Effect of C1q/TNF-Related Protein 9 on Coronary Artery Calcification: An Observational Study
Abstract
:1. Introduction
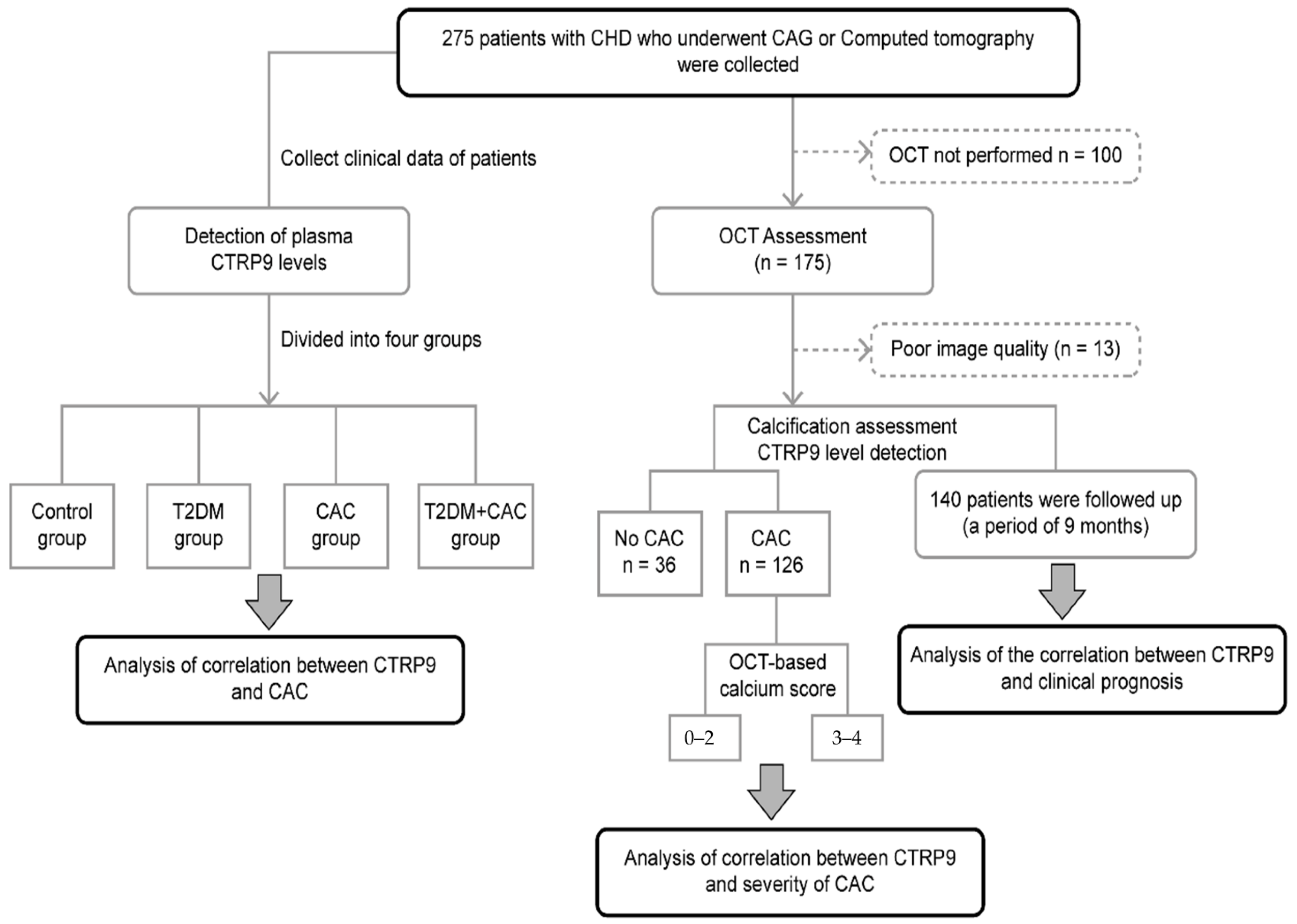
2. Materials and Methods
2.1. Participants and Eligibility
2.2. Clinical and Biochemical Characteristics of the Study Population
2.3. Detection of Plasma CTRP9 Level
2.4. Optical Coherence Tomography Image Acquisition and Analysis
2.4.1. OCT Image Acquisition
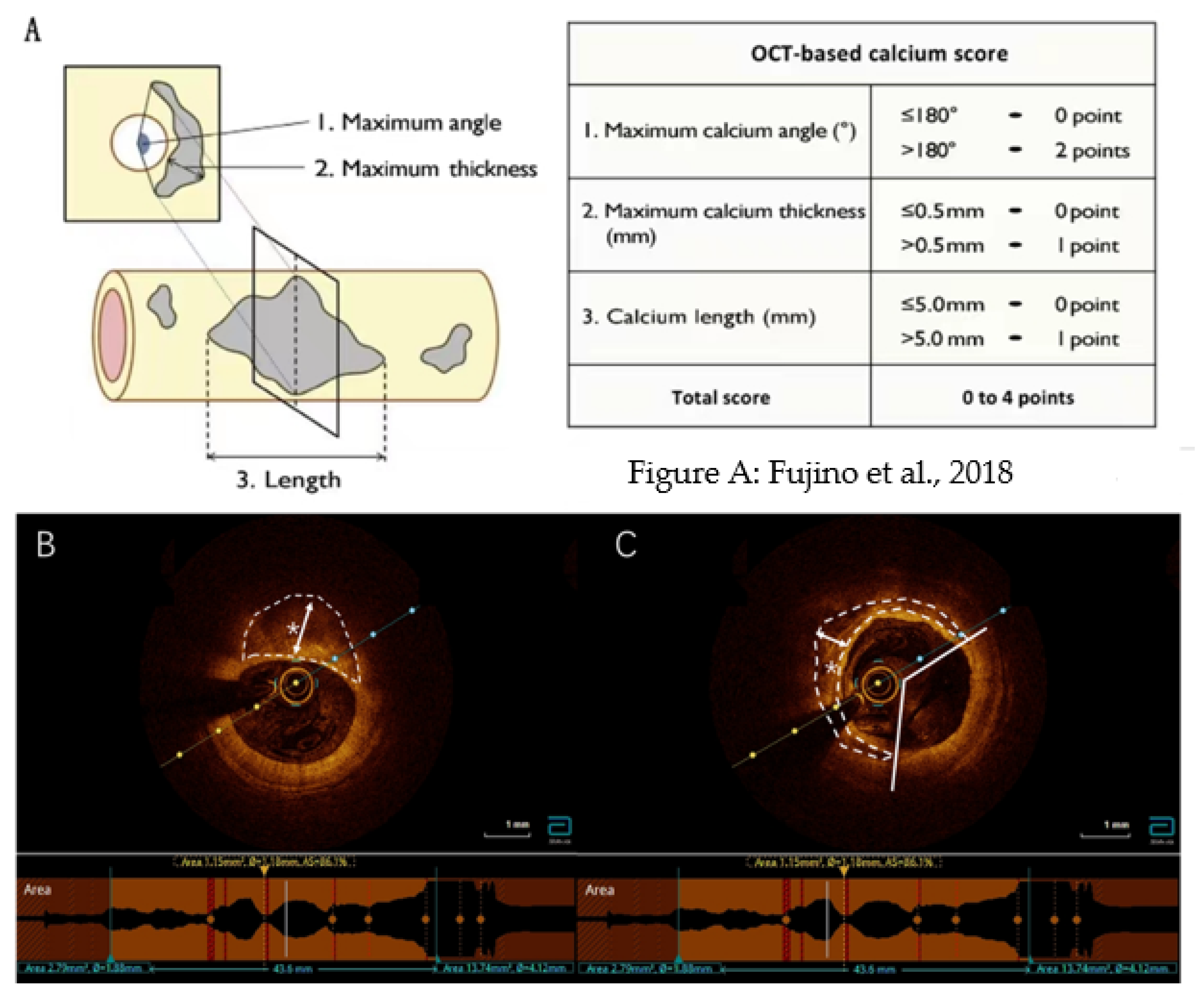
2.4.2. OCT Image Analysis, including Calcium Parameters
2.5. Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. CTRP9 Is a Protective Factor for CAC
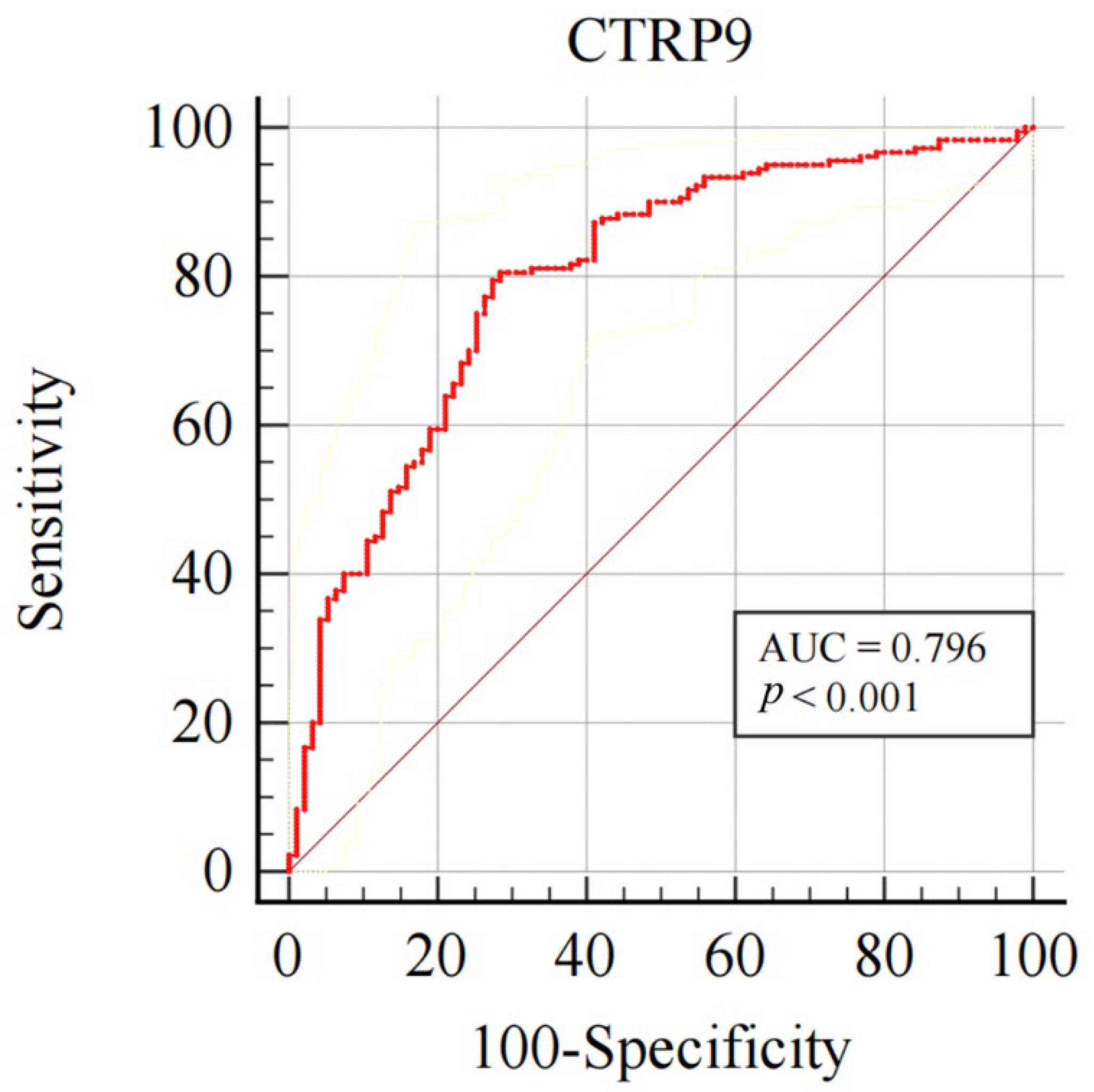
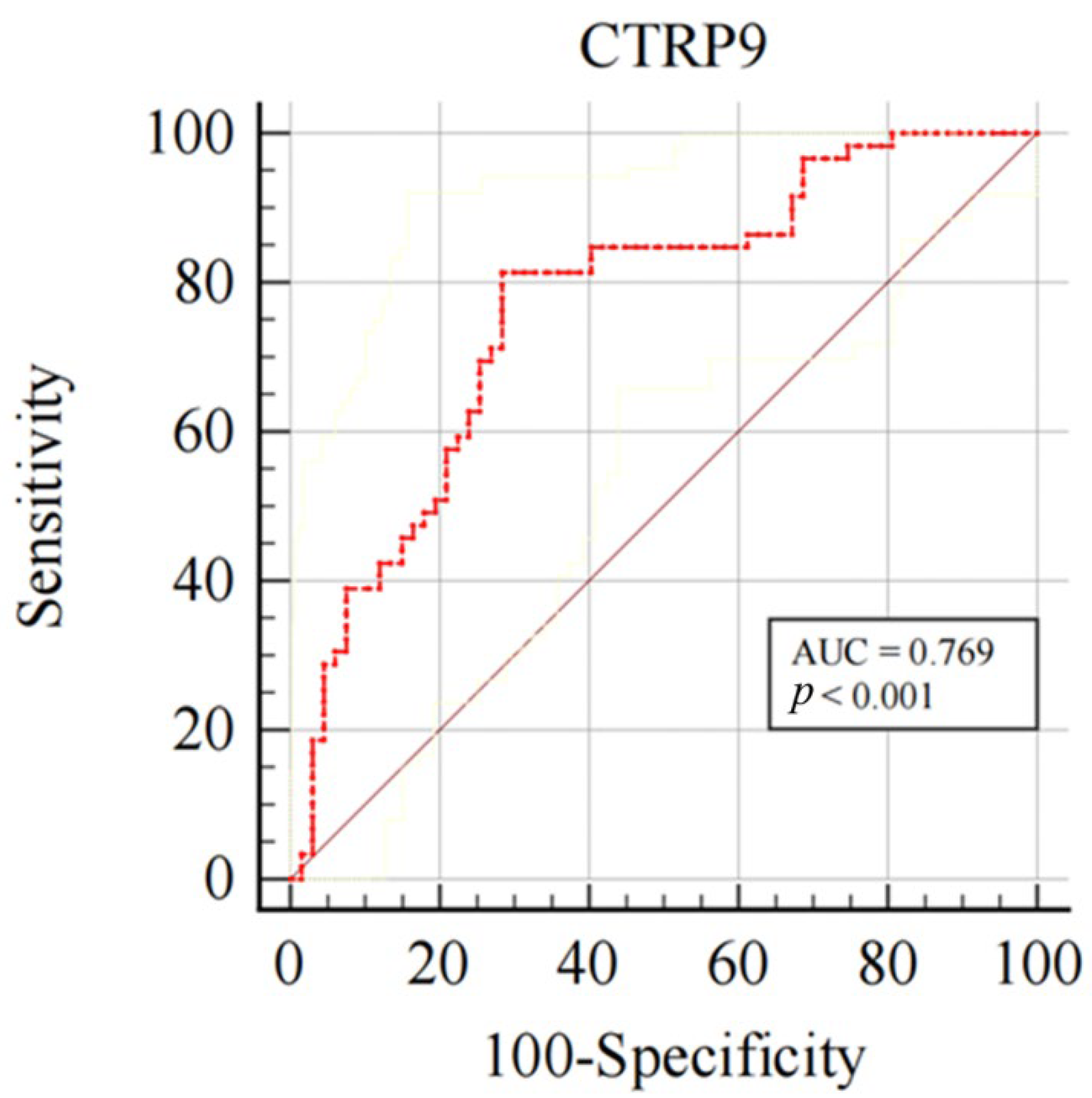
3.3. Plasma CTRP9 Diagnostic Value for CAC
3.4. Correlation Analysis between CTRP9 and Severity of Coronary Artery Calcification
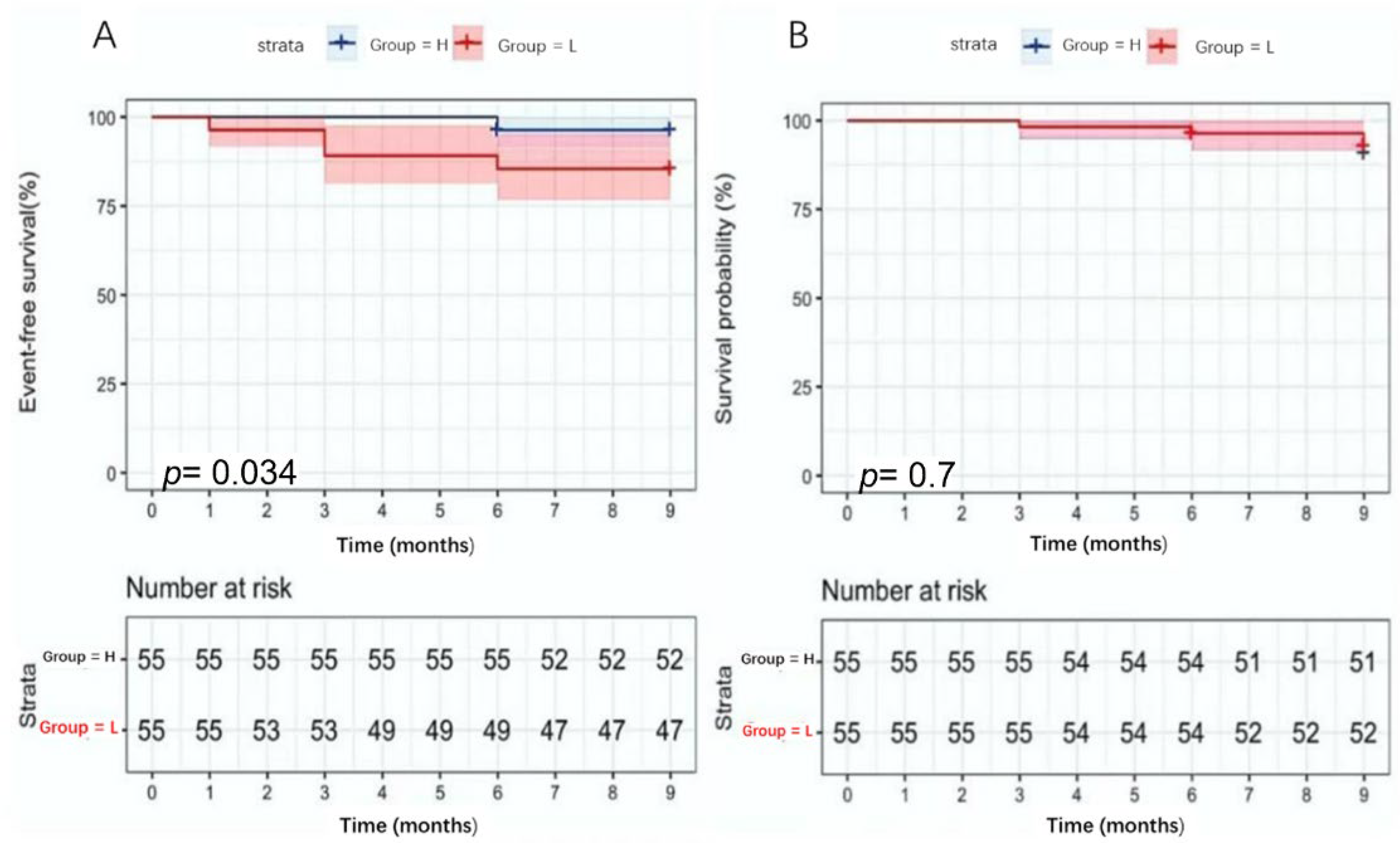
3.5. CTRP9 Predictive Value for Patient Prognosis
4. Discussion
4.1. Vascular Calcification Is a Cell-Mediated Active and Highly Controllable Biological Process
4.2. CTRP9 Might Play an Important Role in CAC Formation in the Context of T2DM
4.3. CTRP9 Is Associated with CAC Severity and Vascular Calcification Scores Can Pre-dict Cardiovascular Events
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| HGB | hemoglobin |
| HbA1c | glycosylated hemoglobin A1c |
| GLU | fasting blood glucose |
| hsCRP | hypersensitive C-reactive protein |
| TC | total cholesterol |
| TG | serum triglycerides |
| HDL-C | high-density lipoprotein cholesterol |
| LDL-C | low-density lipoprotein cholesterol |
| SCR | creatinine |
| ALT | alanine transaminase |
| AST | aspartate transaminase |
| APOA | apolipoprotein A1 |
| ApoB | apolipoprotein B |
| LPa | lipoprotein a |
| MYO | myoglobin |
| CK | creatine kinase |
| CK-MB | creatine kinase-muscle/brain |
| LDH | lactate dehydrogenase |
| GGT | γ-glutamyl transpeptidase |
| β2MG | β2 microglobulin |
| LVEF | left ventricular ejection fraction |
| EDV | end-diastolic volume |
| ESV | end-systolic volume |
| CTRP9 | C1q/TNF-related protein 9 |
References
- Cummins, P.; Bruining, N. Robot-Assisted Telestenting: Brightening the Light of Science. EuroIntervention 2017, 12, 1561–1563. [Google Scholar] [CrossRef] [PubMed]
