Inadvertent Lead Malposition in the Left Heart during Implantation of Cardiac Electric Devices: A Systematic Review
Abstract
1. Introduction
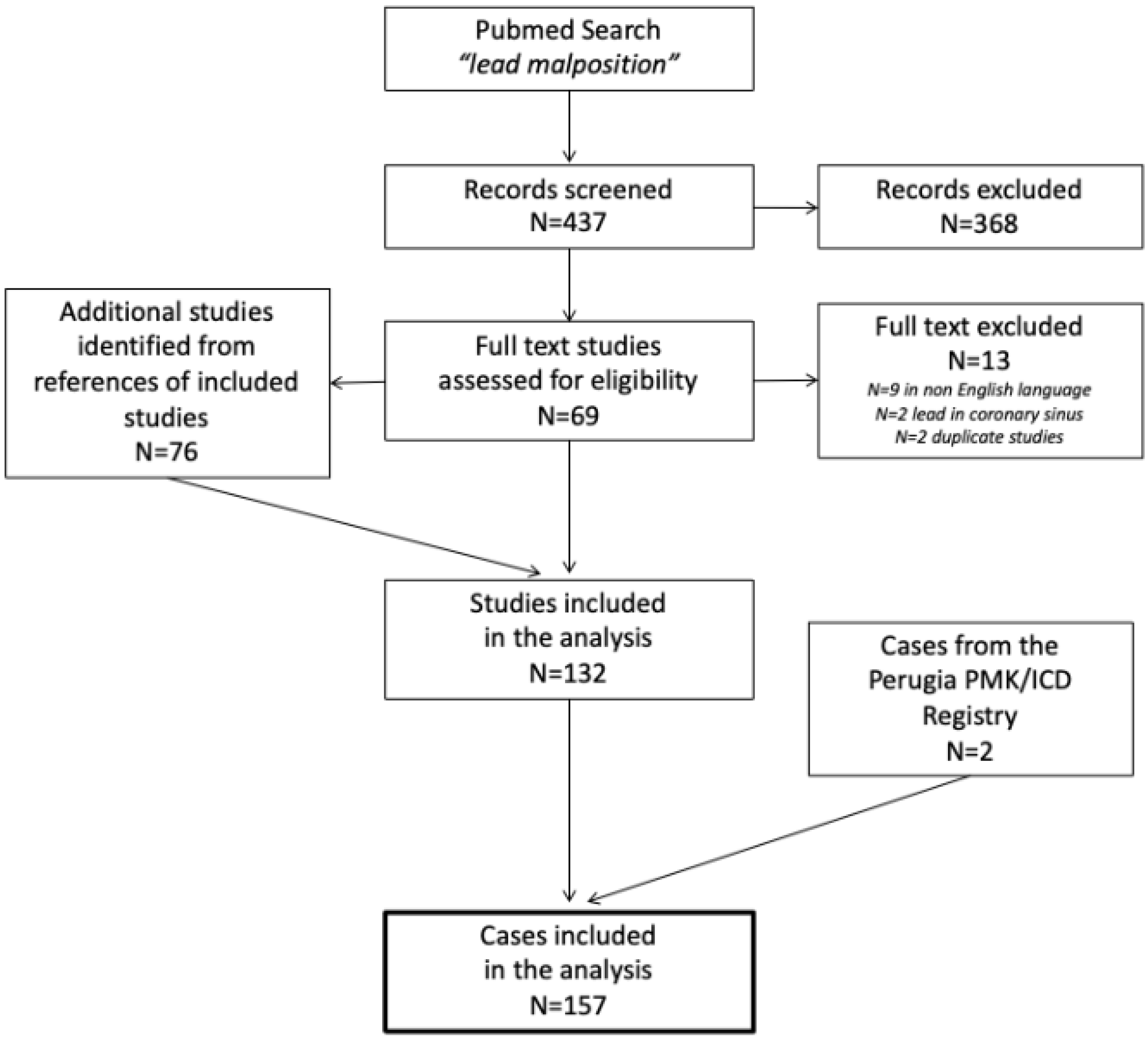
2. Materials and Methods
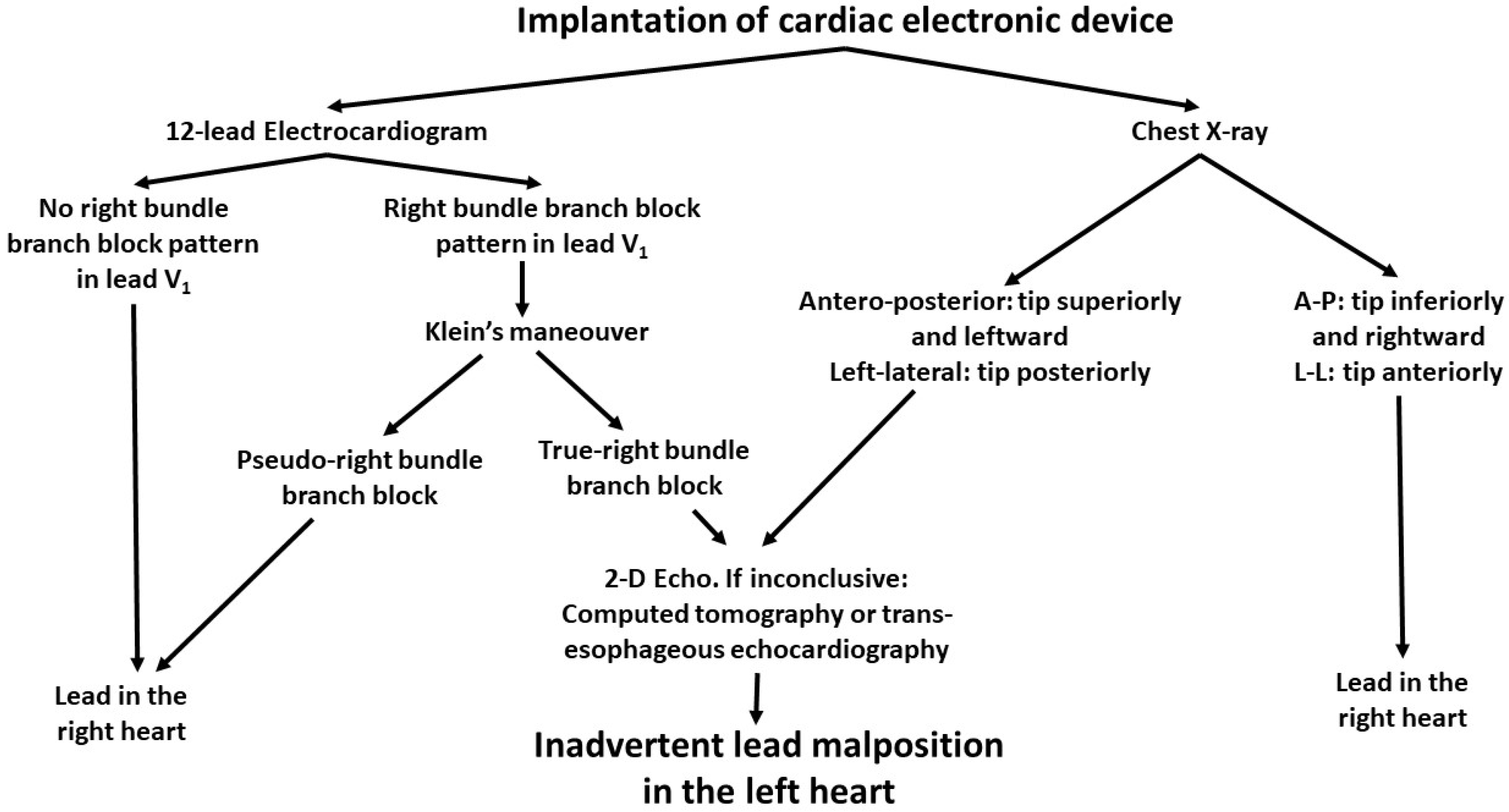
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stillman, M.T.; Richards, A.M. Perforation of the interventricular septum by transvenous pacemaker catheter. Diagnosis by change in pattern of depolarization on the electrocardiogram. Am. J. Cardiol. 1969, 24, 269–273. [Google Scholar] [CrossRef]
- Van Gelder, B.M.; Bracke, F.A.; Oto, A.; Yildirir, A.; Haas, P.C.; Seger, J.J.; Stainback, R.F.; Botman, K.J.; Meijer, A. Diagnosis and management of inadvertently placed pacing and ICD leads in the left ventricle: A multicenter experience and review of the literature. Pacing Clin. Electrophysiol. 2000, 23, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, Y.; Baltodano, P.; Tower, A.; Martinez, C.; Carrillo, R. Management of symptomatic inadvertently placed endocardial leads in the left ventricle. Pacing Clin. Electrophysiol. 2011, 34, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.O.; Beker, B.; Sareli, P.; DiSegni, E.; Dean, H.; Kaplinsky, E. Unusual QRS morphology associated with transvenous pacemakers: The pseudo RBBB pattern. Chest 1985, 87, 517–521. [Google Scholar] [CrossRef]
- Shmuely, H.; Erdman, S.; Strasberg, B.; Rosenfeld, J.B. Seven years of left ventricular pacing due to malposition of pacing electrode. Pacing Clin. Electrophysiol. 1992, 15, 369–372. [Google Scholar] [CrossRef]
- Van Erckelens, F.; Sigmund, M.; Lambertz, H.; Kreis, A.; Reupcke, C.; Hanrath, P. Asymptomatic left ventricular malposition of a transvenous pacemaker lead through a sinus venosus defect: Follow-up over 17 years. Pacing Clin. Electrophysiol. 1991, 14, 989–993. [Google Scholar] [CrossRef]
- Lepore, V.; Pizzarelli, G.; Dernevik, L. Inadvertent transarterial pacemaker insertion: An unusual complication. Pacing Clin. Electrophysiol. 1987, 10, 951–954. [Google Scholar] [CrossRef]
- Kusumoto, F.M.; Schoenfeld, M.H.; Wilkoff, B.L.; Berul, C.I.; Birgersdotter-Green, U.M.; Carrillo, R.; Cha, Y.M.; Clancy, J.; Deharo, J.C.; Ellenbogen, K.A.; et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm 2017, 14, e503–e551. [Google Scholar] [CrossRef]
- van Gelder, B.M.; Scheffer, M.G.; Meijer, A.; Bracke, F.A. Transseptal endocardial left ventricular pacing: An alternative technique for coronary sinus lead placement in cardiac resynchronization therapy. Heart Rhythm 2007, 4, 454–460. [Google Scholar] [CrossRef]
- Sharifi, M.; Sorkin, R.; Lakier, J.B. Left heart pacing and cardioembolic stroke. Pacing Clin. Electrophysiol. 1994, 17, 1691–1696. [Google Scholar] [CrossRef]
- Kosmidou, I.; Karmpaliotis, D.; Kandzari, D.E.; Dan, D. Inadvertent transarterial lead placement in the left ventricle and aortic cusp: Percutaneous lead removal with carotid embolic protection and stent graft placement. Indian Pacing Electrophysiol. J. 2012, 12, 269–273. [Google Scholar] [CrossRef][Green Version]
- Liebold, A.; Aebert, H.; Muscholl, M.; Birnbaum, D.E. Cerebral embolism due to left ventricular pacemaker lead: Removal with cardiopulmonary bypass. Pacing Clin. Electrophysiol. 1994, 17, 2353–2355. [Google Scholar] [CrossRef]
- Overbeck, M.; Kolb, C.; Schmitt, C.; Schomig, A.; Lange, R. Accidental transarterial implantation of dual chamber pacemaker leads in the left ventricle and the right coronary artery. Pacing Clin. Electrophysiol. 2005, 28, 469–471. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Ohlow, M.A.; Roos, M.; Lauer, B.; Von Korn, H.; Geller, J.C. Incidence, predictors, and outcome of inadvertent malposition of transvenous pacing or defibrillation lead in the left heart. Europace 2016, 18, 1049–1054. [Google Scholar] [CrossRef]
