Prosthetic Valve Function after Aortic Valve Replacement for Severe Aortic Stenosis by Transcatheter Procedure versus Surgery
Abstract
:1. Introduction
2. Methods
2.1. Patients
2.2. Treatment Selection
2.3. Data Collection
2.4. Endpoints
2.5. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Operative Outcomes
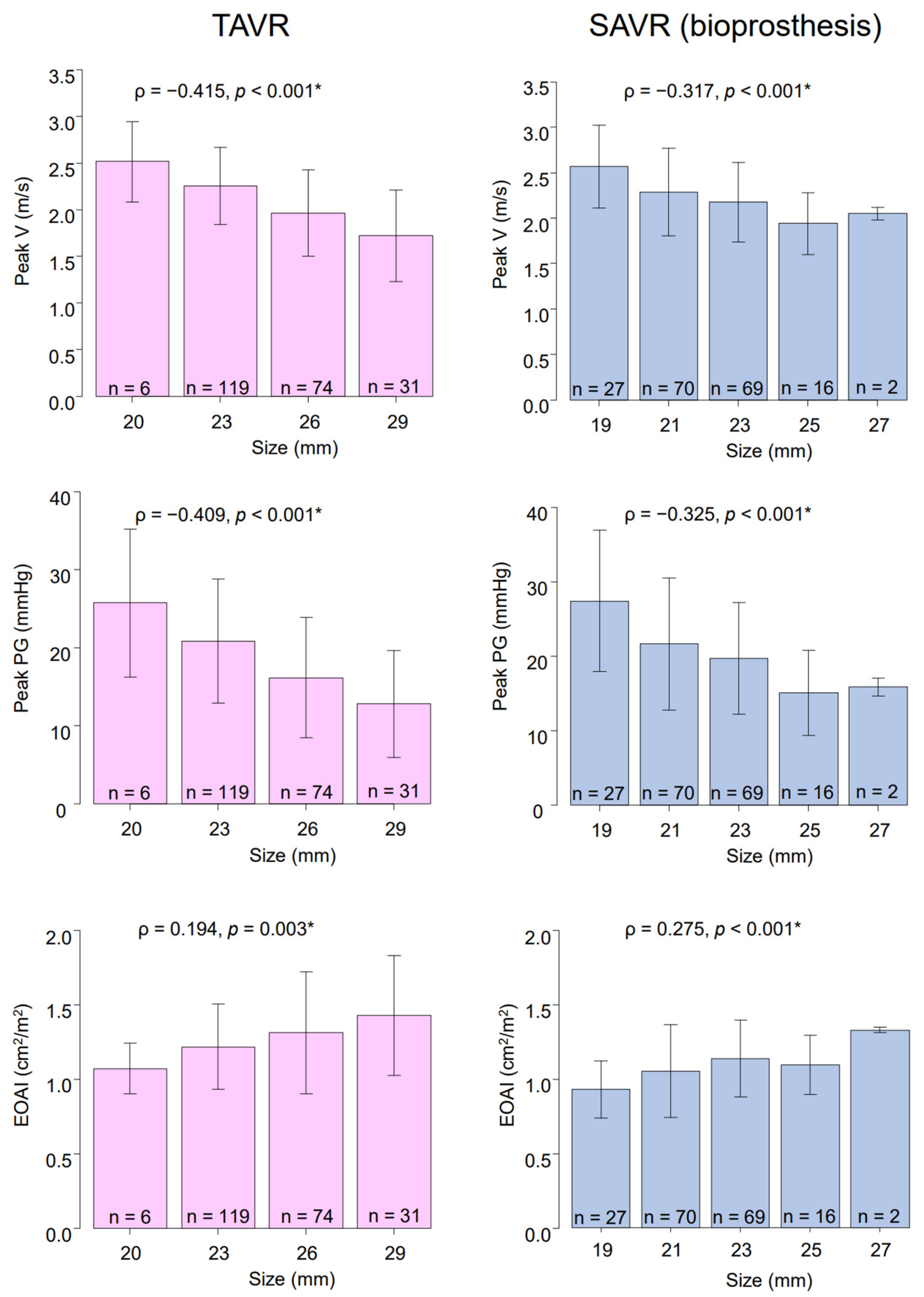
3.3. Postoperative Echocardiographic Findings
3.4. Primary Endpoint
3.5. Secondary Endpoints
3.6. Risk Factors for Primary Endpoint
4. Discussions
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varadarajan, P.; Kapoor, N.; Bansal, R.C.; Pai, R.G. Survival in elderly patients with severe aortic stenosis is dramatically improved by aortic valve replacement: Results from a cohort of 277 patients aged > or =80 years. Eur. J. Cardiothorac. Surg. 2006, 30, 722–727. [Google Scholar] [CrossRef] [Green Version]
- Huber, C.H.; Goeber, V.; Berdat, P.; Carrel, T.; Eckstein, F. Benefits of cardiac surgery in octogenarians—A postoperative quality of life assessment. Eur. J. Cardiothorac. Surg. 2007, 31, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef] [Green Version]
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J., Jr.; Kleiman, N.S.; et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N. Engl. J. Med. 2014, 370, 1790–1798. [Google Scholar] [CrossRef] [Green Version]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef]
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J., Jr.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar]
- Falk, V.; Baumgartner, H.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izumi, C.; Eishi, K.; Ashihara, K.; Arita, T.; Otsuji, Y.; Kunihara, T.; Komiya, T.; Shibata, T.; Seo, Y.; Daimon, M.; et al. JCS/JSCS/JATS/JSVS 2020 Guidelines on the Management of Valvular Heart Disease. Circ. J. 2020, 84, 2037–2119. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Chambers, J.B.; Dumesnil, J.G.; Foster, E.; Gottdiener, J.S.; Grayburn, P.A.; Khandheria, B.K.; Levine, R.A.; Marx, G.R.; Miller, F.A.; et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2009, 22, 975–1014, quiz 82–84. [Google Scholar]
- Pibarot, P.; Dumesnil, J.G. Prosthesis-patient mismatch: Definition, clinical impact, and prevention. Heart 2006, 92, 1022–1029. [Google Scholar] [CrossRef]
- Head, S.J.; Mokhles, M.M.; Osnabrugge, R.L.; Pibarot, P.; Mack, M.J.; Takkenberg, J.J.; Bogers, A.J.; Kappetein, A.P. The impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: A systematic review and meta-analysis of 34 observational studies comprising 27 186 patients with 133 141 patient-years. Eur. Heart J. 2012, 33, 1518–1529. [Google Scholar] [CrossRef] [Green Version]
