Volume Analysis to Predict the Long-Term Evolution of Residual Aortic Dissection after Type A Repair
Abstract
1. Introduction
2. Patients and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Radiological Analysis
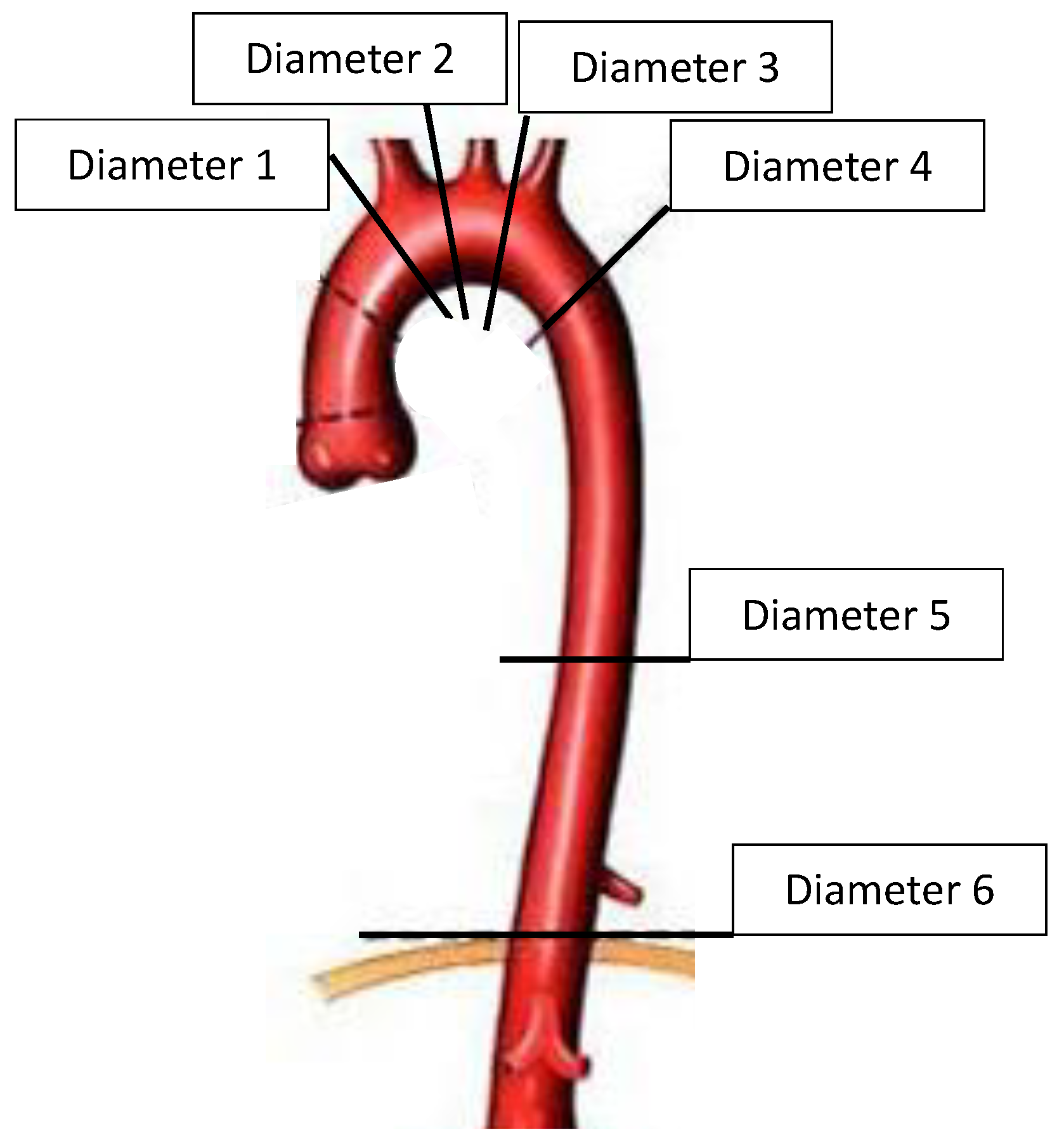
2.2.1. Diameter Measurement
2.2.2. Entry Tear
2.2.3. Volume Calculation
2.2.4. Volume Calculation Validation: Semiautomated Method Coupled with the Standard Method
3. Endpoints
4. Statistical Analysis
5. Results
5.1. Study Population
5.2. Measurement Validation
5.2.1. Diameter
5.2.2. Volume
5.3. Anatomical Risk Factors for the Dissection-Related Events
5.3.1. Univariate Analysis
Aortic Diameter Analysis
Aortic Diameter Evolution Analysis
Entry Tear
Volume Analysis
Volume Evolution Analysis
5.3.2. Multivariate Analysis
6. Comment
Limits
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RAD | residual aortic dissection |
| TAAD | type A aortic dissection |
| CL | centerline |
| CT | computed tomography |
| FL | false lumen |
| TL | true lumen |
| EV | elementary volume |
| CC | coefficient correlation |
| HR | hazard ratio |
References
- Hagan, P.G.; Nienaber, C.A.; Isselbacher, E.M.; Bruckman, D.; Karavite, D.J.; Russman, P.L.; Evangelista, A.; Fattori, R.; Suzuki, T.; Oh, J.K.; et al. The International Registry of Acute Aortic Dissection (IRAD): New insights into an old disease. JAMA 2000, 283, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Nienaber, C.A.; Kische, S.; Rousseau, H.; Eggebrecht, H.; Rehders, T.C.; Kundt, G.; Glass, A.; Scheinert, D.; Czerny, M.; Kleinfeldt, T.; et al. Endovascular repair of type B aortic dissection: Long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ. Cardiovasc. Interv. 2013, 6, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Kimura, N.; Itoh, S.; Yuri, K.; Adachi, K.; Matsumoto, H.; Yamaguchi, A.; Adachi, H. Reoperation for enlargement of the distal aorta after initial surgery for acute type A aortic dissection. J. Thorac. Cardiovasc. Surg. 2015, 149, S91–S98.e1. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-H.; Chen, M.-C.; Wu, Y.-C.; Wang, Y.-C.; Chu, J.-J.; Lin, P.J. Risk factors for descending aortic aneurysm formation in medium-term follow-up of patients with type A aortic dissection. Chest 2003, 124, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Asai, T.; Kinoshita, T. Predictors for Late Reoperation After Surgical Repair of Acute Type A Aortic Dissection. Ann. Thorac. Surg. 2018, 106, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Chikazawa, G.; Hiraoka, A.; Totsugawa, T.; Sakaguchi, T.; Yoshitaka, H. The prognostic impact of distal anastomotic new entry after acute type I aortic dissection repair. Eur. J. Cardiothorac. Surg. 2017, 52, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Lederle, F.A.; Johnson, G.R.; Wilson, S.E.; Littooy, F.N.; Krupski, W.C.; Bandyk, D.; Acher, C.W.; Chute, E.P.; Hye, R.J.; Gordon, I.L.; et al. Yield of repeated screening for abdominal aortic aneurysm after a 4-year interval. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch. Intern. Med. 2000, 160, 1117–1121. [Google Scholar] [CrossRef]
- Fattouch, K.; Sampognaro, R.; Navarra, E.; Caruso, M.; Pisano, C.; Coppola, G.; Speziale, G.; Ruvolo, G. Long-term results after repair of type a acute aortic dissection according to false lumen patency. Ann. Thorac. Surg. 2009, 88, 1244–1250. [Google Scholar] [CrossRef]
- Leontyev, S.; Haag, F.; Davierwala, P.M.; Lehmkuhl, L.; Borger, M.A.; Etz, C.D.; Misfeld, M.; Gutberlet, M.; Mohr, F.W. Postoperative Changes in the Distal Residual Aorta after Surgery for Acute Type A Aortic Dissection: Impact of False Lumen Patency and Size of Descending Aorta. Thorac. Cardiovasc. Surg. 2017, 65, 90–98. [Google Scholar]
- Park, K.-H.; Lim, C.; Choi, J.H.; Chung, E.; Choi, S.I.; Chun, E.J.; Sung, K. Midterm change of descending aortic false lumen after repair of acute type I dissection. Ann. Thorac. Surg. 2009, 87, 103–108. [Google Scholar] [CrossRef]
- Zierer, A.; Voeller, R.K.; Hill, K.E.; Kouchoukos, N.T.; Damiano, R.J., Jr.; Moon, M.R. Aortic enlargement and late reoperation after repair of acute type A aortic dissection. Ann. Thorac. Surg. 2007, 84, 479–486, discussion 486–487. [Google Scholar] [CrossRef]
- Evangelista, A.; Salas, A.; Ribera, A.; Ferreira-González, I.; Cuellar, H.; Pineda, V.; González-Alujas, T.; Bijnens, B.; Permanyer-Miralda, G.; Garcia-Dorado, D. Long-term outcome of aortic dissection with patent false lumen: Predictive role of entry tear size and location. Circulation 2012, 125, 3133–3141. [Google Scholar] [CrossRef]
- Gaudry, M.; Porto, A.; Guivier-Curien, C.; Blanchard, A.; Bal, L.; Resseguier, N.; Omnes, V.; De Masi, M.; Ejargue, M.; Jacquier, A.; et al. Results of a prospective follow-up study after type A aortic dissection repair: A high rate of distal aneurysmal evolution and reinterventions. Eur. J. Cardiothorac. Surg. 2021, 61, 152–159. [Google Scholar] [CrossRef]