- Schmiady, M.O.; Hofmann, M.; Maisano, F.; Morjan, M. Do all roads lead to Rome? Treatment of malposition pacemaker lead in the left ventricle. Eur. J. Cardio-Thorac. Surg. 2020, 57, 1009–1010. [Google Scholar] [CrossRef]
- Stouffer, C.W.; Shillingford, M.S.; Miles, W.M.; Conti, J.B.; Beaver, T.M. Lead astray: Minimally invasive removal of a pacing lead in the left ventricle. Clin. Cardiol. 2010, 33, E109–E110. [Google Scholar] [CrossRef]
- Singh, N.; Madan, H.; Arora, Y.K.; Dutta, R.; Sofat, S.; Bhardwaj, P.; Sharma, R.; Chadha, D.S.; Ghosh, A.K.; Sengupta, S. Malplacement of endocardial pacemaker lead in the left ventricle. Med. J. Armed Forces India 2014, 70, 76–78. [Google Scholar] [CrossRef][Green Version]
- Barold, S.S.; Giudici, M.C. Renewed interest in the significance of the tall R wave in ECG lead V1 during right ventricular pacing. Expert Rev. Med. Devices 2016, 13, 611–613. [Google Scholar] [CrossRef]
- Okmen, E.; Erdinler, I.; Oguz, E.; Akyol, A.; Turek, O.; Cam, N.; Ulufer, T. An electrocardiographic algorithm for determining the location of pacemaker electrode in patients with right bundle branch block configuration during permanent ventricular pacing. Angiology 2006, 57, 623–630. [Google Scholar] [CrossRef]
- Trohman, R.G.; Sharma, P.S. Detecting and managing device leads inadvertently placed in the left ventricle. Clevel. Clin. J. Med. 2018, 85, 69–75. [Google Scholar] [CrossRef]
- Bajaj, R.R.; Fam, N.; Singh, S.M. Inadvertent transarterial pacemaker lead placement. Indian Heart J. 2015, 67, 452–454. [Google Scholar] [CrossRef]
- Mazzetti, H.; Dussaut, A.; Tentori, C.; Dussaut, E.; Lazzari, J.O. Transarterial permanent pacing of the left ventricle. Pacing Clin. Electrophysiol. 1990, 13, 588–592. [Google Scholar] [CrossRef]
- Winner, S.J.; Boon, N.A. Transvenous pacemaker electrodes placed unintentionally in the left ventricle: Three cases. Postgrad. Med. J. 1989, 65, 98–102. [Google Scholar] [CrossRef]
- Zabek, A.; Malecka, B.; Pfitzner, R.; Trystula, M.; Kruszec, P.; Lelakowski, J. Extraction of left ventricular pacing lead inserted via the left subclavian artery. Pol. Arch. Med. Wewn. 2013, 123, 560–561. [Google Scholar] [CrossRef][Green Version]
- Almomani, A.; Abualsuod, A.; Paydak, H.; Peer, W.; Maskoun, W. Chronic lead malposition diagnosis and management: Discussion of two cases and literature review. Clin. Case Rep. 2017, 5, 270–276. [Google Scholar] [CrossRef]
- Ergun, K.; Tufekcioglu, O.; Karabal, O.; Ozdogan, O.U.; Deveci, B.; Golbasi, Z. An unusual cause of stroke in a patient with permanent transvenous pacemaker. Jpn. Heart J. 2004, 45, 873–875. [Google Scholar] [CrossRef]
- Sinha, S.K.; Varm, C.M.; Thakur, R.; Krishna, V.; Goel, A.; Kumar, A.; Jha, M.J.; Mishra, V.; Singh Syal, K. An Unconventional Route of Left Ventricular Pacing. Cardiol. Res. 2015, 6, 324–328. [Google Scholar] [CrossRef]
- Sharifi, M.; Sorkin, R.; Sharifi, V.; Lakier, J.B. Inadvertent malposition of a transvenous-inserted pacing lead in the left ventricular chamber. Am. J. Cardiol. 1995, 76, 92–95. [Google Scholar] [CrossRef]
- Bohm, A.; Banyai, F.; Komaromy, K.; Pinter, A.; Preda, I. Cerebral embolism due to a retained pacemaker lead: A case report. Pacing Clin. Electrophysiol. 1998, 21, 629–630. [Google Scholar] [CrossRef]