- Demer, L.L.; Tintut, Y. Vascular Calcification: Pathobiology of a Multifaceted Disease. Circulation 2008, 117, 2938–2948. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of Smooth Muscle Cells in Vascular Calcification: Implications in Atherosclerosis and Arterial Stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef]
- Leng, W.; Li, D.; Chen, L.; Xia, H.; Tang, Q.; Chen, B.; Gong, Q.; Gao, F.; Bi, F. Retraction of “Novel Bioluminescent Activatable Reporter for Src Tyrosine Kinase Activity in Living Mice”. Theranostics 2020, 10, 11862. [Google Scholar] [CrossRef]
- Leopold, J.A. MicroRNAs Regulate Vascular Medial Calcification. Cells 2014, 3, 963–980. [Google Scholar] [CrossRef]
- Wang, Z.; Li, L.; Du, R.; Yan, J.; Liu, N.; Yuan, W.; Jiang, Y.; Xu, S.; Ye, F.; Yuan, G.; et al. CML/RAGE Signal Induces Calcification Cascade in Diabetes. Diabetol. Metab. Syndr. 2016, 8, 83. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Chen, Z.; Zhang, M. Arterial Calcification and Its Association with Stroke: Implication of Risk, Prognosis, Treatment Response, and Prevention. Front. Cell. Neurosci. 2022, 16, 845215. [Google Scholar] [CrossRef]
- Rennenberg, R.J.; Kessels, A.G.; Schurgers, L.J.; van Engelshoven, J.M.; de Leeuw, P.W.; Kroon, A.A. Vascular calcifications as a marker of increased cardiovascular risk: A meta-analysis. Vasc. Health Risk Manag. 2009, 5, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Yahagi, K.; Kolodgie, F.D.; Lutter, C.; Mori, H.; Romero, M.E.; Finn, A.V.; Virmani, R. Pathology of Human Coronary and Carotid Artery Atherosclerosis and Vascular Calcification in Diabetes Mellitus. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 191–204. [Google Scholar] [CrossRef]
- Appari, M.; Breitbart, A.; Brandes, F.; Szaroszyk, M.; Froese, N.; Korf-Klingebiel, M.; Mohammadi, M.M.; Grund, A.; Scharf, G.M.; Wang, H.; et al. C1q-TNF-Related Protein-9 Promotes Cardiac Hypertrophy and Failure. Circ. Res. 2017, 120, 66–77. [Google Scholar] [CrossRef]
- Liu, M.; Yin, L.; Li, W.; Hu, J.; Wang, H.; Ye, B.; Tang, Y.; Huang, C. C1q/TNF-Related Protein-9 Promotes Macrophage Polarization and Improves Cardiac Dysfunction after Myocardial Infarction. J. Cell. Physiol. 2019, 234, 18731–18747. [Google Scholar] [CrossRef]