- Flameng, W.; Herregods, M.C.; Vercalsteren, M.; Herijgers, P.; Bogaerts, K.; Meuris, B. Prosthesis-patient mismatch predicts structural valve degeneration in bioprosthetic heart valves. Circulation 2010, 121, 2123–2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinning, J.M.; Vasa-Nicotera, M.; Chin, D.; Hammerstingl, C.; Ghanem, A.; Bence, J.; Kovac, J.; Grube, E.; Nickenig, G.; Werner, N. Evaluation and management of paravalvular aortic regurgitation after transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 2013, 62, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Cla Clavel, M.A.; Webb, J.G.; Pibarot, P.; Altwegg, L.; Dumont, E.; Thompson, C.; De Larochellière, R.; Doyle, D.; Masson, J.B.; Bergeron, S.; et al. Comparison of the hemodynamic performance of percutaneous and surgical bioprostheses for the treatment of severe aortic stenosis. J. Am. Coll. Cardiol. 2009, 53, 1883–1891. [Google Scholar] [CrossRef] [Green Version]
- Puri, R.; Byrne, J.; Muller, R.; Baumbach, H.; Eltchaninoff, H.; Redwood, S.; Cheema, A.; Dubois, C.; Ihlberg, L.; Wijeysundera, H.C.; et al. Transcatheter aortic valve implantation in patients with small aortic annuli using a 20 mm balloon-expanding valve. Heart 2017, 103, 148–153. [Google Scholar] [CrossRef] [Green Version]
- Rodés-Cabau, J.; Pibarot, P.; Suri, R.M.; Kodali, S.; Thourani, V.H.; Szeto, W.Y.; Svensson, L.G.; Dumont, E.; Xu, K.; Hahn, R.T.; et al. Impact of aortic annulus size on valve hemodynamics and clinical outcomes after transcatheter and surgical aortic valve replacement: Insights from the PARTNER Trial. Circ. Cardiovasc. Interv. 2014, 7, 701–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasic, M.; Unbehaun, A.; Buz, S.; Drews, T.; Hetzer, R. Annular rupture during transcatheter aortic valve replacement: Classification, pathophysiology, diagnostics, treatment approaches, and prevention. JACC Cardiovasc. Interv. 2015, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, H.B.; Nombela-Franco, L.; Urena, M.; Mok, M.; Pasian, S.; Doyle, D.; DeLarochellière, R.; Côté, M.; Laflamme, L.; DeLarochellière, H.; et al. Coronary obstruction following transcatheter aortic valve implantation: A systematic review. JACC Cardiovasc. Interv. 2013, 6, 452–461. [Google Scholar] [CrossRef]
- Zhang, D.; Mao, X.; Liu, D.; Zhang, J.; Luo, G.; Luo, L. Transcatheter vs surgical aortic valve replacement in low to intermediate surgical risk aortic stenosis patients: A systematic review and meta-analysis of randomized controlled trials. Clin. Cardiol. 2020, 43, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Witberg, G.; Lador, A.; Yahav, D.; Kornowski, R. Transcatheter versus surgical aortic valve replacement in patients at low surgical risk: A meta-analysis of randomized trials and propensity score matched observational studies. Catheter. Cardiovasc. Interv. 2018, 92, 408–416. [Google Scholar] [CrossRef]