- Gamble, J.H.P.; Herring, N.; Ginks, M.; Rajappan, K.; Bashir, Y.; Betts, T.R. Endocardial left ventricular pacing for cardiac resynchronization: Systematic review and meta-analysis. Europace 2018, 20, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Geller, L.; Sallo, Z.; Molnar, L.; Tahin, T.; Ozcan, E.E.; Kutyifa, V.; Osztheimer, I.; Szilagyi, S.; Szegedi, N.; Abraham, P.; et al. Long-term single-centre large volume experience with transseptal endocardial left ventricular lead implantation. Europace 2019, 21, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.M.; Biffi, M.; Geller, L.; Leclercq, C.; Ruffa, F.; Tung, S.; Defaye, P.; Yang, Z.; Gerritse, B.; van Ginneken, M.; et al. ALternate Site Cardiac ResYNChronization (ALSYNC): A prospective and multicentre study of left ventricular endocardial pacing for cardiac resynchronization therapy. Eur. Heart J. 2016, 37, 2118–2127. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.F.; Lever, H. Six uneventful years with a pacing lead in the left ventricle. Heart Rhythm 2013, 10, 614–615. [Google Scholar] [CrossRef]
- Contractor, T.; Co, M.L.; Cooper, J.M.; Mandapati, R.; Abudayyeh, I. Management of inadvertent lead placement in the left ventricle via a patent foramen ovale: A multidisciplinary approach. HeartRhythm Case Rep. 2020, 6, 89–93. [Google Scholar] [CrossRef][Green Version]
- Agnelli, D.; Ferrari, A.; Saltafossi, D.; Falcone, C. Stroke Cardiembolico dovuto a malposizionamento di elettrocatetere in ventricolo sinistro. Descrizione di un caso. Ital. Heart J. Suppl. 2000, 1, 122–125. [Google Scholar]
- Aguilar, J.; Summerson, C. Transarterial permanent pacing of the left ventricle. An unusual complication. Rev. Mex. Cardiol. 2002, 13, 56–58. [Google Scholar]
- Alan, B.; Dusak, A.; Cetincakmak, M.G.; Alan, S. An unusual pacemaker malposition and delayed diagnosis. Dicle Med. J. 2015, 42. [Google Scholar] [CrossRef]
- Alozie, A.; Westphal, B.; Yerebakan, C.; Steinhoff, G. Transient ischaemic attack due to the lead of an implantable defibrillator in the left heart. Interact. Cardiovasc. Thorac. Surg. 2011, 14, 128–130. [Google Scholar] [CrossRef]
- Anastacio, M.M.; Castillo-Sang, M.; Lawton, J.S. Laser Extraction of Pacemaker Lead Traversing a Patent Foramen Ovale and the Mitral Valve. Ann. Thorac. Surg. 2012, 94, 2125–2127. [Google Scholar] [CrossRef]
- Arnar, D.O.; Kerber, R.E. Cerebral Embolism Resulting from a Transvenous Pacemaker Catheter Inadvertently Placed in the Left Ventricle: A Report of Two Cases Confirmed by Echocardiography. Echocardiography 2001, 18, 681–684. [Google Scholar] [CrossRef]
- Adnan Aslam, A.; McIlwain, E.F.; Talano, J.V.; Ferguson, T.B.; McKinnie, J.; Kerut, E.K. An Unusual Case of Embolic Stroke: A Permanent Ventricular Pacemaker Lead Entirely Within the Arterial System Documented by Transthoracic and Transesophageal Echocardiography. Echocardiogr 1999, 16, 373–378. [Google Scholar] [CrossRef]
- Bahadorani, J.N.; Schricker, A.A.; Pretorius, V.G.; Birgersdotter-Green, U.; Dominguez, A.; Mahmud, E. Percutaneous extraction of inadvertently placed left-sided pacemaker leads with complete cerebral embolic protection. Catheter. Cardiovasc. Interv. 2015, 86, 777–785. [Google Scholar] [CrossRef]
- Bauersfeld, U.K.; Thakur, R.K.; Ghani, M.; Yee, R.; Klein, G.J. Malposition of transvenous pacing lead in the left ventricle: Radiographic findings. Am. J. Roentgenol. 1994, 162, 290–292. [Google Scholar] [CrossRef]
- Curnis, A.; Bontempi, L.; Coppola, G.; Cerini, M.; Novo, S.; Dei Cas, L. Undesired left ventricular pacing. G. Ital. Cardiol. 2011, 12, 724–725. [Google Scholar]
- Daher, I.N.; Schwarz, E.R.; Agoston, I.; Rahman, M.A.; Saeed, M.; Ahmad, M. Live Three-Dimensional Echocardiography in Diagnosis of Interventricular Septal Perforation by Pacemaker Lead. Echocardiography 2006, 23, 428–429. [Google Scholar] [CrossRef]
- Ghani, M.; Thakur, R.K.; Boughner, D.; Morillo, C.A.; Yee, R.; Klein, G.J. Malposition of transvenous pacing lead in the left ventricle. Pacing Clin. Electrophysiol. PACE 1993, 16, 1800–1807. [Google Scholar] [CrossRef]
- Feltes Guzman, G.I.; Vivas Balcones, D.; Perez de Isla, L.; Zamorano Gomez, J.L. Long-term pacemaker lead malposition. Role of echocardiography. Rev. Esp. Cardiol. Mar. 2011, 64, 250. [Google Scholar] [CrossRef]