- Rodriguez-Lopez, J.A.; Diethrich, E.B. Diameter or volume? The measure of success after endovascular repair of thoracic aortic dissections. J. Endovasc. Ther. 2009, 16, 39–41. [Google Scholar] [CrossRef]
- Sobocinski, J.; Lombardi, J.V.; Dias, N.V.; Berger, L.; Zhou, Q.; Jia, F.; Resch, T.; Haulon, S. Volume analysis of true and false lumens in acute complicated type B aortic dissections after thoracic endovascular aortic repair with stent grafts alone or with a composite device design. J. Vasc. Surg. 2016, 63, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Kamman, A.V.; Van Herwaarden, J.A.; Orrico, M.; Nauta, F.J.; Heijmen, R.H.; Moll, F.L.; Trimarchi, S. Standardized Protocol to Analyze Computed Tomography Imaging of Type B Aortic Dissections. J. Endovasc. Ther. 2016, 23, 472–482. [Google Scholar] [CrossRef]
- Tolenaar, J.L.; Kern, J.A.; Jonker, F.H.W.; Cherry, K.J.; Tracci, M.C.; Angle, J.F.; Sabri, S.; Trimarchi, S.; Strider, D.; Alaiwaidi, G.; et al. Predictors of false lumen thrombosis in type B aortic dissection treated with TEVAR. Ann. Cardiothorac. Surg. 2014, 3, 255–263. [Google Scholar] [PubMed]
- Melissano, G.; Bertoglio, L.; Rinaldi, E.; Civilini, E.; Tshomba, Y.; Kahlberg, A.; Agricola, E.; Chiesa, R. Volume changes in aortic true and false lumen after the "PETTICOAT" procedure for type B aortic dissection. J. Vasc. Surg. 2012, 55, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Sigman, M.M.; Palmer, O.P.; Ham, S.W.; Cunningham, M.; Weaver, F.A. Aortic morphologic findings after thoracic endovascular aortic repair for type B aortic dissection. JAMA Surg. 2014, 149, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Fillinger, M.F.; Greenberg, R.K.; McKinsey, J.F.; Chaikof, E.L. Society for Vascular Surgery Ad Hoc Committee on TRS. Reporting standards for thoracic endovascular aortic repair (TEVAR). J. Vasc. Surg. 2010, 52, 1022–1033, 1033.e15. [Google Scholar] [CrossRef]
- Czermak, B.V.; Fraedrich, G.; Schocke, M.F.; Steingruber, I.E.; Waldenberger, P.; Perkmann, R.; Rieger, M.; Jaschke, W. Serial CT volume measurements after endovascular aortic aneurysm repair. J. Endovasc. Ther. 2001, 8, 380–389. [Google Scholar] [CrossRef]
- van Prehn, J.; van der Wal, M.B.; Vincken, K.; Bartels, L.W.; Moll, F.L.; van Herwaarden, J.A. Intra- and interobserver variability of aortic aneurysm volume measurement with fast CTA postprocessing software. J. Endovasc. Ther. 2008, 15, 504–510. [Google Scholar] [CrossRef]