- Hagar, A.; Li, Y.; Wei, X.; Peng, Y.; Xu, Y.; Ou, Y.; Wang, Z.; Wang, X.; Shah, J.P.; Sihag, V.; et al. Incidence, Predictors, and Outcome of Paravalvular Leak after Transcatheter Aortic Valve Implantation. J. Interv. Cardiol. 2020, 2020, 8249497. [Google Scholar] [CrossRef] [PubMed]
- Pollari, F.; Dell’Aquila, A.M.; Söhn, C.; Marianowicz, J.; Wiehofsky, P.; Schwab, J.; Pauschinger, M.; Hitzl, W.; Fischlein, T.; Pfeiffer, S. Risk factors for paravalvular leak after transcatheter aortic valve replacement. J. Thorac. Cardiovasc. Surg. 2019, 157, 1406–1415.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamm, A.R.; Hell, M.M.; Geyer, M.; Kreidel, F.; da Rocha e Silva, J.G.; Seidl, M.; Ruf, T.F.; Kornberger, A.; Beiras-Fernandez, A.; Münzel, T.; et al. Minimizing Paravalvular Regurgitation With the Novel SAPIEN 3 Ultra TAVR Prosthesis: A Real-World Comparison Study. Front. Cardiovasc. Med. 2021, 8, 623146. [Google Scholar] [CrossRef] [PubMed]
- Mouillet, G.; Lellouche, N.; Yamamoto, M.; Oguri, A.; Dubois-Rande, J.L.; Van Belle, E.; Gilard, M.; Laskar, M.; Teiger, E. Outcomes following pacemaker implantation after transcatheter aortic valve implantation with CoreValve(®) devices: Results from the FRANCE 2 Registry. Catheter. Cardiovasc. Interv. 2015, 86, E158–E166. [Google Scholar] [CrossRef]
- Nazif, T.M.; Dizon, J.M.; Hahn, R.T.; Xu, K.E.; Babaliaros, V.; Douglas, P.S.; El-Chami, M.F.; Herrmann, H.C.; Mack, M.; Makkar, R.R.; et al. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: The PARTNER (Placement of AoRtic TraNscathetER Valves) trial and registry. JACC Cardiovasc. Interv. 2015, 8, 60–69. [Google Scholar] [CrossRef] [Green Version]
- F Fadahunsi, O.O.; Olowoyeye, A.; Ukaigwe, A.; Li, Z.; Vora, A.N.; Vemulapalli, S.; Elgin, E.; Donato, A. Incidence, Predictors, and Outcomes of Permanent Pacemaker Implantation Following Transcatheter Aortic Valve Replacement: Analysis From the U.S. Society of Thoracic Surgeons/American College of Cardiology TVT Registry. JACC Cardiovasc. Interv. 2016, 9, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- Aljabbary, T.; Qiu, F.; Masih, S.; Fang, J.; Elbaz-Greener, G.; Austin, P.C.; Rodés-Cabau, J.; Ko, D.T.; Singh, S.; Wijeysundera, H.C. Association of Clinical and Economic Outcomes With Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement. JAMA Netw. Open 2018, 1, e180088. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.V.; Omar, W.; Gonzalez, P.E.; Jessen, M.E.; Huffman, L.; Kumbhani, D.J.; Bavry, A.A. Expansion of TAVR into Low-Risk Patients and Who to Consider for SAVR. Cardiol. Ther. 2020, 9, 377–394. [Google Scholar] [CrossRef] [PubMed]