- Harrison, J.L.; Patel, R.; Jogiya, R.; Redwood, S.; Rinaldi, C. Use of a cerebral protection device for the laser extraction of a pacemaker lead traversing a patent foramen ovale. HeartRhythm Case Rep. 2017, 3, 447–449. [Google Scholar] [CrossRef]
- Heck, P.M.; Hoole, S.P.; Cooper, J.P.; Begley, D.A. Inadvertent placement of left ventricular endocardial pacing lead. J. Cardiovasc. Med. 2012, 13, 656–659. [Google Scholar] [CrossRef]
- Hinojos, A.; Ilg, K. Removal of Misplaced Left Ventricular Single Lead Pacemaker in a Patient Presenting with Recurrent Transient Ischemic Attacks. Spartan Med. Res. J. 2017, 2, 6068. [Google Scholar] [CrossRef]
- Kalavakunta, J.K.; Gupta, V.; Paulus, B.; Lapenna, W. An Unusual Cause of Transient Ischemic Attack in a Patient with Pacemaker. Case Rep. Cardiol. 2014, 2014, 1–3. [Google Scholar] [CrossRef]
- Karavelioglu, Y.; Doğan, T.; Kalçık, M.; Yalçınkaya, A. Malposition of an atrial pacemaker lead crossing through patent foramen ovale in a patient with ischemic stroke. Turk Kardiyol. Dern. Ars.-Arch. Turk. Soc. Cardiol. 2015, 44, 87. [Google Scholar] [CrossRef][Green Version]
- Kutarski, A.; Pietura, R.; Tomaszewski, A.; Czajkowski, M.; Boczar, K. Transvenous extraction of an eight-year-old ventricular lead accidentally implanted into the left ventricle. Kardiol. Pol. 2013, 71, 1317–1321. [Google Scholar] [CrossRef]
- McManus, D.D.; Mattei, M.-L.; Rose, K.; Rashkin, J.; Rosenthal, L.S. Inadvertent Lead Placement In The Left Ventricle: A Case Report And Brief Review. Indian Pacing Electrophysiol. J. 2009, 9, 224–228. [Google Scholar]
- Orlov, M.V.; Messenger, J.C.; Tobias, S.; Smith, C.W.; Waider, W.; Winters, R.; Schandling, A.; Castellanet, M. Transesophageal echocardiographic visualization of left ventricular malpositioned pacemaker electrodes: Implications for lead extraction procedures. Pacing Clin. Electrophysiol. 1999, 22, 1407–1409. [Google Scholar] [CrossRef]
- Parikh, S.S.; Traub, D.; Wormer, D.; Huang, D.T. Expressive aphasia in a patient with recent dual-chamber cardioverter-defibrillator implantation: A preventable complication. Cardiol. J. 2011, 18. [Google Scholar]
- Raghavan, C.; Cashion, W.R.; Spencer, W.H. Malposition of transvenous pacing lead in the left ventricle. Clin. Cardiol. 1996, 19, 335–338. [Google Scholar] [CrossRef]
- Rath, C.; Andreas, M.; Khazen, C.; Wiedemann, M.; Habertheuer, A.; Kocher, A. Pacemaker lead malpositioning led to subsequent ischemic strokes despite antiplatelet and anticoagulation therapy. J. Cardiothorac. Surg. 2014, 9, 54. [Google Scholar] [CrossRef]
- Read, P.A.; Bowd, L.M.; Kalra, P.R.; Roberts, P.R. Ventricular tachycardia and amaurosis fugax following inadvertent left ventricular pacing. Int. J. Cardiol. 2005, 99, 479–480. [Google Scholar] [CrossRef]
- Reising, S.; Safford, R.; Castello, R.; Bosworth, V.; Freeman, W.; Kusumoto, F. A Stroke of Bad Luck: Left Ventricular Pacemaker Malposition. J. Am. Soc. Echocardiogr. 2007, 20, 1316.e1–1316.e3. [Google Scholar] [CrossRef] [PubMed]
- Roos, M.; Geller, C.; Ohlow, M. Catch the Important Beats: Unmasking Inadvertently Left Ventricular Pacing. Open J. Clin. Med. Case Rep. 2015, 1, 1–4. [Google Scholar]
- Schiavone, W.A.; Castle, L.W.; Salcedo, E.; Graor, R. Amaurosis Fugax in a Patient with a Left Ventricular Endocardial Pacemaker. Pacing Clin. Electrophysiol. 1984, 7, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Schon, N. Inadvertently Placed Pacing Lead: A Case Report. J. Kardiol. 2007, 14, 228–230. [Google Scholar]
- Schulze, M.R.; Ostermaier, R.; Franke, Y.; Matschke, K.; Braun, M.U.; Strasser, R.H. Aortic Endocarditis Caused by Inadvertent Left Ventricular Pacemaker Lead Placement. Circulation 2005, 112, e361–e363. [Google Scholar] [CrossRef][Green Version]