- Sun, Y.; Yi, W.; Yuan, Y.; Lau, W.B.; Yi, D.; Wang, X.; Wang, Y.; Su, H.; Wang, X.; Gao, E.; et al. C1q/tumor Necrosis Factor-Related protein-9, a Novel Adipocyte-Derived Cytokine, Attenuates Adverse Remodeling in the Ischemic Mouse Heart via Protein Kinase A Activation. Circulation 2013, 128 (Suppl. 1), S113–S120. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Su, H.; Yang, R.; Tan, Y.; Li, B.; Yi, W.; Dong, Q.; Zhang, H.; Xing, W.; Sun, X. C1q/TNF-Related Protein-9 Ameliorates Hypoxia-Induced Pulmonary Hypertension by Regulating Secretion of Endothelin-1 and Nitric Oxide Mediated by AMPK in Rats. Sci. Rep. 2021, 11, 11372. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xin, J.Y.; Liu, Z.L.; Fan, F.; Li, Y.M.; Jin, F.; Wang, Q.S.; Guo, F.Q.; Yu, N.W.; Le, W.D.; et al. Association Between Serum C1q Tumor Necrosis Factor-Related Protein 9 and the Clinical Characteristics and Prognosis of Ischemic Stroke. Neurol. Ther. 2022, 11, 87–101. [Google Scholar] [CrossRef]
- Liang, W.; Moyzis, A.G.; Lampert, M.A.; Diao, R.Y.; Najor, R.H.; Gustafsson, Å.B. Aging is Associated with a Decline in Atg9b-Mediated Autophagosome Formation and Appearance of Enlarged Mitochondria in the Heart. Aging Cell 2020, 19, e13187. [Google Scholar] [CrossRef]
- Kume, T.; Okura, H.; Kawamoto, T.; Yamada, R.; Miyamoto, Y.; Hayashida, A.; Watanabe, N.; Neishi, Y.; Sadahira, Y.; Akasaka, T.; et al. Assessment of the Coronary Calcification by Optical Coherence Tomography. EuroIntervention 2011, 6, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Fujino, A.; Mintz, G.S.; Matsumura, M.; Lee, T.; Kim, S.Y.; Hoshino, M.; Usui, E.; Yonetsu, T.; Haag, E.S.; Shlofmitz, R.A.; et al. A New Optical Coherence Tomography-Based Calcium Scoring System to Predict Stent Underexpansion. EuroIntervention 2018, 13, e2182–e2189. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Matsumura, M.; Mintz, G.S.; Lee, T.; Zhang, W.; Cao, Y.; Fujino, A.; Lin, Y.; Usui, E.; Kanaji, Y.; et al. In Vivo Calcium Detection by Comparing Optical Coherence Tomography, Intravascular Ultrasound, and Angiography. JACC Cardiovasc. Imaging 2017, 10, 869–879. [Google Scholar] [CrossRef]
- Wijns, W.; Shite, J.; Jones, M.R.; Lee, S.W.; Price, M.J.; Fabbiocchi, F.; Barbato, E.; Akasaka, T.; Bezerra, H.; Holmes, D. Optical Coherence Tomography Imaging During Percutaneous Coronary Intervention Impacts Physician Decision-Making: ILUMIEN I Study. Eur. Heart J. 2015, 36, 3346–3355. [Google Scholar] [CrossRef]