- England, A.; Fisher, R.; McWilliams, R.; Torella, F. Estimating the error of CT-based measurements of aortic lumen volume used in endovascular planning. Radiography 2017, 23, 287–291. [Google Scholar] [CrossRef]
- Concistrè, G.; Casali, G.; Santaniello, E.; Montalto, A.; Fiorani, B.; Dell’Aquila, A.; Musumeci, F. Reoperation after surgical correction of acute type A aortic dissection: Risk factor analysis. Ann. Thorac. Surg. 2012, 93, 450–455. [Google Scholar] [CrossRef]
- Kimura, N.; Tanaka, M.; Kawahito, K.; Yamaguchi, A.; Ino, T.; Adachi, H. Influence of patent false lumen on long-term outcome after surgery for acute type A aortic dissection. J. Thorac. Cardiovasc. Surg. 2008, 136, 1160–1166.e3. [Google Scholar] [CrossRef]
- Sayer, D.; Bratby, M.; Brooks, M.; Loftus, I.; Morgan, R.; Thompson, M. Aortic morphology following endovascular repair of acute and chronic type B aortic dissection: Implications for management. Eur. J. Vasc. Endovasc. Surg. 2008, 36, 522–529. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, X.Y.; Rosendahl, U.; Pepper, J.; Mirsadraee, S. Prediction of aortic dilatation in surgically repaired type A dissection: A longitudinal study using computational fluid dynamics. JTCVS Open 2022, 9, 11–27. [Google Scholar] [CrossRef]
- Zhu, Y.; Mirsadraee, S.; Asimakopoulos, G.; Gambaro, A.; Rosendahl, U.; Pepper, J.; Xu, X.Y. Association of hemodynamic factors and progressive aortic dilatation following type A aortic dissection surgical repair. Sci. Rep. 2021, 11, 11521. [Google Scholar] [CrossRef] [PubMed]
- Fatma, K.; Carine, G.C.; Marine, G.; Philippe, P.; Valérie, D. Numerical modelingof residual type B aortic dissection: Longitudinal analysis of favorable and unfavorable evolution. Med. Biol. Eng. Comput. 2022, 60, 769–783. [Google Scholar] [CrossRef]




| Variables | Total n = 54 | Dissection Related Events n = 34 | Non-Dissection Related Events n = 20 | p Value |
|---|---|---|---|---|
| Male sex, n (%) | 35 (64.8) | 23 (67.6) | 12 (60.0) | 0.57 |
| Age, median (range) | 62.5 (37–82) | 64.3 (37–82) | 60.4 (40–75) | 0.19 |
| Hypertension, n (%) | 34 (62.9) | 22 (64.7) | 12 (60.0) | 0.72 |
| Dyslipidemia, n (%) | 6 (11.1) | 3 (8.8) | 3 (15.0) | 0.66 |
| Smoking, n (%) | 18 (33.3) | 9 (26.5) | 9 (45.0) | 0.16 |
| Diabetes, n (%) | 4 (7.4) | 4 (11.8) | 0 (0) | 0.29 |
| COPD, n (%) | 2 (3.7) | 1 (2.9) | 1 (5.0) | >0.99 |
| CAD, n (%) | 2 (3.7) | 1 (2.9) | 1 (5.0) | >0.99 |
| Renal failure, n (%) | 1 (1.8) | 0 (0.0) | 1 (5.0) | 0.37 |
| Marfan syndrome, n (%) | 1 (1.8) | 1 (2.9) | 0 (0) | >0.99 |