- Sivapathasuntharam, D.; Hyde, J.A.J.; Reay, V.; Rajkumar, C. Recurrent strokes caused by a malpositioned pacemaker lead. Age Ageing 2011, 41, 420–421. [Google Scholar] [CrossRef]
- Teshome, M.; Ifedili, I.; Nayyar, M.; Levine, Y.; Holden, A.; Yedlapati, N.; Kabra, R. Diagnosis and management of inadvertently placed pacemaker lead in the left ventricle following sinus venosus atrial septal defect repair surgery. HeartRhythm Case Rep. 2020, 6, 279–282. [Google Scholar] [CrossRef][Green Version]
- Thosani, A.; Raina, A.; Liu, E.; Lasorda, D.; Chenarides, J. Malpositioned endocardial left ventricular pacing lead extraction with transcatheter cerebral embolic protection in the setting of multiple prior embolic strokes. HeartRhythm Case Rep. 2019, 5, 552–554. [Google Scholar] [CrossRef]
- Al-Dashti, R.; Huynh, T.; Rosengarten, M.; Page, P. Transvenous pacemaker malposition in the systemic circulation and pacemaker infection: A case report and review of the literature. Can. J. Cardiol. 2002, 18, 887–890. [Google Scholar]
- Barbarash, S.; Tong, A. Automatic internal cardiac defibrillator lead in the left ventricle. Complex Issues Cardiovasc. Dis. 2016, 2, 111–114. [Google Scholar]
- Benito Martin, E.; Rubin Lopez, J.; Corros Vicente, C.; De La Hera Galarza, J.; Martin Fernandez, M. Malposition of the pacemaker lead in the left ventricle. Rev. Port. Cardiol. 2013, 32, 633–635. [Google Scholar] [CrossRef]
- Bodian, M.; Aw, F.; Bamba, M.N.; Kane, A.; Jobe, M.; Tabane, A.; Mbaye, A.; Sarr, S.A.; Diao, M.; Sarr, M.; et al. Sinus venosus atrial septal defect: A rare cause of misplacement of pacemaker leads. Int. Med. Case Rep. J. 2013, 6, 29–32. [Google Scholar] [CrossRef][Green Version]
- Bracke, F.A.; Meijer, A.; Van Gelder, L. Lead extraction for device related infections: A single-centre experience. Europace 2004, 6, 243–247. [Google Scholar] [CrossRef]
- Calvagna, G.M.; Patanè, S.; Ceresa, F.; Fontana, A.; Sicuso, G.; Vinci, E.; Muscio, G.; Vasquez, L.; Patanè, F. Inadvertent implantation of a pacemaker lead in the left ventricle: A new challenge in cardiology. Int. J. Cardiol. 2015, 202, 914–917. [Google Scholar] [CrossRef]
- Carrizo, A.; Alfie, A.; Amit, G.; Andersen, G.; Leguizamón, J.; Oseroff, O. Transarterial Percutaneous Pacemaker Lead Extraction. Rev. Argent. De Cardiol. 2015, 83, 443–444. [Google Scholar]
- Chun, J.K.; Bode, F.; Wiegand, U.K. Left ventricular malposition of pacemaker lead in Chagas’ disease. Pacing Clin. Electrophysiol. PACE 2004, 27, 1682–1685. [Google Scholar] [CrossRef]
- De Cock, C.C.; van Campen, C.M.; Kamp, O.; Visser, C.A. Successful percutaneous extraction of an inadvertently placed left ventricular pacing lead. Europace 2003, 5, 195–197. [Google Scholar] [CrossRef]
- Deshmukh, A.; Pothineni, N.V.; Pant, S.; Paydak, H. Pacemaker lead malposition: When right is not right! Arch. Cardiovasc. Dis. 2014, 107, 201–202. [Google Scholar] [CrossRef][Green Version]
- Di Tommaso, L.; Iannelli, G.; Mottola, M.; Mannaccio, V.; Poli, V.; Esposito, G.; Morisco, C.; Vosa, C. TEVAR for Iatrogenic Injury of the Distal Aortic Arch after Pacemaker Implantation. EJVES Extra 2013, 26, e17–e19. [Google Scholar] [CrossRef]
- Dissmann, R.; Wolthoff, U.; Zabel, M. Double left ventricular pacing following accidental malpositioning of the right ventricular electrode during implantation of a cardiac resynchronization therapy device. J. Cardiothorac. Surg. 2013, 8, 162. [Google Scholar] [CrossRef]
- Estrada-Quintero, T.; Kross, D.E.; Gorcsan, J. Identification of a malpositioned atrial pacemaker lead across a patent foramen ovale by transesophageal echocardiography. J. Am. Soc. Echocardiogr. 1995, 8, 560–562. [Google Scholar] [CrossRef]