- Souteyrand, G.; Amabile, N.; Mangin, L.; Chabin, X.; Meneveau, N.; Cayla, G.; Vanzetto, G.; Barnay, P.; Trouillet, C.; Rioufol, G.; et al. Mechanisms of Stent Thrombosis Analysed by Optical Coherence Tomography: Insights From the National PESTO French Registry. Eur. Heart J. 2016, 37, 1208–1216. [Google Scholar] [CrossRef] [Green Version]
- Tearney, G.J.; Regar, E.; Akasaka, T.; Adriaenssens, T.; Barlis, P.; Bezerra, H.G.; Bouma, B.; Bruining, N.; Cho, J.M.; Chowdhary, S.; et al. Consensus Standards for Acquisition, Measurement, and Reporting of Intravascular Optical Coherence Tomography Studies: A Report From the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J. Am. Coll. Cardiol. 2012, 59, 1058–1072. [Google Scholar] [CrossRef]
- Fujii, K.; Kubo, T.; Otake, H.; Nakazawa, G.; Sonoda, S.; Hibi, K.; Shinke, T.; Kobayashi, Y.; Ikari, Y.; Akasaka, T. Expert Consensus Statement for Quantitative Measurement and Morphological Assessment of Optical Coherence Tomography: Update. Cardiovasc. Interv. Ther. 2020, 35, 2022. [Google Scholar] [CrossRef]
- Sugiyama, T.; Yamamoto, E.; Fracassi, F.; Lee, H.; Yonetsu, T.; Kakuta, T.; Soeda, T.; Saito, Y.; Yan, B.P.; Kurihara, O.; et al. Calcified Plaques in Patients With Acute Coronary Syndromes. JACC Cardiovasc. Interv. 2019, 12, 531–540. [Google Scholar] [CrossRef]
- Baron, S.J.; Chinnakondepalli, K.; Magnuson, E.A.; Kandzari, D.E.; Puskas, J.D.; Ben-Yehuda, O.; van Es, G.A.; Taggart, D.P.; Morice, M.C.; Lembo, N.J.; et al. Quality-of-Life After Everolimus-Eluting Stents or Bypass Surgery for Left-Main Disease: Results From the EXCEL Trial. J. Am. Coll. Cardiol. 2017, 70, 3113–3122. [Google Scholar] [CrossRef]
- Brilakis, E.S.; Mashayekhi, K.; Burke, M.N. How DECISION-CTO Can Help Guide the Decision to Perform Chronic Total Occlusion Percutaneous Coronary Intervention. Circulation 2019, 139, 1684–1687. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Huang, J.; Liu, Y.; Xie, C.; Zhang, K.; Zhu, X.; Chen, J.; Huang, H. Deletion of Soluble Epoxide Hydrolase Suppressed Chronic Kidney Disease-Related Vascular Calcification by Restoring Sirtuin 3 Expression. Cell Death Dis. 2021, 12, 992. [Google Scholar] [CrossRef]