| TAVR | SAVR | ||
|---|---|---|---|
| Characteristics | (n = 230) | (n = 195) | p-Value |
| Age, year | 84.6 ± 4.3 | 74.0 ± 8.0 | <0.001 |
| Female sex, no. (%) | 166 (72.2) | 82 (42.1) | <0.001 |
| Body mass index | 22.2 ± 3.7 | 23.4 ± 3.9 | <0.001 |
| STS-PROM | 6.6 ± 4.6 | 5.1 ± 5.5 | <0.001 |
| NYHA class III or IV, no. (%) | 60 (26.1) | 55 (28.2) | 0.662 |
| Coronary artery disease, no. (%) | 33 (14.3) | 69 (32.3) | <0.001 |
| Triple vessel disease and/or left main trunk disease, no (%) | 3 (1.3) | 23 (11.8) | <0.001 |
| Cerebral vascular disease/Carotid disease, no. (%) | 60 (26.1) | 32 (16.4) | 0.018 |
| Peripheral vascular disease, no. (%) | 31 (13.5) | 38 (19.5) | 0.113 |
| COPD, no. (%) | 42 (18.3) | 33 (16.9) | 0.799 |
| Creatinine > 2 mg/dL, no. (%) | 2 (0.9) | 39 (20.0) | <0.001 |
| Hemodialysis, no. (%) | 0 (0) | 30 (15.4) | <0.001 |
| Diabetes, no. (%) | 76 (33.0) | 70 (35.9) | 0.541 |
| Atrial fibrillation, no (%) | 33 (14.3) | 33 (16.9) | 0.503 |
| Previous cardiovascular surgery, no. (%) | 12 (5.2) | 9 (4.6) | 0.826 |
| Bicuspid aortic valve, no. (%) | 6 (2.6) | 39 (20.0) | <0.001 |
| Left ventricular ejection fraction, % | 58.1 ± 11.4 | 55.7 ± 13.1 | 0.043 |
| Left ventricular ejection fraction < 30%, no. (%) | 3 (1.3) | 11 (5.6) | 0.014 |
| Emergent/Urgent operation, no. (%) | 8 (3.5) | 23 (11.8) | 0.001 |
| Concomitant CABG/TAVR + PCI, no. (%) | 27 (11.7) | 57 (29.2) | <0.001 |
| Size (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Prosthesis | 19 | 20 | 21 | 22 | 23 | 25 | 26 | 27 | 29 |
| Transcatheter aortic valve replacement | |||||||||
| Sapien XT | - | 1 | - | - | 22 | - | 11 | - | 1 |
| Sapien 3 | - | 5 | - | - | 92 | - | 38 | - | 11 |
| CoreValve | - | 0 | - | - | 0 | - | 1 | - | 1 |
| CoreValve EVOLUTE R | - | 0 | - | - | 4 | - | 18 | - | 13 |
| CoreValve EVOLUTE PRO | - | 0 | - | - | 1 | - | 6 | - | 5 |
| Surgical aortic valve replacement Biological | |||||||||
| Magna Ease | 15 | - | 28 | - | 13 | 8 | - | 0 | 0 |
| INSPIRIS RESILIA | 3 | - | 2 | - | 7 | 0 | - | 0 | 0 |
| Carpentier-Edwards PERIMOUNT | 0 | - | 1 | - | 2 | 1 | - | 1 | 0 |
| Crown PRT | 4 | - | 32 | - | 39 | 6 | - | 0 | 0 |
| SOLO SMART | 1 | - | 4 | - | 3 | 0 | - | 0 | 0 |
| Mitroflow | 2 | - | 0 | - | 0 | 0 | - | 0 | 0 |
| Trifecta | 1 | - | 3 | - | 0 | 0 | - | 0 | 0 |
| Mosaic Ultra | 0 | - | 0 | - | 2 | 0 | - | 0 | 0 |
| AVALUS | 1 | - | 0 | - | 3 | 1 | - | 1 | 0 |
| Mechanical | |||||||||
| SJM Regent | 3 | - | 2 | - | 1 | 0 | - | 0 | 0 |
| ATS | - | 2 | - | 1 | - | - | 0 | - | - |