- Moorthy, N.; Garg, N. Inadvertent temporary pacemaker lead placement in aortic sinus. Heart Views 2013, 14, 182–184. [Google Scholar] [CrossRef]
- Gondi, B.; Nanda, N.C. Real-time, two-dimensional echocardiographic features of pacemaker perforation. Circulation 1981, 64, 97–106. [Google Scholar] [CrossRef]
- Gupta, S.; Annamalaisamy, R.; Coupe, M. Misplacement of Temporary Pacing Wire into the Left Ventricle Via an Anomalous Vein. Hell. J. Cardiol. HJC Hell. Kardiol. Ep. 2010, 51, 175–177. [Google Scholar]
- Iliceto, S.; Di Biase, M.; Antonelli, G.; Favale, S.; Rizzon, P. Two-Dimensional Echocardiographic Recognition of a Pacing Catheter Perforation of the Interventricular Septum. Pacing Clin. Electrophysiol. 1982, 5, 934–936. [Google Scholar] [CrossRef]
- Irvine, J.N.; LaPar, D.J.; Mahapatra, S.; DiMarco, J.P.; Ailawadi, G. Treatment of a malpositioned transcutaneous ventricular pacing lead in the left ventricle via direct aortic puncture. Europace 2011, 13, 1207–1208. [Google Scholar] [CrossRef]
- Judson, P.L.; Moore, T.B.; Swank, M.; Ashworth, H.E. Two-Dimensional Echocardiograms of a Transvenous Left Ventricular Pacing Catheter. Chest 1981, 80, 228–230. [Google Scholar] [CrossRef]
- Letek, A.; Kurzawski, J.; Sadowski, M. The random placement of pacing lead in the left ventricle in a patient with patent foramen ovale. Folia Cardiol. 2016, 11, 535–538. [Google Scholar]
- Lin, J.; Wang, L.; Zhao, Y. Inadvertent left ventricular pacing and perforation by a temporary pacemaker. J. Electrocardiol. 2017, 50, 686–689. [Google Scholar] [CrossRef]
- Miniard, J.K. Ultrasound Diagnosis of Malpositioned Pacemaker Lead Through a Patent Foramen Ovale. J. Diagn. Med. Sonogr. 2001, 17, 172–174. [Google Scholar] [CrossRef]
- Ninot, S.; Sánchez, G.; Mestres, C.-A. An unusual travel of an endocardial pacing lead to the left ventricle. Interact. Cardiovasc. Thorac. Surg. 2003, 2, 624–625. [Google Scholar] [CrossRef]
- Pollock, J.; Pollema, T.; Pretorius, V.; Birgersdotter-Green, U.; Cronin, B. Percutaneous Laser Lead Extraction of an Inadvertently Placed Left-Sided Pacemaker Lead. J. Cardiothorac. Vasc. Anesthesia 2017, 31, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Ross, W.B.; Mohiuddin, S.M.; Pagano, T.; Hughes, D. Malposition of A Transvenous Cardiac Electrode Associated with Amaurosis Fugax. Pacing Clin. Electrophysiol. 1983, 6, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Rovera, C.; Golzio, P.G.; Corgnati, G.; Conti, V.; Franco, E.; Frea, S.; Moretti, C. A pacemaker lead in the left ventricle: An “unexpected” finding? J. Cardiol. Cases Dec 2019, 20, 228–231. [Google Scholar] [CrossRef]
- Ruhela, M.; Bagarhatta, M. Right bundle branch block pacing pattern (complicated and uncomplicated) on ECG with right ventricular pacing in a single patient A case report. J. Indian Coll. Cardiol. 2014, 4, 31–35. [Google Scholar] [CrossRef]
- Sahin, T.; Kilic, T.; Celikyurt, U.; Aygun, F.; Bildirici, U.; Agacdiken, A. Asymptomatic malposition of pacemaker lead associated with thrombus. Clin. Res. Cardiol. Off. J. Ger. Card. Soc. 2009, 98, 71–73. [Google Scholar] [CrossRef]
- Santarpia, G.; Passafaro, F.; Pasceri, E.; Mongiardo, A.; Curcio, A.; Indolfi, C. Inadvertent defibrillator lead placement into the left ventricle after MitraClip implantation: A case report. Medicine 2018, 97, e0733. [Google Scholar] [CrossRef]
- Sarubbi, B.; Scognamiglio, G.; Fusco, F.; Melillo, E.; D’Alto, M.; Russo, M.G. A “long-standing” malpositioned pacing lead. Long-term follow-up after extraction. Monaldi Arch. Chest Dis. Arch. Monaldi Mal. Torace 2018, 88, 927. [Google Scholar]
- Seki, H.; Fukui, T.; Shimokawa, T.; Manabe, S.; Watanabe, Y.; Chino, K.; Takanashi, S. Malpositioning of a pacemaker lead to the left ventricle accompanied by posterior mitral leaflet injury. Interact. Cardiovasc. Thorac. Surg. 2008, 8, 235–237. [Google Scholar] [CrossRef]