- Orringer, C.E.; Blaha, M.J.; Blankstein, R.; Budoff, M.J.; Goldberg, R.B.; Gill, E.A.; Maki, K.C.; Mehta, L.; Jacobson, T.A. The National Lipid Association Scientific Statement on Coronary Artery Calcium Scoring to Guide Preventive Strategies for ASCVD Risk Reduction. J. Clin. Lipidol. 2021, 15, 33–60. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Zhao, Y.; Budoff, M.; Nasir, K.; Blumenthal, R.S.; Bertoni, A.G.; Wong, N.D. Coronary Artery Calcium Score for Long-Term Risk Classification in Individuals with Type 2 Diabetes and Metabolic Syndrome From the Multi-ethnic Study of Atherosclerosis. JAMA Cardiol. 2017, 2, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.H.; Zhang, D.W.; Zheng, X.L.; Tang, C.K. C1q Tumor Necrosis Factor-Related Protein 9 in Atherosclerosis: Mechanistic Insights and Therapeutic Potential. Atherosclerosis 2018, 276, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Lee, M.J.; Kang, Y.M.; Jang, J.E.; Leem, J.; Lee, Y.L.; Seol, S.M.; Yoon, H.K.; Lee, W.J.; Park, J.Y. Association of Serum C1q/TNF-Related Protein-9 Concentration with Arterial Stiffness in Subjects with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2014, 99, E2477–E2484. [Google Scholar] [CrossRef] [Green Version]
- Miyatake, N.; Adachi, H.; Nomura-Nakayama, K.; Okada, K.; Okino, K.; Hayashi, N.; Fujimoto, K.; Furuichi, K.; Yokoyama, H. Circulating CTRP9 Correlates with the Prevention of Aortic Calcification in Renal Allograft Recipients. PLoS ONE 2020, 15, e0226526. [Google Scholar] [CrossRef] [PubMed]
- Shisen, J.; Leung, D.Y.; Juergens, C.P. Gender and age differences in the prevalence of coronary artery calcification in 953 Chinese subjects. Heart Lung Circ. 2005, 14, 69–73. [Google Scholar] [CrossRef]
- Cereda, A.; Toselli, M.; Palmisano, A.; Vignale, D.; Leone, R.; Nicoletti, V.; Gnasso, C.; Mangieri, A.; Khokhar, A.; Campo, G.; et al. The hidden interplay between sex and COVID-19 mortality: The role of cardiovascular calcification. Geroscience 2021, 43, 2215–2229. [Google Scholar] [CrossRef]
- Leopold, J.A. Vascular Calcification: An Age-Old Problem of Old Age. Circulation 2013, 127, 2380–2382. [Google Scholar] [CrossRef]
- Silver, S.A.; Adhikari, N.K.; Bell, C.M.; Chan, C.T.; Harel, Z.; Kitchlu, A.; Meraz-Muñoz, A.; Norman, P.A.; Perez, A.; Zahirieh, A.; et al. Nephrologist Follow-Up Versus Usual Care After an Acute Kidney Injury Hospitalization (Fusion): A Randomized Controlled Trial. Clin. J. Am. Soc. Nephrol. 2021, 16, 1005–1014. [Google Scholar] [CrossRef]