| On-X | 0 | - | 0 | - | 2 | 0 | - | 0 | 0 |
| TAVR | SAVR | ||
|---|---|---|---|
| Parameters | (n = 230) | (n = 195) | p-Value |
| Intra-aortic balloon pump, no. (%) | 3 (1.3) | 14 (7.2) | 0.002 |
| Extracorporeal membrane oxygenation, no. (%) | 1 (0.4) | 0 (0.0) | 1 |
| Intraoperative bleeding, mL | 116.0 ± 342.6 | 861.0 ± 615.1 | <0.001 |
| Transfusion (red blood cell), mL | 338.1 ± 349.4 | 1063.4 ± 673.9 | <0.001 |
| Reoperation for bleeding, no. (%) | 7 (3.0) | 11 (5.6) | 0.229 |
| New-onset atrial fibrillation, no. (%) | 13 (5.7) | 59 (30.3) | <0.001 |
| Permanent pacemaker implantation, no. (%) | 23 (10.0) | 3 (1.5) | <0.001 |
| Newly induced renal replacement therapy, no. (%) | 3 (1.3) | 5 (2.6) | 0.479 |
| Prosthetic valve endocarditis, no. (%) | 1 (0.4) | 6 (3.1) | 0.051 |
| Peripheral vascular complication, no. (%) | 15 (6.5) | 0 (0.0) | <0.001 |
| Extubation in operation room, no. (%) | 183 (79.6) | 0 (0.0) | <0.001 |
| Intubation time, hours | 9.1 ± 51.3 | 17.4 ± 29.3 | 0.049 |
| Intensive care unit stay, days | 1.4 ± 3.5 | 3.2 ± 3.8 | <0.001 |
| Echocardiographic findings | |||
| Peak velocity through aortic valve, m/s | 2.1 ± 0.5 | 2.3 ± 0.5 | <0.001 |
| Mean pressure gradient, mmHg | 10.0 ± 4.8 | 11.6 ± 4.9 | <0.001 |
| Peak pressure gradient, mmHg | 18.5 ± 8.5 | 21.4 ± 8.7 | <0.001 |
| Effective orifice area index, cm2/m2 | 1.27 ± 0.35 | 1.06 ± 0.27 | <0.001 |
| Effective orifice area index < 0.85 cm2/m2, no. (%) | 20 (8.7) | 44 (22.6) | <0.001 |
| Effective orifice area index < 0.65 cm2/m2, no. (%) | 2 (0.9) | 7 (3.6) | 0.087 |
| ≥Trivial paravalvular leakage, no. (%) | 201 (87.4) | 16 (8.2) | <0.001 |
| ≥Mild paravalvular leakage, no. (%) | 49 (21.3) | 0 (0.0) | <0.001 |
| Univariate | Multivariable | ||
|---|---|---|---|
| Parameters | p-Value | p-Value | Hazard Ratio (95% CI) |
| Age | 0.763 | ||
| Female sex | 0.135 | ||
| Body-mass index | 0.671 | ||
| STS-PROM | <0.001 | 0.042 | 1.038 (1.001–1.076) |
| NYHA class III or IV | 0.046 | 0.721 | 0.921 (0.587–1.445) |
| Coronary artery disease | 0.036 | 0.7 | 1.130 (0.607–2.105) |
| Triple vessel disease and/or left main trunk disease | 0.449 | ||
| Cerebral vascular disease/Carotid disease | 0.7 | ||
| Peripheral vascular disease | <0.001 | 0.048 | 1.595 (1.004–2.534) |
| COPD | 0.802 | ||
| Creatinine > 2 mg/dL | <0.001 | 0.707 | 1.190 (0.481–2.946) |
| Hemodialysis | <0.001 | 0.487 | 1.398 (0.544–3.595) |
| Diabetes | 0.186 | ||
| Atrial fibrillation | 0.032 | 0.066 | 1.524 (0.973–2.388) |
| Previous cardiovascular surgery | 0.531 | ||
| Bicuspid aortic valve | 0.832 | ||
| Left ventricular ejection fraction | 0.263 | ||
| Left ventricular ejection fraction < 30% | 0.092 | 0.432 | 1.424 (0.590–3.433) |