- Seethala, S.; Kumar, A.; Adhar, C.; Generalovich, T. A Rare Cause of Cardiac Tamponade: Left Ventricular Pacemaker Malposition. Open Cardiovasc. Imaging J. 2011, 3, 1–3. [Google Scholar] [CrossRef][Green Version]
- Splittgerber, F.H.; Ulbricht, L.J.; Reifschneider, H.-J.; Probst, H.; Gülker, H.; Minale, C. Left Ventricular Malposition of a Transvenous Cardioverter Defibrillator Lead: A Case Report. Pacing Clin. Electrophysiol. 1993, 16, 1066–1069. [Google Scholar] [CrossRef]
- Syed, A.; Salim, S.; Castillo, R. Incidental Finding of Malpositioned Pacing Lead in the Left Ventricle in a Patient With Subacute Subdural Hematoma. Cardiol. Res. 2012, 3, 187–188. [Google Scholar] [CrossRef][Green Version]
- Tobin, A.M.; Grodman, R.S.; Fisherkeller, M.; Nicolosi, R. Two-Dimensional Echocardiographic Localization of a Malpositioned Pacing Catheter. Pacing Clin. Electrophysiol. 1983, 6, 291–299. [Google Scholar] [CrossRef]
- Velankar, P.; Alchalabi, S.; Bala, S.; Chang, S. Transarterial direct left ventricolar pacing. MDCVJ 2014, 10, 255–256. [Google Scholar]
- Velibey, Y.; Yaylak, B.; Guvenc, T.S.; Cinier, G.; Kalenderoglu, K.; Guzelburc, O.; Yildirimturk, O. Inadvertent Left Ventricle Endocardial or Uncomplicated Right Ventricular Pacing: How to Differentiate in the Emergency Department. J. Emerg. Med. 2018, 54, e91–e95. [Google Scholar] [CrossRef]
- Wynn, G.J.; Weston, C.; Cooper, R.J.; Somauroo, J.D. Inadvertent left ventricular pacing through a patent foramen ovale: Identification, management and implications for postpacemaker implantation checks. BMJ Case Rep. 2013, 2013. [Google Scholar] [CrossRef]

| Number of Cases | 157 | |
| Age at diagnosis, years (SD) | 68 (14) | |
| Male gender, N (%) | 74 (47) | |
| Atrial Fibrillation, N (%) | 23 (15) | |
| Hypertension, N (%) | 23 (15) | |
| Ischemic Heart Disease, N (%) | 23 (15) | |
| Diabetes, N (%) | 11 (7) | |
| Mechanical Heart Valve, N (%) | 2 (1) | |
| History of Stroke/TIA, N (%) | 5 (3) | |
| Heart Failure, N (%) | 14 (9) | |
| Baseline Antithrombotic Therapy | No Antithrombotic therapy, N (%) | 55 (35) |
| Antiplatelets, N (%) | 24 (15) | |
| Anticoagulants, N (%) | 22 (14) | |
| Unknown, N (%) | 56 (36) | |
| Indication for implant | Sick Sinus Syndrome, N (%) | 45 (29) |
| Atrioventricular block, N (%) | 64 (41) | |
| Primary prevention, N (%) | 11 (7) | |
| Secondary prevention, N (%) | 6 (4) | |
| Other, N (%) | 18 (11) | |
| Unknown, N (%) | 13 (8) | |
| Right sided implant, N (%) | 38 (24) | |
| Type of device | Pacemaker, N (%) | 138 (88) |
| Implantable Cardioverter Defibrillator, N (%) | 16 (10) | |
| Cardiac Resynchronization Therapy, N (%) | 3 (2) | |
| Number of Cases | 157 | |
| Time to diagnosis, days (IQR) | 365 (30–1642) | |
| Symptoms at diagnosis | Asymptomatic, N (%) | 73 (46) |
| Transient ischemic attack or Stroke, N (%) | 48 (31) | |
| Heart Failure, N (%) | 23 (15) | |
| Endocarditis, N (%) | 2 (1) | |
| Other, N (%) | 11 (7) | |
| Confirmation of Malposition | By Fluoroscopy, N (%) | 3 (2) |
| By Chest X-ray, N (%) | 28 (18) | |
| By Transthoracic Echocardiography, N (%) | 95 (60) | |
| By Transesophageal Echocardiography, N (%) | 23 (15) | |
| By Computed Tomography, N (%) | 4 (2) | |
| Unknown, N (%) | 2 (3) | |
| Cause of Malposition | Interatrial septum perforation, N (%) | 31 (20) |
| Patent foramen ovale, N (%) | 46 (29) | |
| Atrial Septal Defect, N (%) | 25 (16) | |
| Interventricular septum perforation, N (%) | 7 (4) | |
| Arterial puncture, N (%) | 38 (24) | |
| Complex congenital heart disease, N (%) | 6 (4) | |
| Other, N (%) | 4 (3) | |
| QRS Transition | Number of Cases (%) |
|---|---|
| V1, N (%) | 1 (2) |
| V2, N (%) | 3 (5) |