- Jennings, G.L.; Audehm, R.; Bishop, W.; Chow, C.K.; Liaw, S.T.; Liew, D.; Linton, S.M. National Heart Foundation of Australia: Position Statement on Coronary Artery Calcium Scoring for the Primary Prevention of Cardiovascular Disease in Australia. Med. J. Aust. 2021, 214, 434–439. [Google Scholar] [CrossRef]
- Lehmann, N.; Erbel, R.; Mahabadi, A.A.; Rauwolf, M.; Möhlenkamp, S.; Moebus, S.; Kälsch, H.; Budde, T.; Schmermund, A.; Stang, A.; et al. Value of Progression of Coronary Artery Calcification for Risk Prediction of Coronary and Cardiovascular Events: Result of the HNR Study (Heinz Nixdorf Recall). Circulation 2018, 137, 665–679. [Google Scholar] [CrossRef]
- Peng, A.W.; Mirbolouk, M.; Orimoloye, O.A.; Osei, A.D.; Dardari, Z.; Dzaye, O.; Budoff, M.J.; Shaw, L.; Miedema, M.D.; Rumberger, J.; et al. Long-Term All-Cause and Cause-Specific Mortality in Asymptomatic Patients With CAC ≥1000: Results From the CAC Consortium. JACC Cardiovasc. Imaging 2020, 13, 83–93. [Google Scholar] [CrossRef]
- Gottlieb, I.; Miller, J.M.; Arbab-Zadeh, A.; Dewey, M.; Clouse, M.E.; Sara, L.; Niinuma, H.; Bush, D.E.; Paul, N.; Vavere, A.L.; et al. The Absence of Coronary Calcification Does Not Exclude Obstructive Coronary Artery Disease or the Need for Revascularization in Patients Referred for Conventional Coronary Angiography. J. Am. Coll. Cardiol. 2010, 55, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Vrachimis, A.; Honold, L.; Faust, A.; Hermann, S.; Schäfers, M. New Molecular Probes of Vascular Inflammation. Q. J. Nucl. Med. Mol. Imaging 2016, 60, 194–204. [Google Scholar]
| Variables | Control Group | CAC Group | T2DM Group | T2DM with CAC Group | p |
|---|---|---|---|---|---|
| Age (years) | 56.5 ± 9.1 b | 60.7 ± 8.4 a | 60.1 ± 8.2 | 64.4 ± 7.5 a,b | <0.001 |
| Sex (male/female) | 59 (31,28) | 92 (68,24) | 36(26,10) | 88 (60,28) | 0.044 |
| SCR (mg/dl) | 67.0 (62.0,77.0) | 71.0 (64.0,79.8) | 78.5 (67.0,88.5) a | 69.0 (59.3,78.8) c | 0.024 |
| Hypertension, n (%) | 27 (45.8) | 59 (64.1) | 24 (66.7) | 62 (70.5) | 0.020 |
| Smoking, n (%) | 8 (13.6) | 15 (16.3) | 15 (41.7) | 29 (33.0) | 0.001 |
| Heart failure, n (%) | 2(3.4) | 5 (5.4) | 5 (13.9) | 20 (22.7) | <0.001 |
| Systolic BP (mmHg) | 132.0 ± 18.3 | 137.8 ± 19.3 | 135.9 ± 18.5 | 134.6 ± 17.1 | 0.285 |
| Diastolic BP (mmHg) | 78.1 ± 10.7 | 78.5 ± 12.7 | 76.3 ± 10.5 | 77.7 ± 10.3 | 0.794 |
