivariate Log Regression | |||||
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Unadjusted log regression | ||||||
| Apelin to NT-proBNP ratio < 0.82 × 10−2 units | 1.32 | 1.12–2.12 | 0.001 | 1.44 | 1.18–2.77 | 0.001 |
| LV hypertrophy | 1.16 | 1.10–1.19 | 0.046 | 1.03 | 1.00–1.05 | 0.14 |
| LVEF | 1.03 | 1.00–1.06 | 0.62 | - | ||
| BMI > 34 кг/м2 | 1.09 | 1.02–1.14 | 0.044 | 1.07 | 1.01–1.10 | 0.036 |
| Apelin > 4.5 ng/mL | 1.06 | 1.01–1.10 | 0.046 | 1.04 | 1.01–1.07 | 0.040 |
| NT-proBNP > 458 pmol/mL | 1.24 | 1.06–1.33 | 0.001 | 1.17 | 1.02–1.26 | 0.042 |
| Age | 1.03 | 1.02–1.05 | 0.048 | 1.03 | 1.00–1.04 | 0.16 |
| Smoking | 1.04 | 0.98–1.07 | 0.92 | - | ||
| E/e’ > 11 units | 1.12 | 1.06–1.20 | 0.001 | 1.04 | 1.01–1.06 | 0.044 |
| LAVI > 34 mL/m2 | 1.20 | 1.11–1.36 | 0.001 | 1.06 | 1.02–1.13 | 0.042 |
| SGLT2i | 0.98 | 0.95–1.05 | 0.92 | - | ||
| ACEI/ARBs | 0.99 | 0.91–1.09 | 0.93 | - | ||
| Obesity-adjusted log regression | ||||||
| Apelin to NT-proBNP ratio < 0.82 × 10−2 units | 1.37 | 1.12–3.15 | 0.001 | 1.44 | 1.18–2.77 | 0.001 |
| LV hypertrophy | 1.04 | 1.03–1.07 | 0.046 | 1.02 | 1.00–1.04 | 0.72 |
| E/e’ > 11 units | 1.02 | 1.00–1.05 | 0.92 | - | ||
| LAVI > 34 mL/m2 | 1.08 | 1.01–1.12 | 0.050 | 1.08 | 1.00–1.10 | 0.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berezin, A.A.; Fushtey, I.M.; Berezin, A.E. Discriminative Utility of Apelin-to-NT-Pro-Brain Natriuretic Peptide Ratio for Heart Failure with Preserved Ejection Fraction among Type 2 Diabetes Mellitus Patients. J. Cardiovasc. Dev. Dis. 2022, 9, 23. https://doi.org/10.3390/jcdd9010023
Berezin AA, Fushtey IM, Berezin AE. Discriminative Utility of Apelin-to-NT-Pro-Brain Natriuretic Peptide Ratio for Heart Failure with Preserved Ejection Fraction among Type 2 Diabetes Mellitus Patients. Journal of Cardiovascular Development and Disease. 2022; 9(1):23. https://doi.org/10.3390/jcdd9010023
Chicago/Turabian StyleBerezin, Alexander A., Ivan M. Fushtey, and Alexander E. Berezin. 2022. "Discriminative Utility of Apelin-to-NT-Pro-Brain Natriuretic Peptide Ratio for Heart Failure with Preserved Ejection Fraction among Type 2 Diabetes Mellitus Patients" Journal of Cardiovascular Development and Disease 9, no. 1: 23. https://doi.org/10.3390/jcdd9010023
APA StyleBerezin, A. A., Fushtey, I. M., & Berezin, A. E. (2022). Discriminative Utility of Apelin-to-NT-Pro-Brain Natriuretic Peptide Ratio for Heart Failure with Preserved Ejection Fraction among Type 2 Diabetes Mellitus Patients. Journal of Cardiovascular Development and Disease, 9(1), 23. https://doi.org/10.3390/jcdd9010023