| Bicuspid aortic valve, n (%) | 1 (1.8) | 0 (0) | 1 (5.0) | 0.37 |
| Follow-up (months), median (range) | 69.0 (32–144) | 76.0 (32–131) | 66.5 (34–144) | 0.47 |
| Initial type A aortic repair, n (%) | ||||
| Ascending aortic replacement | 2 (3.7) | 1 (2.9) | 1 (5.0) | 0.05 |
| Hemiarch replacement | 42 (77.8) | 24 (70.6) | 18 (90.0) | |
| Partial aortic arch replacement | 6 (11.1) | 5 (14.7) | 1 (5.0) | |
| Total aortic arch replacement | 4 (7.4) | 4 (11.8) | 0 (0.0) |
| T1 | T2 | T3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Levels | Group 1 | Group 2 | p-Value | Group 1 | Group 2 | p-Value | Group 1 | Group 2 | p-Value | |
| Total aortic diameter mm, med (range) | D 1 | 35.1 [32.4–40.9] | 34.0 [29.0–38.7] | 0.201 | 35.5 [32.4–40.2] | 33.8 [30.8–37.8] | 0.186 | 35.8 [31.9–41.9] | 32.9 [30.0–39.9] | 0.116 |
| D 2 | 43.1 [36.1–45.4] | 37.3 [33.7–41.0] | 0.033 * | 41.7 [36.4–48.0] | 37.9 [33.3–40.7] | 0.023 * | 43.4 [36.5–51.6] | 37.4 [31.7–42.7] | 0.018 * | |
| D 3 | 40.9 [35.7–44.9] | 35.2 [34.1–37.5] | 0.001 * | 41.4 [36.3–46.3] | 36.2 [32.5–37.0] | <0.001 * | 44.6 [36.0–47.5] | 35.9 [31.3–38.8] | <0.001 * | |
| D 4 | 39.5 [36.9–42.9] | 34.1 [31.0–36.8] | <0.001 * | 44.0 [40.1–49.3] | 34.4 [32.5–38.1] | <0.001 * | 48.4 [43.6–53.5] | 37.5 [32.9–40.4] | <0.001 * | |
| D 5 | 35.8 [33.4–38.7] | 31.8 [29.5–34.1] | <0.001 * | 39.0 [35.2–43.3] | 32.2 [29.2–34.3] | <0.001 * | 42.2 [36.8–46.2] | 34.3 [30.2–37.0] | <0.001 * | |
| D 6 | 31.8 [30.2–34.6] | 29.3 [27.7–32.6] | 0.009 * | 33.0 [31.7–36.5] | 30.0 [28.7–33.1] | 0.001 * | 35.4 [33.2–37.7] | 30.7 [29.1–34.0] | <0.001 * | |
| True lumen mm, med (range) | D 1 | 34.2 [30.9–37.5] | 31.5 [28.6–35.7] | 0.380 | 35.7 [31.6–38.6] | 32.5 [28.6–37.7] | 0.158 | 36.0 [33.7–41.0] | 35.2 [32.2–39.7] | 0.814 |
| D 2 | 36.4 [35.2–39.2] | 31.5 [27.2–34.8] | 0.001 * | 35.9 [34.4–40.0] | 34.5 [28.4–36.1] | 0.034 * | 37.0 [34.1–40.7] | 33.8 [30.3–36.5] | 0.020 * | |
| D 3 | 34.6 [30.7–38.1] | 29.2 [27.4–32.0] | <0.001 * | 36.8 [32.0–40.2] | 31.2 [29.7–32.8] | 0.001 * | 37.2 [32.8–40.8] | 30.4 [28.9–32.8] | <0.001 * | |
| D 4 | 28.4 [25.5–32.0] | 27.2 [23.8–29.2] | 0.209 | 32.0 [27.7–36.3] | 28.1 [26.5–31.3] | 0.025 * | 33.3 [29.3–37.2] | 29.1 [26.6–32.1] | 0.003 * | |
| D 5 | 27.8 [23.1–30.5] | 24.8 [23.5–28.6] | 0.352 | 28.4 [25.2–30.3] | 26.7 [24.2–31.4] | 0.599 | 29.5 [27.0–32.1] | 27.5 [26.4–29.1] | 0.051 | |