| HR (bpm) | 70.3 + 10.4 c | 69.0 ± 9.2c | 65.1 ± 6.5 a,b | 69.2 ± 10.4 c | 0.010 |
| BMI (kg/m2) | 25.3 ± 2.9 | 24.6 ± 3.1c | 26.6 ± 2.9 b | 25.7 ± 2.9 | 0.003 |
| HGB | 133.0 ± 14.7 | 131.3 ± 15.1 | 135.4 ± 13.2 | 131.1 ± 14.0 | 0.417 |
| HbA1c (%) | 5.7 (5.5,6.0)c | 5.9 (5.5,6.1) c | 7.6 (6.7,8.5) a,b | 7.7 (6.8,8.5) a,b | <0.001 |
| GLU | 4.8 (4.4,5.3) | 4.7 (4.3,5.3) | 6.0 (5.3,7.5) a,b | 6.6 (5.7,7.9) a,b | <0.001 |
| hsCRP | 1.5 (0.8,5.3) | 2.1 (1.0,4.9) | 1.7 (1.0,4.6) | 1.9 (1.1,3.5) | 0.736 |
| TC (mmol/L) | 3.7 (3.3,4.4) | 3.6 (3.1,4.3) | 3.5 (3.1,5.0) | 3.5 (2.9,4.3) | 0.576 |
| TG (mmol/L) | 1.3 (1.0,1.9) | 1.4 (1.0,2.0) | 1.2 (1.0,1.7) | 1.47 (1.1,2.2) | 0.074 |
| HDL-C (mmol/L) | 1.0 (0.8,1.2) | 1.0 (0.9,1.2) | 1.1 (0.8,1.1) | 0.9 (0.8,1.1) | 0.051 |
| LDL-C (mmol/L) | 2.19 (1.8,2.7) | 2.2 (1.6,2.5) | 2.0 (1.7,3.0) | 2.0 (1.5,2.8) | 0.723 |
| ALT | 20.6 (12.8,31.5) | 18.9 (12.9,25.0) | 17.0 (12.6,24.8) | 20.3 (13.4,33.7) | 0.406 |
| AST | 19.3 (15.5,26.6) | 19.5 (16.9,27.3) | 15.1 (13.7,18.9) a,b | 18.0 (13.7,25.0) b | 0.001 |
| APOA (g/L) | 1.2 ± 0.2 | 1.2 ± 0.3 | 1.1 ± 0.2 | 1.1 ± 0.2 b | 0.016 |
| ApoB (g/L) | 0.8 (0.6,1.0) | 0.77 (0.6,0.9) | 0.70 (0.6,0.9) | 0.74 (0.6,1.0) | 0.573 |
| LPa (mg/L) | 11.9 (6.3,28.5) | 15.4 (6.7,31.1) | 11.0 (4.9,22.2) | 10.3 (5.3,31.4) | 0.546 |
| MYO | 45.0 (40.0,55.0) | 53.5 (42.5,66.8) a | 48.0 (40.3,66.3) | 44.0 (37.0,51.0) b | 0.001 |
| CK | 67.0 (50.0,89.0) | 72.5 (54.0,104.8) | 64.0 (42.5,89.0) | 68.5 (48.0,91.0) | 0.327 |
| CKMB | 18.0 (13.0,23.0) | 18.0 (15.0,23.8) | 17.5 (16.0,22.0) | 18.0 (14.0,22.0) | 0.623 |
| LDH | 173.0 (158.0,199.0) | 181.5 (166.0,208.8) c | 166.5 (142.3,187.2) b | 177.0 (154.3,207.8) | 0.024 |
| GGT | 23.0 (17.0,33.0) | 21.5 (16.0,33.8) | 20.0 (16.3,25.0) | 22.5 (15.00,37.75) | 0.734 |
| β2MG | 1.70 (1.56,2.10) | 1.90 (1.60,2.20) | 2.10 (1.81,2.30) a,b | 2.0 (1.7,2.2) a | 0.005 |
| LVEF% | 61.9 (59.9,66.7) | 63.3 (60.5,67.3) | 66.1 (60.0,70.4) | 63.6 (60.1,68.0) | 0.377 |
| E/A | 0.8 (0.6,1.1) | 0.8 (0.6,1.1) | 0.8 (0.7,1.1) | 0.7 (0.6,0.9) | 0.098 |
| EDV | 101.0 (84.0,115.0) | 97.5 (86.0,117.5) | 99.5 (81.3,121.8) | 102.0 (83.2,118.8) | 0.910 |
| ESV | 37.0 (29.0,44.0) | 36.0 (29.3,45.0) | 34.5 (26.8,46.3) | 36.0 (28.0,47.8) | 0.937 |
| E/e’ | 11.0 (9.0,14.2) | 11.5 (9.8,13.8) | 12.4 (10.5,14.0) | 12.9 (11.0,15.5) a,b | 0.039 |