| V3, N (%) | 13 (20) |
| V4, N (%) | 17 (26) |
| V5, N (%) | 20 (31) |
| V6, N (%) | 10 (16) |
| QRS pattern in lead V1 | |
| R, N (%) | 26 (40) |
| qR, N (%) | 5 (8) |
| QS, N (%) | 1 (2) |
| Rr’, N (%) | 20 (31) |
| rR’, N (%) | 12 (19) |
| QRS pattern in lead L1 | |
| QS, N (%) | 19 (30) |
| rS, N (%) | 30 (47) |
| Rs, N (%) | 6 (9) |
| R, N (%) | 8 (12) |
| rs, N (%) | 1 (2) |
| QRS pattern in lead V6 | |
| QS, N (%) | 19 (30) |
| rS, N (%) | 37 (59) |
| R, N (%) | 3 (5) |
| RS, N (%) | 4 (6) |
| QRS pattern in lead aVL | |
| QS, N (%) | 13 (20) |
| rS, N (%) | 27 (42) |
| R, N (%) | 18 (28) |
| qR, N (%) | 1 (2) |
| RS, N (%) | 4 (6) |
| QRS pattern in lead III | |
| QS, N (%) | 14 (23) |
| rS, N (%) | 11 (18) |
| Rr’, N (%) | 2 (3) |
| R, N (%) | 19 (31) |
| rR’, N (%) | 2 (3) |
| qR, N (%) | 12 (19) |
| RS, N (%) | 2 (3) |
| Conservative Treatment n = 55 | Lead Extraction n = 93 | p-Value | ||
|---|---|---|---|---|
| Age, years (IQR) | 74 (67–79) | 69 (62–76) | 0.014 | |
| Year of report (IQR) | 2006 (1998–2011) | 2011 (2003–2015) | 0.002 | |
| Time from implantation, days (IQR) | 875 (292–2281) | 90 (2–690) | <0.0001 | |
| Male gender | 23 (42%) | 48 (52%) | ns | |
| Symptoms at diagnosis | Asymptomatic, N (%) | 27 (49%) | 41 (44%) | ns |
| TIA/Stroke, N (%) | 15 (27%) | 32 (34%) | 0.105 | |
| Heart Failure, N (%) | 9 (16%) | 12 (13%) | ns | |
| Congenital heart disease | 12 (22%) | 18 (19%) | ns | |
| Transarterial lead | 12 (22%) | 25 (27%) | ns | |
| Antithrombotic therapy at diagnosis | No antithrombotics, N (%) | 24 (52%) | 30 (56%) | ns |
| Antiplatelets, N (%) | 11 (24%) | 13 (24%) | ns | |
| Anticoagulants, N (%) | 11 (24%) | 11 (20%) | ns | |
| Follow-up, months (IQR) | 36 (12–72) | 2 (1–6) | <0.0001 | |
| Variable | Odds Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| Age ≤ 75 years old | 4.4 | 1.0–6.8 | 0.001 |
| Dwelling time ≤ 1 year | 10.7 | 4.1–27.5 | <0.0001 |
| TIA/Stroke at ILMLH diagnosis | 2.7 | 1.0–6.8 | 0.042 |
| Congenital Heart disease | 1.6 | 0.6–4.4 | 0.328 |
| Year of the report | 1.0 | 0.9–1.1 | 0.085 |
| Male gender | 1.1 | 0.5–2.5 | 0.842 |
| Transarterial lead | 1.7 | 0.6–4.9 | 0.328 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spighi, L.; Notaristefano, F.; Piraccini, S.; Giuffrè, G.; Barengo, A.; D’Ammando, M.; Notaristefano, S.; Bagliani, G.; Zingarini, G.; Angeli, F.; et al. Inadvertent Lead Malposition in the Left Heart during Implantation of Cardiac Electric Devices: A Systematic Review. J. Cardiovasc. Dev. Dis. 2022, 9, 362. https://doi.org/10.3390/jcdd9100362
Spighi L, Notaristefano F, Piraccini S, Giuffrè G, Barengo A, D’Ammando M, Notaristefano S, Bagliani G, Zingarini G, Angeli F, et al. Inadvertent Lead Malposition in the Left Heart during Implantation of Cardiac Electric Devices: A Systematic Review. Journal of Cardiovascular Development and Disease. 2022; 9(10):362. https://doi.org/10.3390/jcdd9100362
Chicago/Turabian StyleSpighi, Lorenzo, Francesco Notaristefano, Silvia Piraccini, Giuseppe Giuffrè, Alberto Barengo, Matteo D’Ammando, Salvatore Notaristefano, Giuseppe Bagliani, Gianluca Zingarini, Fabio Angeli, and et al. 2022. "Inadvertent Lead Malposition in the Left Heart during Implantation of Cardiac Electric Devices: A Systematic Review" Journal of Cardiovascular Development and Disease 9, no. 10: 362. https://doi.org/10.3390/jcdd9100362
APA StyleSpighi, L., Notaristefano, F., Piraccini, S., Giuffrè, G., Barengo, A., D’Ammando, M., Notaristefano, S., Bagliani, G., Zingarini, G., Angeli, F., Verdecchia, P., & Cavallini, C. (2022). Inadvertent Lead Malposition in the Left Heart during Implantation of Cardiac Electric Devices: A Systematic Review. Journal of Cardiovascular Development and Disease, 9(10), 362. https://doi.org/10.3390/jcdd9100362