| Emergent/Urgent operation | 0.284 | ||
| Concomitant CABG/TAVR+PCI | 0.082 | 0.844 | 1.069 (0.553–2.065) |
| TAVR | 0.36 |
| Univariate | Multivariable | ||
|---|---|---|---|
| Parameters | p-Value | p-Value | Hazard Ratio (95% CI) |
| Intra-aortic balloon pump | 0.056 | 0.438 | 1.389 (0.606–3.183) |
| Extracorporeal membrane oxygenation | 0.994 | ||
| Intraoperative bleeding (L) | 0.026 | 0.926 | 1.016 (0.723–1.429) |
| Transfusion (red blood cell) (L) | 0.095 | 0.469 | 1.143 (0.796–1.641) |
| Reoperation for bleeding | 0.72 | ||
| Newly onset atrial fibrillation | 0.303 | ||
| Permanent pacemaker implantation | 0.022 | 0.032 | 2.105 (1.066–4.154) |
| Newly induced renal replacement therapy | <0.001 | 0.033 | 2.811 (1.085–7.280) |
| Prosthetic valve endocarditis | <0.001 | <0.001 | 6.984 (3.094–15.770) |
| Peripheral vascular complication | 0.204 | ||
| Intubation time (h) | 0.071 | 0.072 | 0.995 (0.989–1.000) |
| Intensive care unit stay (days) | <0.001 | 0.006 | 1.124 (1.035–1.221) |
| Echocardiographic findings | |||
| Peak velocity through aortic valve (m/s) | 0.51 | ||
| Mean pressure gradient (mmHg) | 0.87 | ||
| Peak pressure gradient (mmHg) | 0.821 | ||
| Effective orifice area index (cm2/m2) | 0.701 | ||
| Effective orifice area index < 0.85 cm2/m2 | 0.009 | 0.371 | 1.269 (0.753–2.140) |
| Effective orifice area index < 0.65 cm2/m2 | 0.02 | 0.17 | 2.072 (0.731–5.873) |
| ≥Trivial paravalvular leakage | 0.2 | ||
| ≥Mild paravalvular leakage | 0.019 | 0.001 | 2.301 (1.381–3.834) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, S.; Sairenchi, T.; Hirota, S.; Niitsuma, K.; Yokoyama, S.; Kanno, Y.; Kanazawa, Y.; Tezuka, M.; Takei, Y.; Tsuchiya, G.; et al. Prosthetic Valve Function after Aortic Valve Replacement for Severe Aortic Stenosis by Transcatheter Procedure versus Surgery. J. Cardiovasc. Dev. Dis. 2022, 9, 355. https://doi.org/10.3390/jcdd9100355
Saito S, Sairenchi T, Hirota S, Niitsuma K, Yokoyama S, Kanno Y, Kanazawa Y, Tezuka M, Takei Y, Tsuchiya G, et al. Prosthetic Valve Function after Aortic Valve Replacement for Severe Aortic Stenosis by Transcatheter Procedure versus Surgery. Journal of Cardiovascular Development and Disease. 2022; 9(10):355. https://doi.org/10.3390/jcdd9100355
Chicago/Turabian StyleSaito, Shunsuke, Toshimi Sairenchi, Shotaro Hirota, Ken Niitsuma, Shohei Yokoyama, Yasuyuki Kanno, Yuta Kanazawa, Masahiro Tezuka, Yusuke Takei, Go Tsuchiya, and et al. 2022. "Prosthetic Valve Function after Aortic Valve Replacement for Severe Aortic Stenosis by Transcatheter Procedure versus Surgery" Journal of Cardiovascular Development and Disease 9, no. 10: 355. https://doi.org/10.3390/jcdd9100355