| CTRP9 (pg/mL) | 206.0 (159.7,263.1) b,c | 80.9 (61.1,116.3) a | 80.7 (67.4,126.1) a | 68.1 (52.9,96.5) a,b,c | <0.001 |
| Parameters | Unmatched Cohort | p | Matched Cohort | p | ||
|---|---|---|---|---|---|---|
| No CAC (n = 95) | CAC (n = 180) | No CAC (n = 68) | CAC (n = 68) | |||
| CTRP9 (pg/mL) | 168.4 (99.3,233.4) | 72.7 (56.9,102.4) | <0.001 | 140.0 (79.5,214.4) | 71.9 (59.7,101.4) | <0.001 |
| Age (years) | 58.0 (52.0,63.0) | 63.0 (57.0,68.0) | <0.001 | 59.2 ± 8.2 | 60.2 ± 8.5 | 0.473 |
| HbA1c (%) | 6.0 (5.6,7.1) | 6.3 (5.8,7.7) | 0.023 | 6.1 (5.6,7.4) | 6.2 (5.7,7.0) | 0.993 |
| LDH | 169 (151.0,192.0) | 179.0 (161.0,208.0) | 0.019 | 170 (147.200.5) | 180.5 (163.2,209.0) | 0.060 |
| Male (%) | 57.0 (60.0%) | 128 (71.1%) | 0.079 | 52( 76.5%) | 48 (70.6%) | 0.624 |
| Hypertension, n (%) | 51 (53.7%) | 121 (67.2%) | 0.036 | 48 (70.6%) | 44 (64.7%) | 0.632 |
| Diabetes, n (%) | 34 (35.8%) | 86 (47.8%) | 0.073 | 34 (50%) | 32 (47.1%) | 0.872 |
| Health Status Assessments | L-CTRP9 Group (n = 72) | H-CTRP9 Group (n = 68) | p |
|---|---|---|---|
| SAQ Physical limitation score | 66.7 (49.4,79.4) | 71.1 (58.9,80.0) | 0.329 |
| SAQ Quality of life score | 75.0 (50.0,100.0) | 100.0 (50.0,100.0) | 0.594 |
| SAQ angina frequency score | 95.0 (80.0,100.0) | 100.0 (90.0,100.0) | 0.021 |
| SAQ quality of life score | 80.0 (65.0,80.0) | 75.0 (65.0,80.0) | 0.380 |
| SAQ Treatment satisfaction score | 58.3 (41.7,75.0) | 58.3 (41.7,72.9) | 0.751 |
| Rose dyspnea score | 2.0 (0.0,3.0) | 1.5 (0,2.0) | 0.363 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Ma, Y.; Jin, X.; Lu, R.; Hu, H.; Gu, G. Effect of C1q/TNF-Related Protein 9 on Coronary Artery Calcification: An Observational Study. J. Cardiovasc. Dev. Dis. 2022, 9, 313. https://doi.org/10.3390/jcdd9100313
Liu D, Ma Y, Jin X, Lu R, Hu H, Gu G. Effect of C1q/TNF-Related Protein 9 on Coronary Artery Calcification: An Observational Study. Journal of Cardiovascular Development and Disease. 2022; 9(10):313. https://doi.org/10.3390/jcdd9100313
Chicago/Turabian StyleLiu, Demin, Yanan Ma, Xiaoxue Jin, Rui Lu, Haijuan Hu, and Guoqiang Gu. 2022. "Effect of C1q/TNF-Related Protein 9 on Coronary Artery Calcification: An Observational Study" Journal of Cardiovascular Development and Disease 9, no. 10: 313. https://doi.org/10.3390/jcdd9100313