| D 6 | 25.7 [23.4–28.0] | 24.2 [20.4–27.9] | 0.244 | 27.0 [24.3–29.3] | 26.2 [21.7–29.2] | 0.565 | 27.3 [25.6–30.8] | 27.2 [23.9–28.5] | 0.629 | |
| False lumen mm, med (range) | D 1 | 32.8 [17.2–40.5] | 35.7 [32.2–37.8] | 0.526 | 39.7 [26.9–48.5] | 33.6 [20.2–40.8] | 0.386 | 40.5 [24.1–52.7] | 36.3 [24.7–41.0] | 0.409 |
| D 2 | 37.1 [32.9–40.7] | 33.2 [26.6–36.6] | 0.043 * | 37.4 [30.7–43.4] | 34.5 [29.6–36.1] | 0.086 | 38.2 [32.9–43.4] | 33.8 [29.4–37.3] | 0.053 | |
| D 3 | 36.6 [31.8–40.2] | 29.6 [26.6–33.4] | 0.001 * | 36.5 [31.3–41.1] | 31.0 [26.7–32.5] | 0.002 * | 37.2 [31.6–41.4] | 32.1 [27.7–33.4] | 0.002 * | |
| D 4 | 39.1 [35.4–41.8] | 34.1 [30.9–35.7] | 0.001 * | 42.4 [38.7–48.5] | 34.6 [32.1–38.9] | <0.001 * | 45.3 [41.6–51.8] | 37.3 [32.9–38.4] | <0.001 * | |
| D 5 | 35.0 [33.1–38.7] | 31.7 [29.2–33.3] | <0.001 * | 38.3 [34.3–40.9] | 32.3 [29.7–33.9] | <0.001 * | 39.9 [35.5–45.8] | 33.4 [30.8–35.5] | <0.001 * | |
| D 6 | 31.3 [29.1–33.7] | 28.5 [26.3–32.1] | 0.025 * | 32.7 [31.1–35.7] | 29.3 [27.3–31.5] | <0.001 * | 33.9 [32.1–36.8] | 28.7 [27.3–32.5] | 0.001 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaudry, M.; Guivier-Curien, C.; Blanchard, A.; Porto, A.; Bal, L.; Omnes, V.; De Masi, M.; Lu, C.; Jacquier, A.; Piquet, P.; et al. Volume Analysis to Predict the Long-Term Evolution of Residual Aortic Dissection after Type A Repair. J. Cardiovasc. Dev. Dis. 2022, 9, 349. https://doi.org/10.3390/jcdd9100349
Gaudry M, Guivier-Curien C, Blanchard A, Porto A, Bal L, Omnes V, De Masi M, Lu C, Jacquier A, Piquet P, et al. Volume Analysis to Predict the Long-Term Evolution of Residual Aortic Dissection after Type A Repair. Journal of Cardiovascular Development and Disease. 2022; 9(10):349. https://doi.org/10.3390/jcdd9100349
Chicago/Turabian StyleGaudry, Marine, Carine Guivier-Curien, Arnaud Blanchard, Alizée Porto, Laurence Bal, Virgile Omnes, Mariangela De Masi, Charlotte Lu, Alexis Jacquier, Philippe Piquet, and et al. 2022. "Volume Analysis to Predict the Long-Term Evolution of Residual Aortic Dissection after Type A Repair" Journal of Cardiovascular Development and Disease 9, no. 10: 349. https://doi.org/10.3390/jcdd9100349
APA StyleGaudry, M., Guivier-Curien, C., Blanchard, A., Porto, A., Bal, L., Omnes, V., De Masi, M., Lu, C., Jacquier, A., Piquet, P., & Deplano, V. (2022). Volume Analysis to Predict the Long-Term Evolution of Residual Aortic Dissection after Type A Repair. Journal of Cardiovascular Development and Disease, 9(10), 349. https://doi.org/10.3390/jcdd9100349





